Research on Bacterial Diversity and Antibiotic Resistance in the Dairy Farm Environment in a Part of Shandong Province
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Identification of Environmental Bacteria
2.3. Antimicrobial Susceptibility Testing
2.4. Detections of Resistance Genes
2.5. Statistical Analysis
3. Results
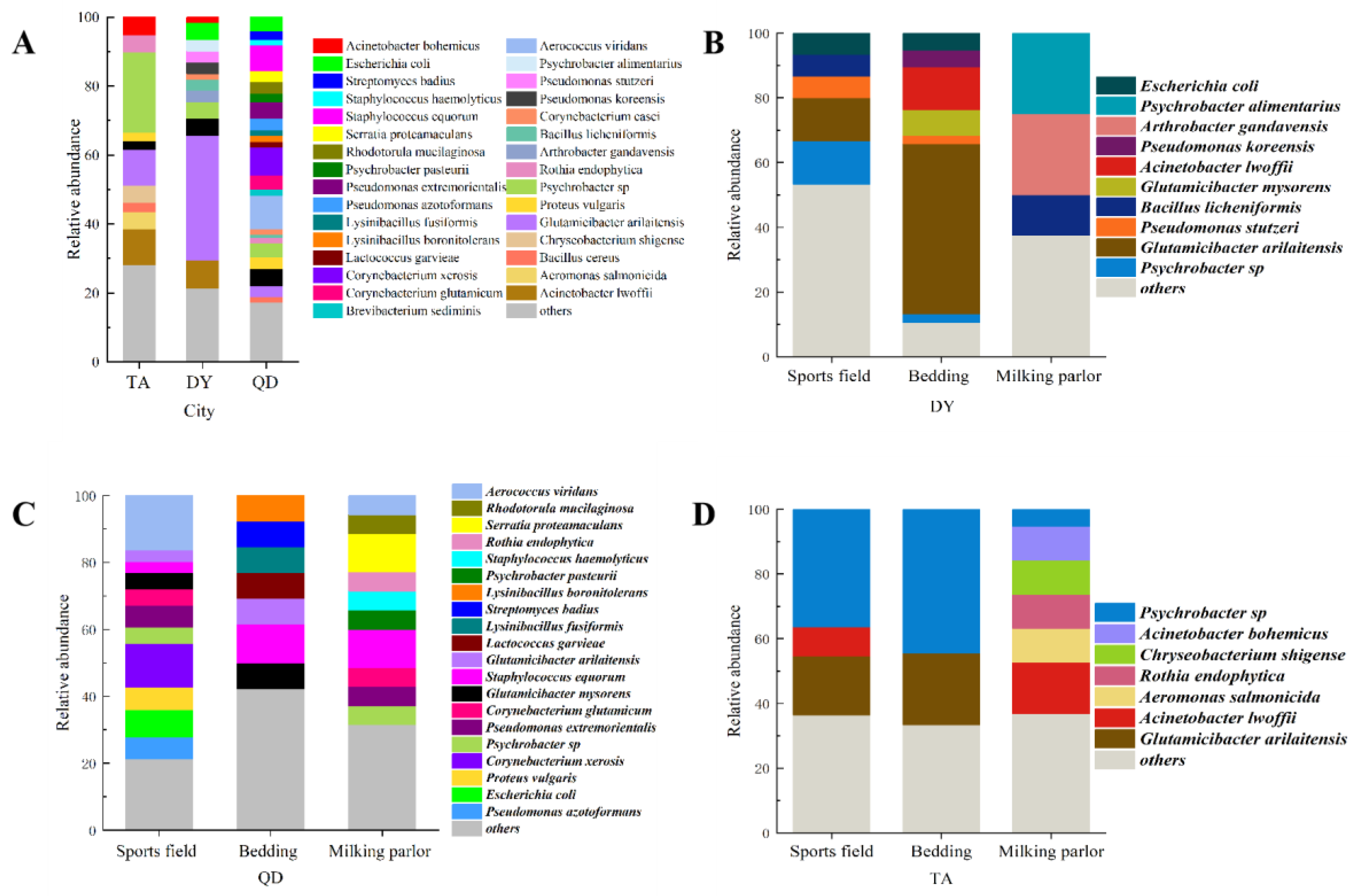
3.1. Isolation and Identification of Bacteria in the Dairy Environment
3.2. Antimicrobial Susceptibility Tests of Isolated Strains
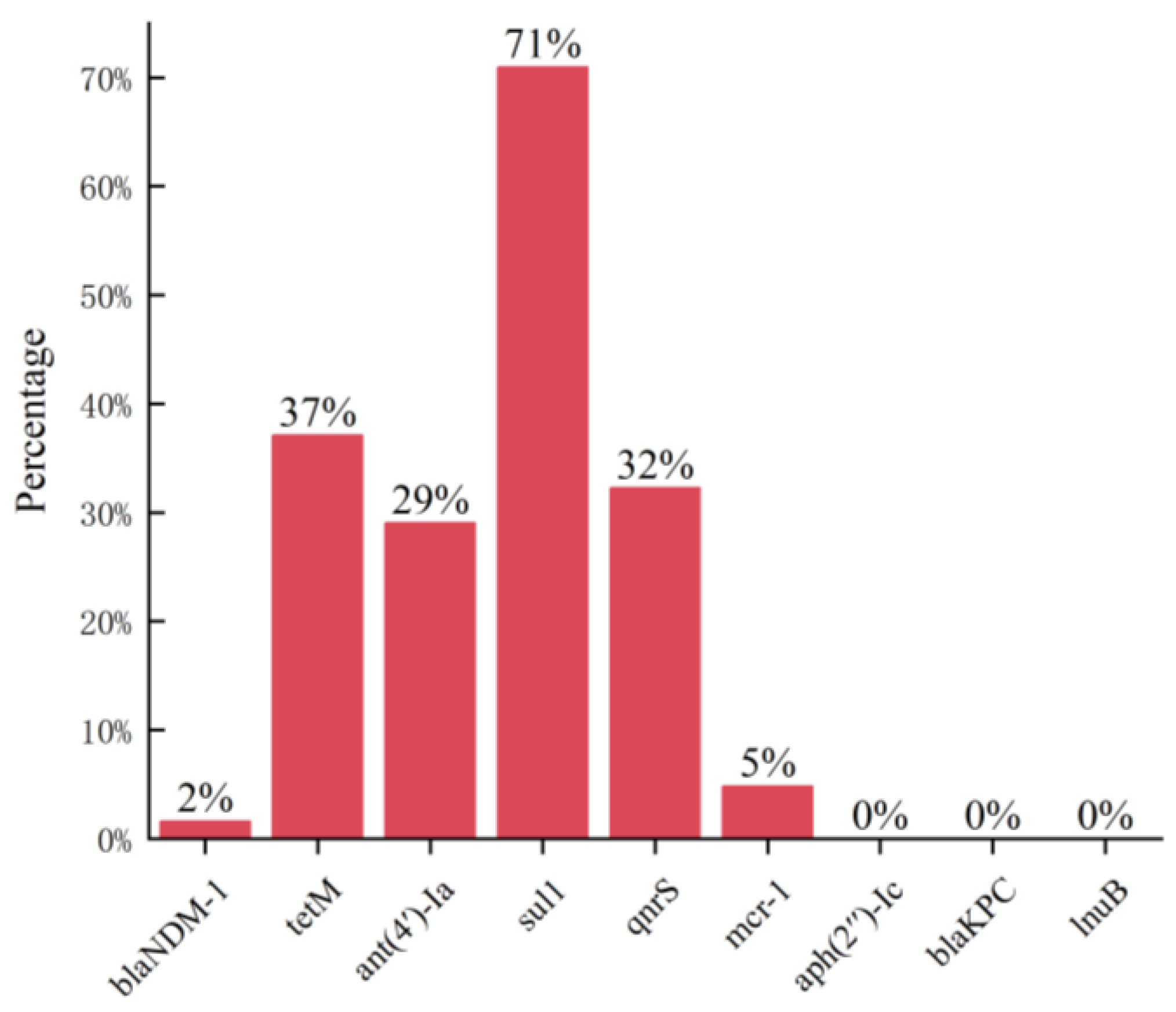
3.3. Detection of Antibiotic Resistance Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Redding, L.E.; Bender, J.; Baker, L. Quantification of antibiotic use on dairy farms in Pennsylvania. J. Dairy Sci. 2019, 102, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Tangcharoensathien, V.; Chanvatik, S.; Sommanustweechai, A. Complex determinants of inappropriate use of antibiotics. Bull. World Health Organ. 2018, 96, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Wernli, D.; Jorgensen, P.S.; Morel, C.M.; Carroll, S.; Harbarth, S.; Levrat, N.; Pittet, D. Mapping global policy discourse on antimicrobial resistance. BMJ Glob. Health 2017, 2, e000378. [Google Scholar] [CrossRef] [PubMed]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Leger, D.F.; Newby, N.C.; Reid-Smith, R.; Anderson, N.; Pearl, D.L.; Lissemore, K.D.; Kelton, D.F. Estimated antimicrobial dispensing frequency and preferences for lactating cow therapy by Ontario dairy veterinarians. Can. Vet. J. 2017, 58, 26–34. [Google Scholar] [PubMed]
- Oliver, J.P.; Gooch, C.A.; Lansing, S.; Schueler, J.; Hurst, J.J.; Sassoubre, L.; Crossette, E.M.; Aga, D.S. Invited review: Fate of antibiotic residues, antibiotic-resistant bacteria, and antibiotic resistance genes in US dairy manure management systems. J. Dairy. Sci. 2020, 103, 1051–1071. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- El Garch, F.; Youala, M.; Simjee, S.; Moyaert, H.; Klee, R.; Truszkowska, B.; Rose, M.; Hocquet, D.; Valot, B.; Morrissey, I.; et al. Antimicrobial susceptibility of nine udder pathogens recovered from bovine clinical mastitis milk in Europe 2015–2016: VetPath results. Vet. Microbiol. 2020, 245, 108644. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, R.; Liu, D.; Walsh, T.R.; Zhang, R.; Lv, Y.; Ke, Y.; Ji, Q.; Wei, R.; Liu, Z. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, C.; Cui, C.-Y.; Zhang, Y.; Liu, X.; Cui, Z.-H.; Ma, X.-Y.; Feng, Y.; Fang, L.-X.; Lian, X.-L. Plasmid-encoded tet (X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019, 4, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gao, G.F.; Zhu, B. The antibiotic resistome: Gene flow in envir onments, animals and human beings. Front. Med. 2017, 11, 161–168. [Google Scholar] [CrossRef]
- Woolhouse, M.; Ward, M.; Van Bunnik, B.; Farrar, J. Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Zhang, W.; Helbling, D.E.; Xu, N.; Sun, W.; Ni, J. Animal production predominantly contributes to antibiotic profiles in the Yangtze River. Water Res. 2023, 242, 120214. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Stępień-Pyśniak, D.; Hauschild, T.; Dec, M.; Marek, A.; Brzeski, M.; Kosikowska, U. Antimicrobial resistance and genetic diversity of Enterococcus faecalis from yolk sac infections in broiler chicks. Poult. Sci. 2021, 100, 101491. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Singh, A.; Chowdhary, P.; Pandey, A.; Gupta, P. Occurrence of emerging sulfonamide resistance (sul1 and sul2) associated with mobile integrons-integrase (intI1 and intI2) in riverine systems. Sci. Total Environ. 2021, 751, 142217. [Google Scholar] [CrossRef] [PubMed]
- Doma, A.O.; Popescu, R.; Mitulețu, M.; Muntean, D.; Dégi, J.; Boldea, M.V.; Radulov, I.; Dumitrescu, E.; Muselin, F.; Puvača, N. Comparative evaluation of qnrA, qnrB, and qnrS genes in Enterobacteriaceae ciprofloxacin-resistant cases, in swine units and a hospital from Western Romania. Antibiotics 2020, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xie, M.; Zhang, J.; Yang, Z.; Liu, L.; Liu, X.; Zheng, Z.; Chan, E.W.-C.; Chen, S. Genetic characterization of mcr-1-bearing plasmids to depict molecular mechanisms underlying dissemination of the colistin resistance determinant. J. Antimicrob. Chemother. 2017, 72, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Hou, Q.; Song, J.; Liu, R.; Qian, Y.; Huang, G. Groundwater antibiotics contamination in an alluvial-pluvial fan, North China Plain: Occurrence, sources, and risk assessment. Environ. Res. 2023, 235, 116653. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, S.; Lin, H.; Li, K.; Li, Z.; Wang, J.; Gaze, W.H.; Zou, J. Uncovering the prevalence and drivers of antibiotic resistance genes in soils across different land-use types. J. Environ. Manag. 2023, 344, 118920. [Google Scholar] [CrossRef] [PubMed]
- Motallebirad, T.; Mardanshah, O.; Safarabadi, M.; Ghaffari, K.; Orouji, M.A.; Abedi, B.; Azadi, D. Screening, molecular identification, population diversity, and antibiotic susceptibility pattern of Actinomycetes species isolated from meat and meat products of slaughterhouses, restaurants, and meat stores of a developing country, Iran. Front. Microbiol. 2023, 14, 1134368. [Google Scholar] [CrossRef] [PubMed]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Zaatout, N.; Hezil, D. A meta-analysis of the global prevalence of methicillin-resistant Staphylococcus aureus (MRSA) isolated from clinical and subclinical bovine mastitis. J. Appl. Microbiol. 2022, 132, 140–154. [Google Scholar] [CrossRef]
- Hocaoglu, M.; Turgut, A.; Eminoglu, E.M.; Yilmaz Karadag, F.; Karateke, A. Maternal ventriculoperitoneal shunt infection due to Corynebacterium xerosis following caesarean section. J. Obstet. Gynaecol. 2019, 39, 400–402. [Google Scholar] [CrossRef]
- Baker, M.; Zhang, X.; Maciel-Guerra, A.; Dong, Y.; Wang, W.; Hu, Y.; Renney, D.; Hu, Y.; Liu, L.; Li, H.; et al. Machine learning and metagenomics reveal shared antimicrobial resistance profiles across multiple chicken farms and abattoirs in China. Nat. Food 2023, 4, 707–720. [Google Scholar] [CrossRef]
- Fang, G.Y.; Liu, X.Q.; Mu, X.J.; Huang, B.W.; Jiang, Y.J. Distinct increase in antimicrobial resistance genes among Vibrio parahaemolyticus in recent decades worldwide. Chemosphere 2023, 340, 139905. [Google Scholar] [CrossRef] [PubMed]


| gene | 5′-3′, F/R | bp |
|---|---|---|
| blaNDM-1 | GTCTGGCAGACTTCCTATCTC GGTTCGACAACGCATTGGCATAAG | 268 |
| blakpc | CGTCTAGTTCTGCTGTCTTG CTTGTCATCCTTGTTAGGCG | 798 |
| lnuB | CCTACCTATTGTTTGTGGAA ATAACGTTACTCTCCTATTC | 925 |
| tetM | GTGGACAAAGGTACAACGAG CGGTAAAGTTCGTCACACAC | 406 |
| ant(4′)-Ia | CAAACTGCTAAATCGGTAGAAGCC GGAAAGTTGACCAGACATTACGAACT | 294 |
| aph(2″)-Ic | CCACAATGATAATGACTCAGTTCCC CCACAGCTTCCGATAGCAAGAG | 444 |
| sul1 | TTCGGCATTCTGAATCTCAC ATGATCTAACCCTCGGTCTC | 822 |
| qnrS | ACGACATTCGTCAACTGGAA TTAATTGGCACCCTGTAGGC | 417 |
| mcr-1 | CGGTCAGTCCGTTTGTTC CTTGGTCGGTCTGTAGGG | 309 |
| Species | blaNDM-1 | tetM | ant(4′)-Ia | sul1 | qnrS | mcr-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Acinetobacter lwoffii | 9 | 1 | 11.11 | 1 | 11.11 | 6 | 66.67 | 6 | 66.67 | 5 | 55.56 | 0 | 0.00 |
| Escherichia coli | 8 | 0 | 0.00 | 0 | 0.00 | 2 | 25.00 | 1 | 12.50 | 6 | 75.00 | 0 | 0.00 |
| Proteus vulgaris | 5 | 0 | 0.00 | 0 | 0.00 | 4 | 80.00 | 4 | 80.00 | 4 | 80.00 | 0 | 0.00 |
| Corynebacterium xerosis | 9 | 0 | 0.00 | 7 | 77.78 | 0 | 0.00 | 8 | 88.89 | 0 | 0.00 | 0 | 0.00 |
| Aerococcus viridans | 13 | 0 | 0.00 | 8 | 61.54 | 0 | 0.00 | 7 | 53.85 | 1 | 11.11 | 3 | 33.33 |
| Bacillus cereus | 3 | 0 | 0.00 | 0 | 0.00 | 2 | 75.00 | 2 | 75.00 | 2 | 75.00 | 0 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Song, K.; Liu, X.; Xu, H.; Wang, X.; Cheng, G.; Zheng, P.; Liu, J. Research on Bacterial Diversity and Antibiotic Resistance in the Dairy Farm Environment in a Part of Shandong Province. Animals 2024, 14, 160. https://doi.org/10.3390/ani14010160
Cui Y, Song K, Liu X, Xu H, Wang X, Cheng G, Zheng P, Liu J. Research on Bacterial Diversity and Antibiotic Resistance in the Dairy Farm Environment in a Part of Shandong Province. Animals. 2024; 14(1):160. https://doi.org/10.3390/ani14010160
Chicago/Turabian StyleCui, Yuehui, Kaimin Song, Xiaoting Liu, Huiling Xu, Xiaozhou Wang, Guodong Cheng, Pimiao Zheng, and Jianzhu Liu. 2024. "Research on Bacterial Diversity and Antibiotic Resistance in the Dairy Farm Environment in a Part of Shandong Province" Animals 14, no. 1: 160. https://doi.org/10.3390/ani14010160





