Characterization of Two Gonadal Genes, zar1 and wt1b, in Hermaphroditic Fish Asian Seabass (Lates calcarifer)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Ethics
2.2. RNA Extraction and Reverse Transcription
2.3. Cloning of cDNA Fragments
2.4. Sequence and Phylogenetic Analysis
2.5. Expression Analysis of Lczar1 and Lcwt1b in Tissues via RT-qPCR
2.6. In Situ Hybridization
3. Results
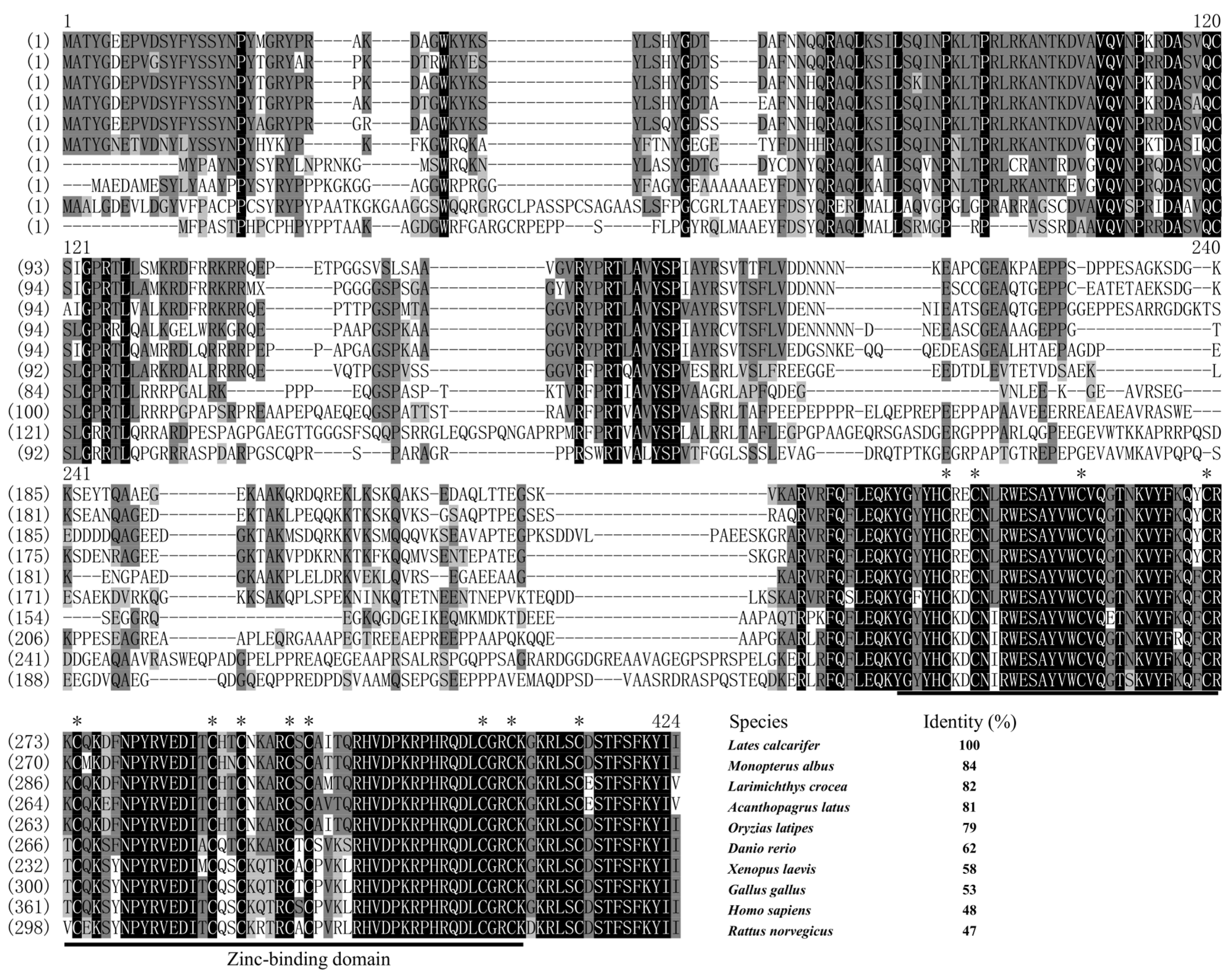
3.1. Sequence Alignment and Phylogenetic Analysis of zar1 and wt1b cDNA
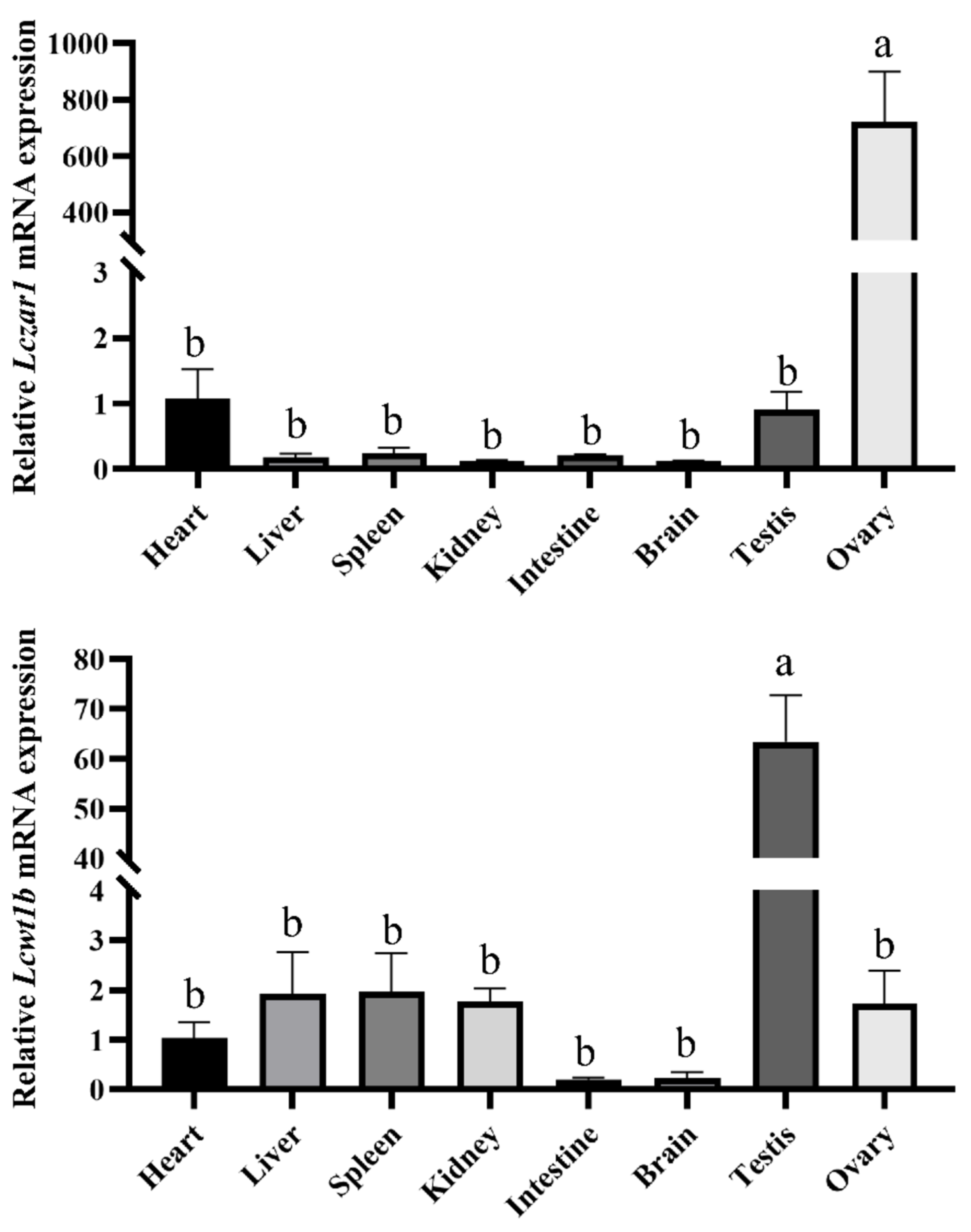
3.2. The mRNA Expression Profiles of Lczar1 and Lcwt1b
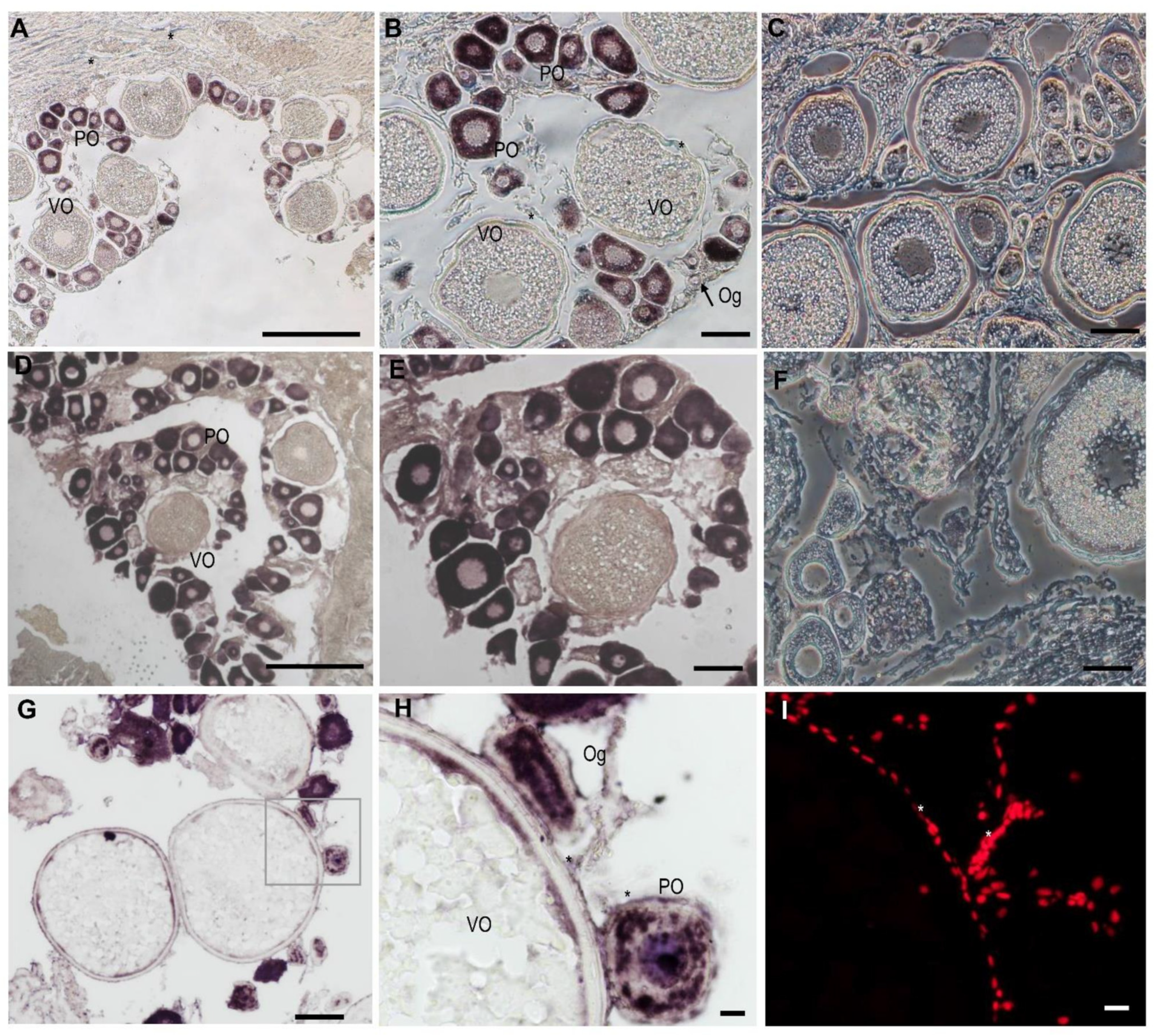
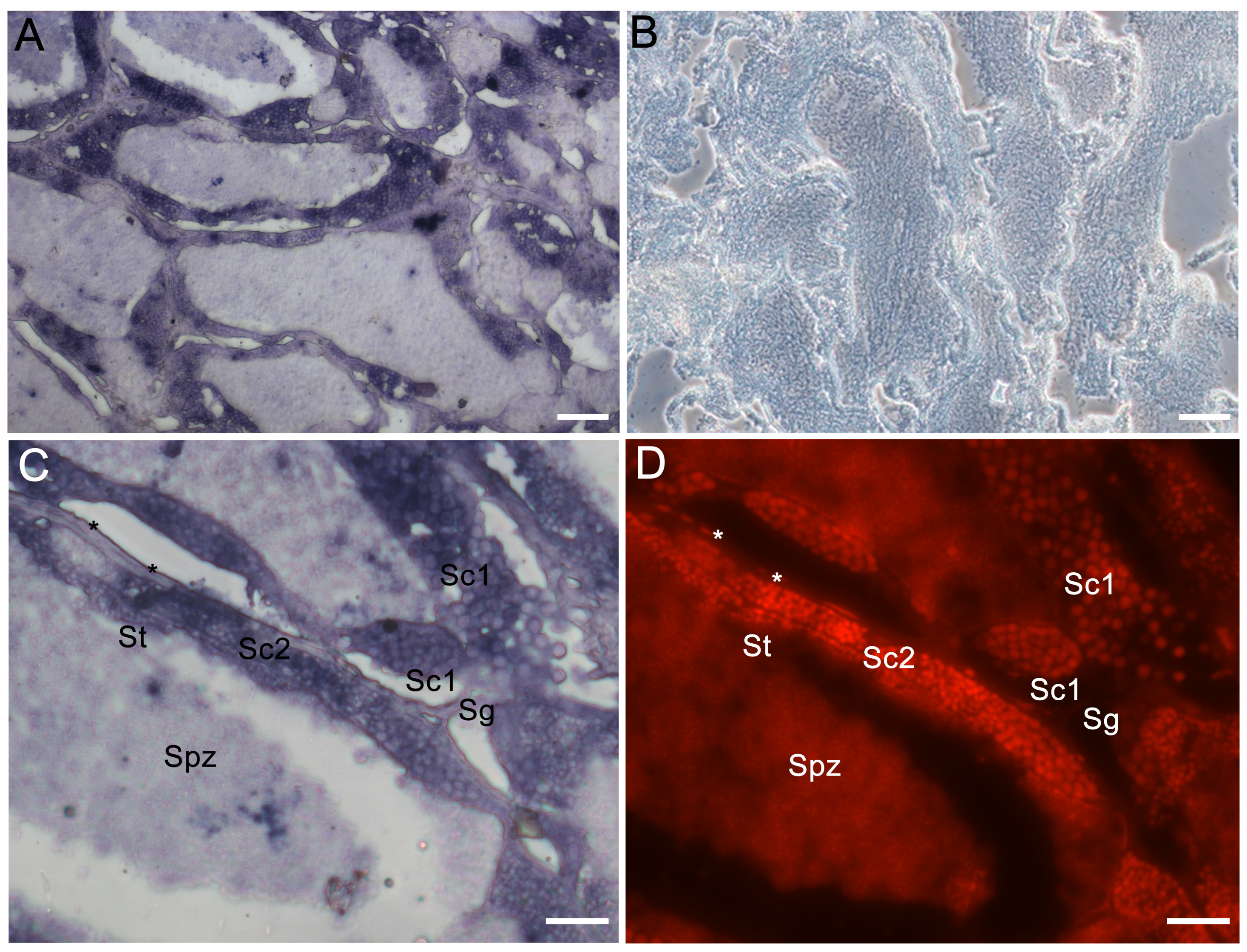
3.3. The Cellular Localization of Lczar1 and Lcwt1b in Gonads
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dodson, A.E.; Kennedy, S. Phase separation in germ cells and development. Dev. Cell 2020, 55, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Robles, V.; Riesco, M.F.; Psenicka, M.; Saito, T.; Valcarce, D.G.; Cabrita, E.; Herráez, P. Biology of teleost primordial germ cells (PGCs) and spermatogonia: Biotechnological applications. Aquaculture 2017, 472, 4–20. [Google Scholar] [CrossRef]
- Williamson, A.; Lehmann, R. Germ cell development in Drosophila. Annu. Rev. Cell Dev. Biol. 1996, 12, 365–391. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Liu, M.; Tao, Y.; Wu, X.; Yang, Y.; Wang, T.; Meng, Z.; Xu, H.; Liu, X. Pou5f1 and Nanog are reliable germ cell-specific genes in gonad of a protogynous hermaphroditic fish, orange-spotted grouper (Epinephelus coioides). Genes 2022, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Wu, X.; Liu, M.; Zhong, C.; Xu, H.; Li, S.; Lin, H.; Liu, X. Identification and characterization of germ cell genes vasa and dazl in a protogynous hermaphrodite fish, orange-spotted grouper (Epinephelus coioides). Gene Expr. Patterns 2020, 35, 119095. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-H.; Wang, Y.; Lu, W.-J.; Li, Z.; Liu, X.-C.; Li, S.-S.; Zhou, L.; Gui, J.-F. Divergent expression patterns and function implications of four nanos genes in a hermaphroditic fish, Epinephelus coioides. Int. J. Mol. Sci. 2017, 18, 685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Duan, J.; Cheng, N.; Nagahama, Y. Specific expression of Olpiwi1 and Olpiwi2 in medaka (Oryzias latipes) germ cells. Biochem. Biophys. Res. Commun. 2012, 418, 592–597. [Google Scholar] [CrossRef]
- Li, X.-Y.; Mei, J.; Ge, C.-T.; Liu, X.-L.; Gui, J.-F. Sex determination mechanisms and sex control approaches in aquaculture animals. Sci. China Life Sci. 2022, 65, 1091–1122. [Google Scholar] [CrossRef]
- Chen, M.; Cen, C.; Wang, N.; Shen, Z.; Wang, M.; Liu, B.; Li, J.; Cui, X.; Wang, Y.; Gao, F. The functions of Wt1 in mouse gonad development and somatic cells differentiation. Biol. Reprod. 2022, 107, 269–274. [Google Scholar] [CrossRef]
- Wu, Y.-K.; Fan, H.-Y. Revisiting ZAR proteins: The understudied regulator of female fertility and beyond. Cell. Mol. Life Sci. 2022, 79, 92. [Google Scholar] [CrossRef]
- Wu, X.M.; Viveiros, M.M.; Eppig, J.J.; Bai, Y.C.; Fitzpatrick, S.L.; Matzuk, M.M. Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat. Genet. 2003, 33, 187–191. [Google Scholar] [CrossRef]
- Wu, X.; Wang, P.; Brown, C.A.; Zilinski, C.A.; Matzuk, M.M. Zygote arrest 1 (Zar1) is an evolutionarily conserved gene expressed in vertebrate ovaries. Biol. Reprod. 2003, 69, 861–867. [Google Scholar] [CrossRef]
- Brevini, T.A.L.; Cillo, F.; Colleoni, S.; Lazzari, G.; Galli, C.; Gandolfi, F. Expression pattern of the maternal factor zygote arrest 1 (Zar1) in bovine tissues, oocytes, and embryos. Mol. Reprod. Dev. 2004, 69, 375–380. [Google Scholar] [CrossRef]
- Uzbekova, S.; Roy-Sabau, M.; Dalbiès-Tran, R.; Perreau, C.; Papillier, P.; Mompart, F.; Thelie, A.; Pennetier, S.; Cognie, J.; Cadoret, V.; et al. Zygote arrest 1 gene in pig, cattle and human: Evidence of different transcript variants in male and female germ cells. Reprod. Biol. Endocrinol. 2006, 4, 12. [Google Scholar] [CrossRef]
- Wang, D.A.N.; Xie, S.-Y.; Zhang, W.E.I.; Sun, C.-X.; Huang, T.A.O.; Wang, A.-S.; Han, X.-L.; Sun, G.-R.; Li, M. Cloning and expression analysis of zygote arrest 1 (Zar1) in New Zealand white rabbits. J. Genet. 2017, 96, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, G.; Argiriou, A.; Avdi, M. Expression of chicken zygote arrest 1 (Zar1) and Zar1-like genes during sexual maturation and embryogenesis. Vet. Res. Commun. 2010, 34, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.M.; Cook, J.M.; Kotter, C.V.; Khat, T.; Silva, K.D.; Ferreyros, M.; Holt, J.W.; Knight, J.D.; Charlesworth, A. Zar1 represses translation in Xenopus oocytes and binds to the TCS in maternal mRNAs with different characteristics than Zar2. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2013, 1829, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Yuan, Y.; Cheng, F.; Fang, J.; Zhou, F.; Ma, W.; Jiang, Y.; Huang, X.; Wang, Y.; Shan, L.; et al. Translation repression by maternal RNA binding protein zar1 is essential for early oogenesis in zebrafish. Development 2017, 144, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Ji, S.-Y.; Zhu, Y.-Z.; Wu, Y.-W.; Shen, L.; Fan, H.-Y. ZAR1 and ZAR2 are required for oocyte meiotic maturation by regulating the maternal transcriptome and mRNA translational activation. Nucleic Acids Res. 2019, 47, 11387–11402. [Google Scholar] [CrossRef] [PubMed]
- Pennetier, S.; Uzbekova, S.; Perreau, C.; Papillier, P.; Mermillod, P.; Dalbiès-Tran, R. Spatio-temporal expression of the germ cell marker genes mater, zar1, gdf9, bmp15,and vasa in adult bovine tissues, oocytes, and preimplantation embryos1. Biol. Reprod. 2004, 71, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgio, L.; Strumbo, B.; Brevini, T.A.L.; Ronchi, S.; Simonic, T. A putative protein structurally related to zygote arrest 1 (Zar1), Zar1-like, is encoded by a novel gene conserved in the vertebrate lineage. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 150, 233–239. [Google Scholar] [CrossRef]
- Richter, A.M.; Kiehl, S.; Köger, N.; Breuer, J.; Stiewe, T.; Dammann, R.H. ZAR1 is a novel epigenetically inactivated tumour suppressor in lung cancer. Clin. Epigenetics 2017, 9, 60. [Google Scholar] [CrossRef]
- Shinojima, Y.; Terui, T.; Hara, H.; Kimura, M.; Igarashi, J.; Wang, X.; Kawashima, H.; Kobayashi, Y.; Muroi, S.; Hayakawa, S.; et al. Identification and analysis of an early diagnostic marker for malignant melanoma: ZAR1 intra-genic differential methylation. J. Dermatol. Sci. 2010, 59, 98–106. [Google Scholar] [CrossRef]
- Watanabe, T.; Yachi, K.; Ohta, T.; Fukushima, T.; Yoshino, A.; Katayama, Y.; Shinojima, Y.; Terui, T.; Nagase, H. Aberrant hypermethylation of non-promoter zygote arrest 1 (Zar1) in human brain tumors. Neurol. Med. Chir. 2010, 50, 1062–1069. [Google Scholar] [CrossRef]
- Pan, Z.; Zhu, C.; Chang, G.; Wu, N.; Ding, H.; Wang, H. Differential expression analysis and identification of sex-related genes by gonad transcriptome sequencing in estradiol-treated and non-treated Ussuri catfish Pseudobagrus ussuriensis. Fish Physiol. Biochem. 2021, 47, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Bobe, J.; Nguyen, T.; Mahé, S.; Monget, P. In silico identification and molecular characterization of genes predominantly expressed in the fish oocyte. BMC Genom. 2008, 9, 499. [Google Scholar] [CrossRef]
- He, F.; Jiang, D.; Chen, H.; Deng, S.; Wu, T.; Tian, C.; Shi, H.; Zhu, C.; LI, G. cDNA Cloning and mRNA expression analysis of zar1 in spotted scat (Scatophagus argus). J. Guangdong Ocean Univ. 2019, 39, 1–7. [Google Scholar]
- Horiuchi, M.; Hagihara, S.; Kume, M.; Chushi, D.; Hasegawa, Y.; Itakura, H.; Yamashita, Y.; Adachi, S.; Ijiri, S. Morphological and molecular gonadal sex differentiation in the wild Japanese eel Anguilla japonica. Cells 2022, 11, 1554. [Google Scholar] [CrossRef]
- Natoli, T.A.; Alberta, J.A.; Bortvin, A.; Taglienti, M.E.; Menke, D.B.; Loring, J.; Jaenisch, R.; Page, D.C.; Housman, D.E.; Kreidberg, J.A. Wt1 functions in the development of germ cells in addition to somatic cell lineages of the testis. Dev. Biol. 2004, 268, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Hammes, A.; Guo, J.K.; Lutsch, G.; Leheste, J.R.; Landrock, D.; Ziegler, U.; Gubler, M.C.; Schedl, A. Two splice variants of the Wilms’ tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 2001, 106, 319–329. [Google Scholar] [CrossRef]
- Call, K.M.; Glaser, T.; Ito, C.Y.; Buckler, A.J.; Pelletier, J.; Haber, D.A.; Rose, E.A.; Kral, A.; Yeger, H.; Lewis, W.H.; et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell 1990, 63, 509–520. [Google Scholar] [CrossRef]
- Cen, C.; Chen, M.; Zhou, J.; Zhang, L.; Duo, S.; Jiang, L.; Hou, X.; Gao, F. Inactivation of Wt1 causes pre-granulosa cell to steroidogenic cell transformation and defect of ovary development. Biol. Reprod. 2020, 103, 60–69. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L.; Cui, X.; Lin, X.; Li, Y.; Wang, Y.; Wang, Y.; Qin, Y.; Chen, D.; Han, C.; et al. Wt1 directs the lineage specification of sertoli and granulosa cells by repressing Sf1 expression. Development 2017, 144, 44–53. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, J.; Wang, X.; Yang, J.; Chen, D.; Huff, V.; Liu, Y.X. Wt1 functions in ovarian follicle development by regulating granulosa cell differentiation. Hum. Mol. Genet. 2014, 23, 333–341. [Google Scholar] [CrossRef]
- Wang, X.N.; Li, Z.S.; Ren, Y.; Jiang, T.; Wang, Y.Q.; Chen, M.; Zhang, J.; Hao, J.X.; Wang, Y.B.; Sha, R.N.; et al. The Wilms tumor gene, Wt1, is critical for mouse spermatogenesis via regulation of sertoli cell polarity and is associated with non-obstructive azoospermia in humans. PLoS Genet. 2013, 9, e1003645. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, M.; Wen, Q.; Li, Y.; Wang, Y.; Wang, Y.; Qin, Y.; Cui, X.; Yang, L.; Huff, V.; et al. Reprogramming of Sertoli cells to fetal-like Leydig cells by Wt1 ablation. Proc. Natl. Acad. Sci. USA 2015, 112, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Bollig, F.; Mehringer, R.; Perner, B.; Hartung, C.; Schäfer, M.; Schartl, M.; Volff, J.N.; Winkler, C.; Englert, C. Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev. Dyn. 2006, 235, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-J.; Lin, G.; Chung, B.-c. Parallel early development of zebrafish interrenal glands and pronephros:differential control bywt1andff1b. Development 2003, 130, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-D.; Wagner, N.; Schedl, A. The complex life of WT1. J. Cell Sci. 2003, 116, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, K.; Wang, Y.; Zhao, Y.; Lin, F.; Liu, X.; Zhang, Y.; Quan, F. Wilms’ tumor (WT1) (±KTS) variants decreases the progesterone secretion of bovine ovarian theca cells. Domest. Anim. Endocrinol. 2021, 74, 106521. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, K.M.; Scholz, H. WT1 in adipose tissue: From development to adult physiology. Front. Cell Dev. Biol. 2022, 10, 854120. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.K.; Pham, J.; Imam, J.S.; MacLean, J.A.; Murali, D.; Furuta, Y.; Sinha-Hikim, A.P.; Wilkinson, M.F. Tissue-specific RNAi reveals that WT1 expression in nurse cells controls germ cell survival and spermatogenesis. Genes Dev. 2006, 20, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.W.; McInnes, L.; Kreidberg, J.; Hastie, N.D.; Schedl, A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 1999, 126, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Klüver, N.; Herpin, A.; Braasch, I.; Driessle, J.; Schartl, M. Regulatory back-up circuit of medaka Wt1 co-orthologs ensures PGC maintenance. Dev. Biol. 2009, 325, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Athauda, S.; Anderson, T. Effect of temperature and salinity on sex inversion in Asian seabass (Lates calcarifer): Relationship with plasma sex steroids concentration and aromatase activity of gonad and brain. Aquac. Res. 2014, 45, 787–797. [Google Scholar] [CrossRef]
- Banh, Q.Q.T.; Guppy, J.L.; Domingos, J.A.; Budd, A.M.; Pinto, R.C.C.; Marc, A.F.; Jerry, D.R. Induction of precocious females in the protandrous barramundi (Lates calcarifer) through implants containing 17β-estradiol-effects on gonadal morphology, gene expression and DNA methylation of key sex genes. Aquaculture 2021, 539, 736601. [Google Scholar] [CrossRef]
- Roberts, B.H.; Morrongiello, J.R.; Morgan, D.L.; King, A.J.; Saunders, T.M.; Crook, D.A. Faster juvenile growth promotes earlier sex change in a protandrous hermaphrodite (barramundi Lates calcarifer). Sci. Rep. 2021, 11, 2276. [Google Scholar] [CrossRef]
- Domingos, J.A.; Budd, A.M.; Banh, Q.Q.; Goldsbury, J.A.; Zenger, K.R.; Jerry, D.R. Sex-specific dmrt1 and cyp19a1 methylation and alternative splicing in gonads of the protandrous hermaphrodite barramundi. PLoS ONE 2018, 13, e0204182. [Google Scholar] [CrossRef]
- Ravi, P.; Jiang, J.; Liew, W.C.; Orbán, L. Small-scale transcriptomics reveals differences among gonadal stages in Asian seabass (Lates calcarifer). Reprod. Biol. Endocrinol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Xu, H.; Lim, M.; Dwarakanath, M.; Hong, Y. Vasa identifies germ cells and critical stages of oogenesis in the Asian seabass. Int. J. Biol. Sci. 2014, 10, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Bienz, M. The PHD finger, a nuclear protein-interaction domain. Trends Biochem. Sci. 2006, 31, 35–40. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Yang, Y.; Chen, K.; Li, Y.; Zhu, X.; Ye, H.; Xu, H. Transcriptome profiling of the ovarian cells at the single-cell resolution in adult Asian seabass. Front. Cell Dev. Biol. 2021, 9, 647892. [Google Scholar] [CrossRef]
- Geffroy, B.; Guilbaud, F.; Amilhat, E.; Beaulaton, L.; Vignon, M.; Huchet, E.; Bardonnet, A. Sexually dimorphic gene expressions in eels: Useful markers for early sex assessment in a conservation context. Sci. Rep. 2016, 6, 34041. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Wang, L.; Ma, H.; Hostuttler, M.; Rexroad, C.E.; Yao, J. Molecular characterization and expression analysis of zar1 and zar1-like genes in rainbow trout. Biol. Reprod. 2012, 87, 304. [Google Scholar] [CrossRef]
- Nakatsuru, Y.; Minami, K.; Yoshikawa, A.; Zhu, J.-J.; Oda, H.; Masahito, P.; Okamoto, N.; Nakamura, Y.; Ishikawa, T. Eel WT1 sequence and expression in spontaneous nephroblastomas in Japanese eel. Gene 2000, 245, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Pritchardjones, K.; Bickmore, W.; Hastie, N.; Bard, J. The expression of the Wilms-tumor gene, Wt1, in the developing mammalian embryo. Mech. Dev. 1993, 40, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Schalling, M.; Buckler, A.; Rogers, A.; Haber, D.; Housman, D. Expression of the Wilms-tumor gene Wt1 in the murine urogenital system. Genes. Dev. 1991, 5, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Maiti, S.; Alam, N.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; Lecureuil, C.; Guillou, F.; Huff, V. The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc. Natl. Acad. Sci. USA 2006, 103, 11987–11992. [Google Scholar] [CrossRef]
- Zheng, Q.-S.; Wang, X.-N.; Wen, Q.; Zhang, Y.; Chen, S.-R.; Zhang, J.; Li, X.-X.; Sha, R.-N.; Hu, Z.-Y.; Gao, F.; et al. Wt1 deficiency causes undifferentiated spermatogonia accumulation and meiotic progression disruption in neonatal mice. Reproduction 2014, 147, 45–52. [Google Scholar] [CrossRef]


| Primer Name | Sequences (5′ to 3′) | Product Length (bp) | Tm (°C) | Purpose | |
|---|---|---|---|---|---|
| Lczar1-CDS | Forward | CGGTTGGTTGAACAAAATGGC | 1192 | 56 | ISH, RT-PCR |
| Reverse | GTGCGGTTCTCACCATCAAT | ||||
| Lcwt1b-CDS | Forward | ACTGTCTCAAACCGCCTTCA | 1521 | 56 | ISH, RT-PCR |
| Reverse | GCAAGCACTAGTTGAGGTGC | ||||
| Lczar1-DL | Forward | CTACGATGGGAGAGTGCCTATG | 247 | 60 | RT-qPCR |
| Reverse | AGCTGAAAGTGCTGTCGCAGG | ||||
| Lcwt1b-DL | Forward | ACATGAGGACACCCTGTCACC | 289 | 60 | RT-qPCR |
| Reverse | GTACTGCGCACTGACGAGCAT | ||||
| Lcβ-actin-DL | Forward | CGGAATCCACGAGACCACCTAC | 268 | 60 | RT-qPCR |
| Reverse | ACTCCTGCTTGCTGATCCACAT | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Zhu, H.; Ban, W.; Li, Y.; Chen, R.; Li, L.; Zhang, X.; Chen, K.; Xu, H. Characterization of Two Gonadal Genes, zar1 and wt1b, in Hermaphroditic Fish Asian Seabass (Lates calcarifer). Animals 2024, 14, 508. https://doi.org/10.3390/ani14030508
Cui H, Zhu H, Ban W, Li Y, Chen R, Li L, Zhang X, Chen K, Xu H. Characterization of Two Gonadal Genes, zar1 and wt1b, in Hermaphroditic Fish Asian Seabass (Lates calcarifer). Animals. 2024; 14(3):508. https://doi.org/10.3390/ani14030508
Chicago/Turabian StyleCui, Han, Haoyu Zhu, Wenzhuo Ban, Yulin Li, Ruyi Chen, Lingli Li, Xiaoling Zhang, Kaili Chen, and Hongyan Xu. 2024. "Characterization of Two Gonadal Genes, zar1 and wt1b, in Hermaphroditic Fish Asian Seabass (Lates calcarifer)" Animals 14, no. 3: 508. https://doi.org/10.3390/ani14030508






