Effects of Dietary Protein Levels on Sheep Gut Metabolite Profiles during the Lactating Stage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval, Animals, Research Design, and Animal Husbandry
2.2. Metabolite Extraction and LC-MS/MS Analysis
2.3. Bioinformatic Analysis
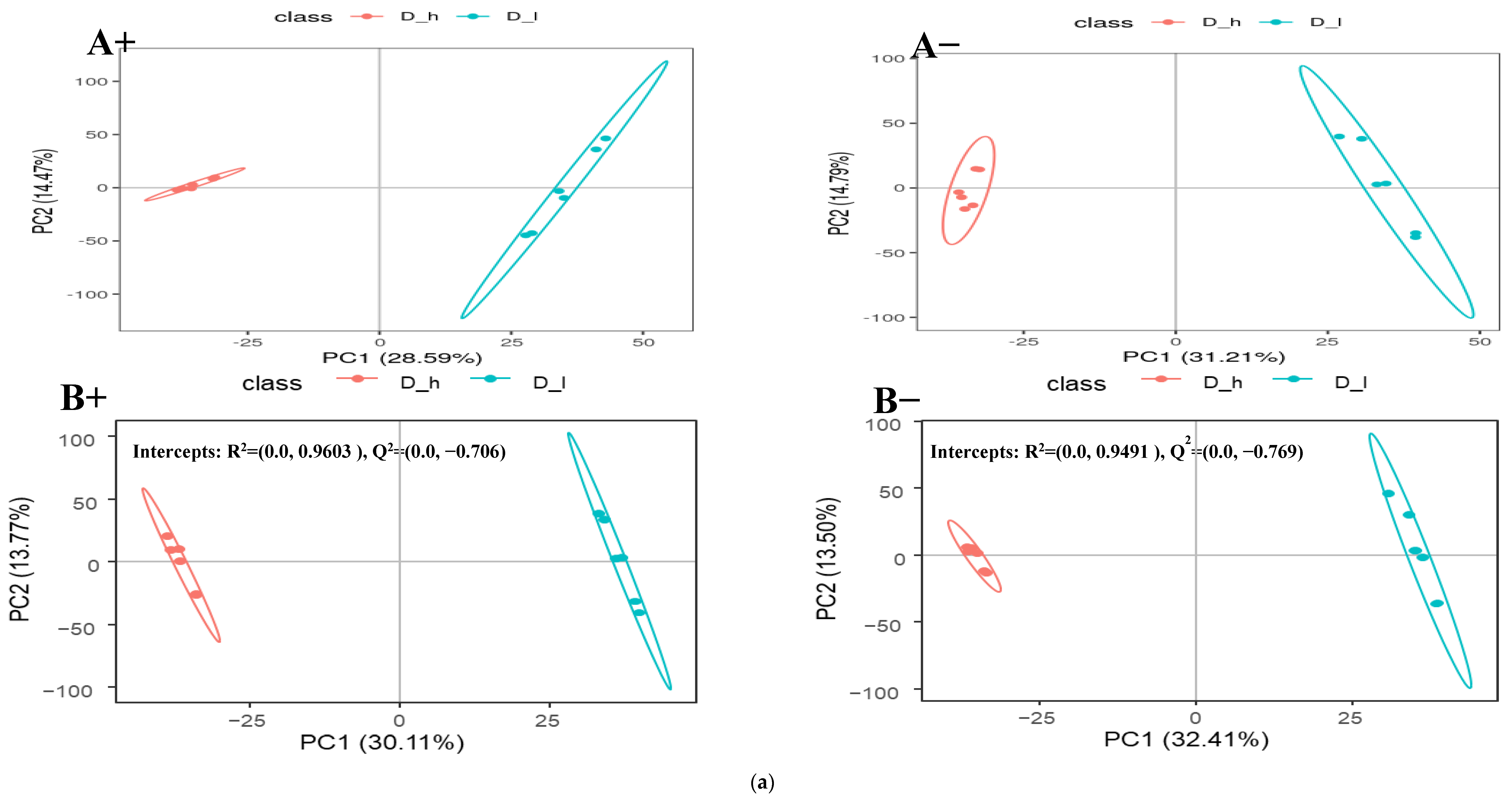
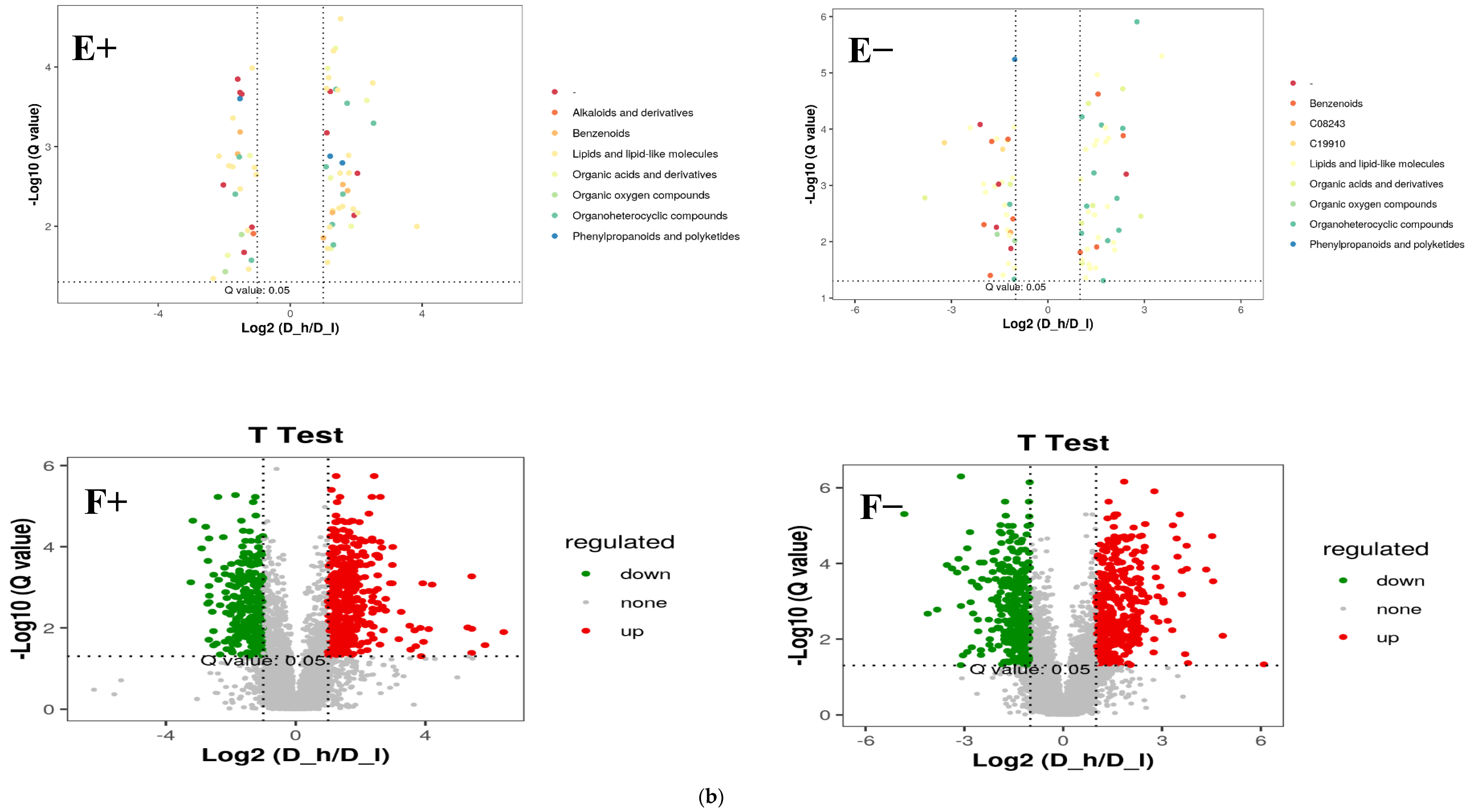
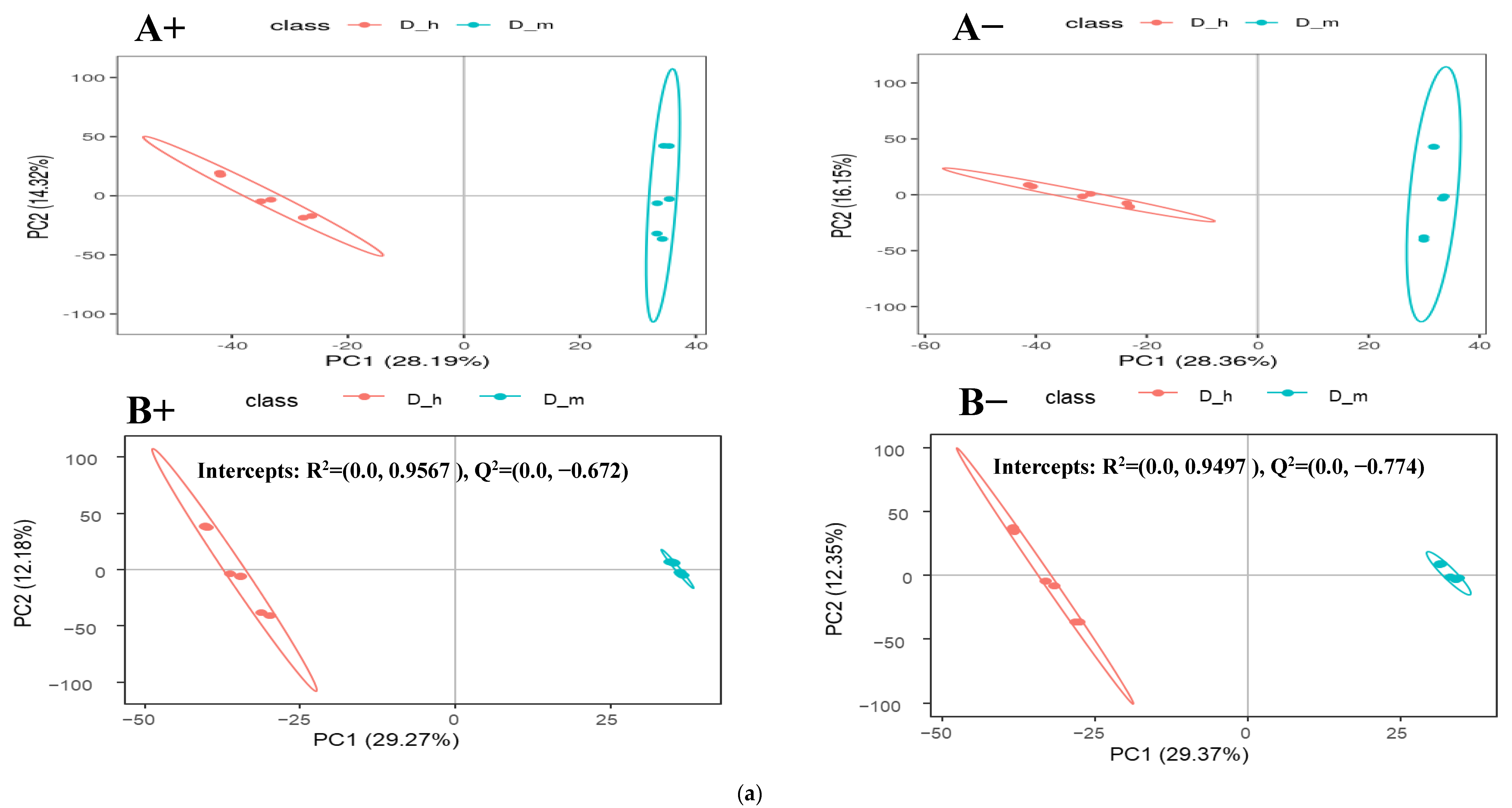
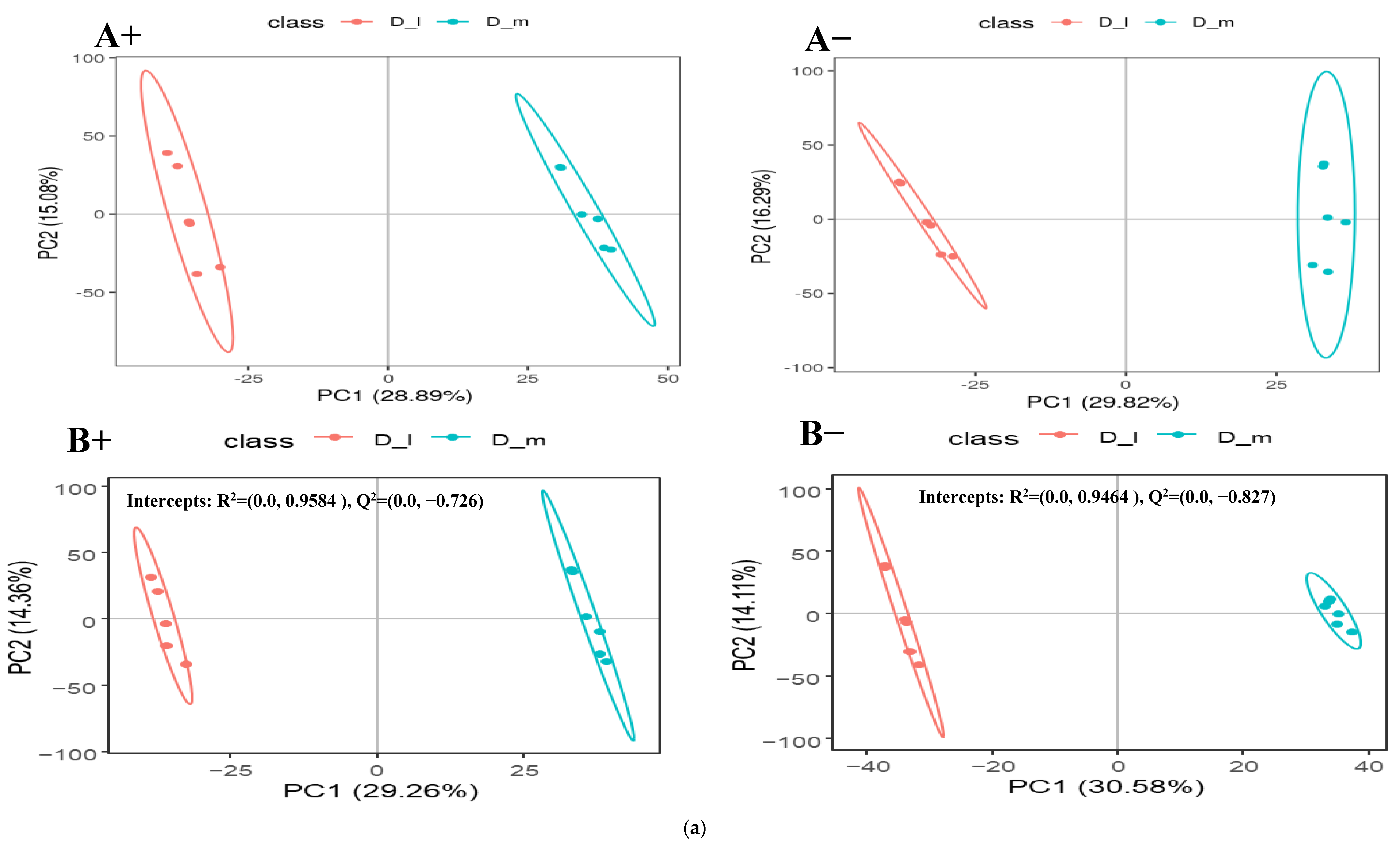
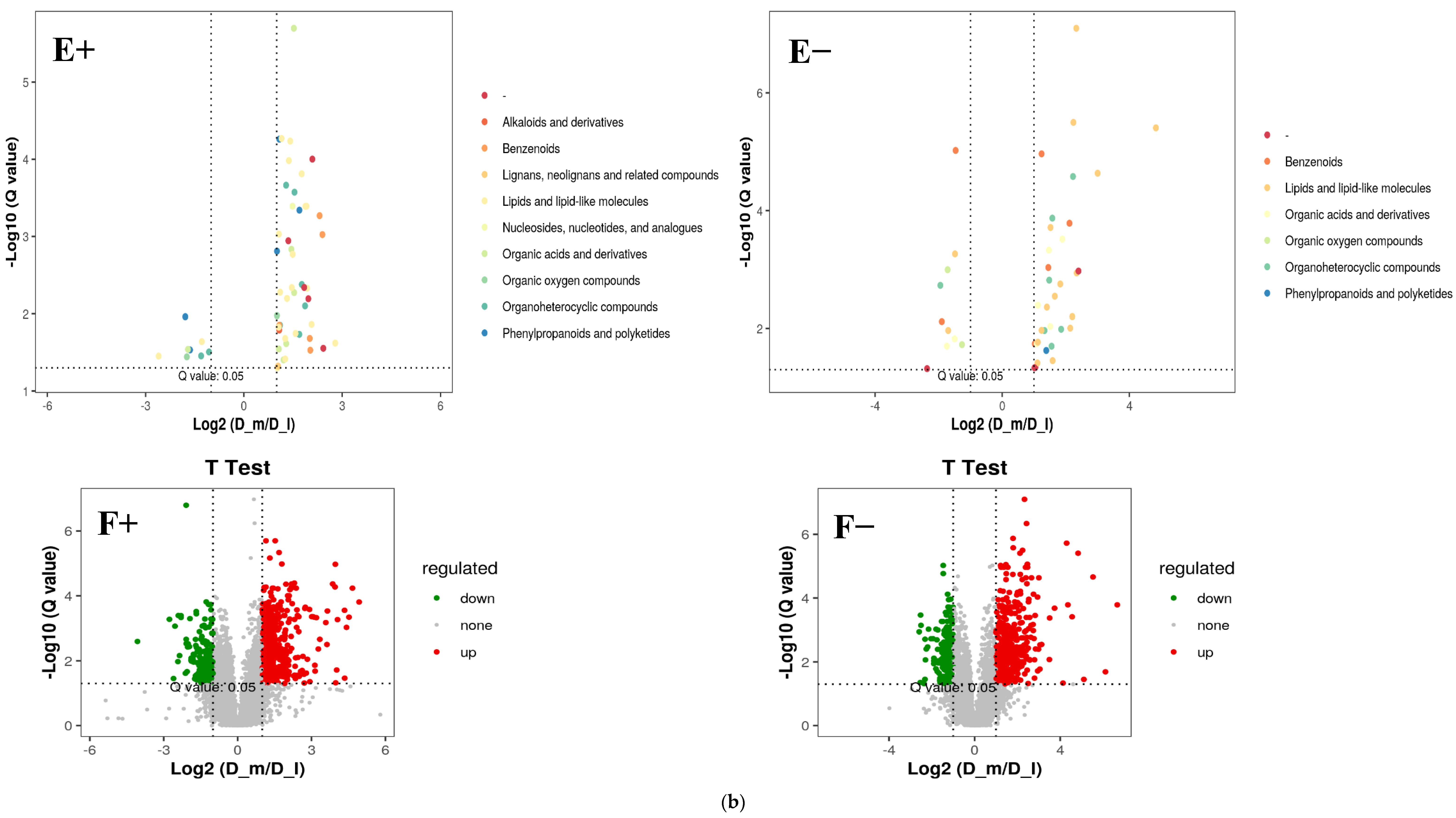
3. Results
3.1. Identification and Quantification of Metabolites in the Fecal Samples
3.2. Fecal Metabolomic Profiles
3.3. Metabolic Pathways of Differential Metabolites


4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Q.; Qiao, Q.; Gao, Y.; Hou, J.; Hu, M.; Du, Y.; Zhao, K.; Li, X. Gut microbiota and their role in health and metabolic disease of dairy cow. Front. Nutr. 2021, 8, 701511. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Liu, H.; Zhao, J.; Gu, X.; Wei, X.; Zhang, X.; Ma, N.; Johnston, L.J.; Bai, Y.; Zhang, W.; et al. Amino acids metabolism by rumen microorganisms: Nutrition and ecology strategies to reduce nitrogen emissions from the inside to the outside. Sci. Total Environ. 2021, 800, 149596. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.; Bello, I.; Mukaila, T.; Monono, E.; Hammed, A. Developing rumen mimicry process for biological ammonia synthesis. Bioprocess Biosyst. Eng. 2023, 46, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E. Effect of dietary protein and processing on gut microbiota—A systematic review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, T.; Zhang, X.; Zhao, N.; Hu, L.; Liu, H.; Zhang, Q.; Geng, Y.; Kang, S.; Xu, S. The response of ruminal microbiota and metabolites to different dietary protein levels in Tibetan sheep on the Qinghai-Tibetan plateau. Front. Vet. Sci. 2022, 9, 922817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yao, J.; Yang, L.; et al. Dietary supplementation of inulin ameliorates subclinical mastitis via regulation of rumen microbial community and metabolites in dairy cows. Microbiol. Spectr. 2021, 9, e0010521. [Google Scholar] [CrossRef]
- Owens, F.N.; Basalan, M. Ruminal fermentation. In Rumenology; Springer: Cham, Switzerland, 2016; pp. 63–102. [Google Scholar]
- Du, Z.; Sun, L.; Chen, C.; Lin, J.; Yang, F.; Cai, Y. Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed Sci. Technol. 2021, 275, 114766. [Google Scholar] [CrossRef]
- Cao, Y.; Yao, J.; Sun, X.; Liu, S.; Martin, G.B. Amino acids in the nutrition and production of sheep and goats. Adv. Exp. Med. Biol. 2021, 1285, 63–79. [Google Scholar]
- Katongole, C.B.; Yan, T. Effect of varying dietary crude protein level on feed intake, nutrient digestibility, milk production, and nitrogen use efficiency by lactating Holstein-Friesian cows. Animals 2020, 10, 2439. [Google Scholar] [CrossRef]
- Smith, L.; Villaret-Cazadamont, J.; Claus, S.P.; Canlet, C.; Guillou, H.; Cabaton, N.J.; Ellero-Simatos, S. Important considerations for sample collection in metabolomics studies with a special focus on applications to liver functions. Metabolites 2020, 10, 104. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted metabolomics strategies—Challenges and emerging directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Han, R.H.; Han, X. Novel advances in shotgun lipidomics for biology and medicine. Prog. Lipid Res. 2016, 61, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Bouatra, S.; Guo, A.C.; Psychogios, N.; Mandal, R.; Dunn, S.M.; Ametaj, B.N.; Wishart, D.S. The bovine ruminal fluid metabolome. Metabolomics 2013, 9, 360–378. [Google Scholar] [CrossRef]
- Jorge-Smeding, E.; Polakof, S.; Bonnet, M.; Durand, S.; Centeno, D.; Pétéra, M.; Taussat, S.; Cantalapiedra-Hijar, G. Untargeted metabolomics confirms the association between plasma branched chain amino acids and residual feed intake in beef heifers. PLoS ONE 2022, 17, e0277458. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.J.; Boerman, J.P. Invited Review: Quantifying protein mobilization in dairy cows during the transition period. Appl. Anim. Sci. 2020, 36, 389–396. [Google Scholar] [CrossRef]
- Liang, J.; Ali, S.; Lv, C.; Yang, H.; Zhao, X.; Ni, X.; Li, C.; Danzeng, B.; Wang, Y.; Quan, G. Dietary protein levels modulate the gut microbiome composition through fecal samples derived from lactating ewes. Front. Endocrinol. 2023, 14, 1194425. [Google Scholar] [CrossRef]
- Zhao, X.; Ali, S.; Hassan, M.F.; Bashir, M.A.; Ni, X.; Lv, C.; Yang, H.; Danzeng, B.; Quan, G. Effects of graded levels of dietary protein supplementation on milk yield, body weight gain, blood biochemical parameters, and gut microbiota in lactating ewes. Front. Vet. Sci. 2023, 10, 1223450. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Murtaza, G.; Metwally, E.; Yang, H.; Kalhoro, M.S.; Kalhoro, D.H.; Chughtai, M.I.; Yin, Y. Role of dietary amino acids and nutrient sensing system in pregnancy associated disorders. Front. Pharmacol. 2020, 11, 586979. [Google Scholar] [CrossRef]
- Zhang, H.B.; Wang, Z.S.; Peng, Q.H.; Tan, C.; Zou, H.W. Effects of different levels of protein supplementary diet on gene expressions related to intramuscular deposition in early-weaned yaks. Anim. Sci. J. 2014, 85, 411–419. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, T.; Wang, X.; Geng, Y.; Zhao, N.; Hu, L.; Liu, H.; Kang, S.; Xu, S. Effect of dietary protein levels on dynamic changes and interactions of ruminal microbiota and metabolites in yaks on the Qinghai-Tibetan plateau. Front. Microbiol. 2021, 12, 684340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, J.; Li, J.V.; Zhou, N.Y.; Tang, H.; Wang, Y. Gut microbiota composition modifies fecal metabolic profiles in mice. J. Proteome Res. 2013, 12, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yin, Y.; Wu, G. Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp. Biol. Med. 2015, 240, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Calsamiglia, S.; Stern, M. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, S. Protein degradation in the forestomachs of ruminants. J. Anim. Sci. 1979, 49, 1615–1630. [Google Scholar] [CrossRef]
- Li, H.; Yu, Q.; Li, T.; Shao, L.; Su, M.; Zhou, H.; Qu, J. Rumen microbiome and metabolome of Tibetan sheep (Ovis aries) reflect animal age and nutritional requirement. Front. Vet. Sci. 2020, 7, 609. [Google Scholar] [CrossRef]
- Liu, Y.; Pei, Z.; Pan, T.; Wang, H.; Chen, W.; Lu, W. Indole metabolites and colorectal cancer: Gut microbial tryptophan metabolism, host gut microbiome biomarkers, and potential intervention mechanisms. Microbiol. Res. 2023, 272, 127392. [Google Scholar] [CrossRef]
- Alcazar, O.; Hernandez, L.F.; Tschiggfrie, A.; Muehlbauer, M.J.; Bain, J.R.; Buchwald, P.; Abdulreda, M.H. Feasibility of localized metabolomics in the study of pancreatic islets and diabetes. Metabolites 2019, 9, 207. [Google Scholar] [CrossRef]
- Kepert, I.; Fonseca, J.; Müller, C.; Milger, K.; Hochwind, K.; Kostric, M.; Fedoseeva, M.; Ohnmacht, C.; Dehmel, S.; Nathan, P.; et al. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J. Allergy Clin. Immunol. 2017, 139, 1525–1535. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.; Ma, N.; Johnston, L.J.; Wu, C.; Ma, X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: A therapeutic target to control intestinal inflammation. Med. Res. Rev. 2021, 41, 1061–1088. [Google Scholar] [CrossRef] [PubMed]
- Chikagwa-Malunga, S.K.; Adesogan, A.T.; Sollenberger, L.E.; Phatak, S.C.; Szabo, N.J.; Kim, S.C.; Huisden, C.M.; Littell, R.C. Nutritional characterization of Mucuna pruriens: 4. Does replacing soybean meal with Mucuna pruriens in lamb diets affect ruminal, blood and tissue l-dopa concentrations? Anim. Feed Sci. Technol. 2009, 148, 124–137. [Google Scholar] [CrossRef]
- Gilbreath, K.R.; Bazer, F.W.; Satterfield, M.C.; Wu, G. Amino acid nutrition and reproductive performance in ruminants. Adv. Exp. Med. Biol. 2021, 1285, 43–61. [Google Scholar] [PubMed]
- Khan, M.Z.; Liu, S.; Ma, Y.; Ma, M.; Ullah, Q.; Khan, I.M.; Wang, J.; Xiao, J.; Chen, T.; Khan, A.; et al. Overview of the effect of rumen-protected limiting amino acids (methionine and lysine) and choline on the immunity, antioxidative, and inflammatory status of periparturient ruminants. Front. Immunol. 2023, 13, 1042895. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, A.; Fitzsimmons, C.; Mandal, R.; Piri-Moghadam, H.; Zheng, J.; Guo, A.; Li, C.; Guan, L.L.; Wishart, D.S. The bovine metabolome. Metabolites 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, G.; Peng, W.; Xu, Y.; Zhang, Y.; Ge, Y.; Jing, Y.; Gong, Z. Corosolic acid isolated from Eriobotrya japonica leaves reduces glucose level in human hepatocellular carcinoma cells, zebrafish and rats. Sci. Rep. 2019, 9, 4388. [Google Scholar] [CrossRef] [PubMed]
- Senbagalakshmi, P.; Muthukrishnan, S.; Jebasingh, T.; Kumar, T.S.; Rao, M.; Kumar, T.S.; Rao, M.V. Squalene, Biosynthesis and its role in production of bioactive compounds, a Proper Scientific Challenge—A Review. J. Emerg. Technol. Innov. Res. 2019, 6, 505–526. [Google Scholar]
- Mu, T.; Hu, H.; Ma, Y.; Feng, X.; Zhang, J.; Gu, Y. Regulation of key genes for milk fat synthesis in ruminants. Front. Nutr. 2021, 8, 765147. [Google Scholar] [CrossRef]
- Mandal, P.; Lanaridi, O.; Warth, B.; Ansari, K.M. Metabolomics as an emerging approach for deciphering the biological impact and toxicity of food contaminants: The case of mycotoxins. Crit. Rev. Food Sci. Nutr. 2023, 1–25. [Google Scholar] [CrossRef]
- Fabrile, M.P.; Ghidini, S.; Conter, M.; Varrà, M.O.; Ianieri, A.; Zanardi, E. Filling gaps in animal welfare assessment through metabolomics. Front. Vet. Sci. 2023, 10, 1129741. [Google Scholar] [CrossRef]
- Cynober, L.A. Plasma amino acid levels with a note on membrane transport: Characteristics, regulation, and metabolic significance. Nutrition 2002, 18, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, L.; Hou, S.; Raza, S.H.; Wang, Z.; Yang, B.; Sun, S.; Ding, B.; Gui, L.; Simal-Gandara, J.; et al. Effects of different feeding regimes on muscle metabolism and its association with meat quality of Tibetan sheep. Food Chem. 2022, 374, 131611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, W.; Jiang, L.; Mao, S. Comparative metabolome analysis of ruminal changes in Holstein dairy cows fed low-or high-concentrate diets. Metabolomics 2017, 13, 74. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zuo, S.; Peng, S.; Wang, Z.; Zhang, Y.; Luo, H. Untargeted and targeted metabolomics profiling of muscle reveals enhanced meat quality in artificial pasture grazing Tan lambs via rescheduling the rumen bacterial community. J. Agric. Food Chem. 2021, 69, 846–858. [Google Scholar] [CrossRef]

| Ingredients | Diets 1 | ||
|---|---|---|---|
| D_l | D_m | D_h | |
| Corn grains | 28.2 | 26.0 | 19.05 |
| Soybean meal | 5.4 | 8.65 | 18.6 |
| Corn starch | 8.65 | 7.6 | 4.7 |
| Corn silage | 40.0 | 35.0 | 34.0 |
| Wheat straw | 13.0 | 10.0 | 10.0 |
| Bean powder | 2.0 | 10.0 | 11.0 |
| Calcium carbonate | 0.55 | 0.6 | 0.65 |
| Calcium hydrogen phosphate | 0.55 | 0.5 | 0.35 |
| Salt | 0.3 | 0.3 | 0.3 |
| Baking soda | 0.35 | 0.35 | 0.35 |
| Premix | 1.0 | 1.0 | 1.0 |
| Total | 100.0 | 100 | 100.0 |
| Nutrients | Diets 1 | ||
|---|---|---|---|
| D_l | D_m | D_h | |
| Metabolizable energy (MJ/kg) | 9.45 | 9.47 | 9.47 |
| Crude protein (%) | 8.58 | 10.34 | 13.93 |
| Neutral detergent fiber (%) | 32.17 | 32.01 | 32.52 |
| Acid detergent fiber (%) | 17.24 | 17.71 | 18.44 |
| Calcium (%) | 0.69 | 0.71 | 0.71 |
| Phosphorus (%) | 0.38 | 0.39 | 0.39 |
| Superclass | Metabolites | Pairwise Comparison | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D_h and D_I | D_h and D_m | D_I and D_m | |||||||||||
| VIP | CV | p-Value | RG | VIP | CV | p-Value | RG | VIP | CV | p-Value | RG | ||
| Benzenoids | 2-methoxy-4-pentadecylbenzoic acid | 2.11 | 0.14 | 0.01 | Up | - | - | - | - | 1.82 | 0.14 | 0.01 | Up |
| 2-Phenylethanaminium | 2.10 | 0.01 | 0.00 | Up | - | - | - | - | 3.02 | 0.01 | 0.00 | Up | |
| C.I. Pigment Red 149 | 2.13 | 0.05 | 0.00 | Down | - | - | - | - | - | - | - | - | |
| Styrene | 2.25 | 0.03 | 0.00 | Up | - | - | - | - | 3.08 | 0.03 | 0.00 | Up | |
| Lipids and lipid-like molecules | (22E,24R)-Stigmasta-4,22-diene-3,6-dione | 2.49 | 0.04 | 0.01 | Up | 1.79 | 0.04 | 0.00 | Up | - | - | - | - |
| 11-Hydroxyeicosatetraenoate glyceryl ester | 2.35 | 0.06 | 0.00 | Up | - | - | - | - | 2.20 | 0.06 | 0.00 | Up | |
| 12-Ketodeoxycholic acid | 2.22 | 0.29 | 0.01 | Up | - | - | - | - | 1.84 | 0.05 | 0.01 | Up | |
| 1-Heptadecanoyl-sn-glycero-3-phosphocholine | 1.83 | 0.07 | 0.03 | Down | 1.71 | 0.07 | 0.02 | Down | - | - | - | - | |
| 2-Linoleoylglycerol | 1.65 | 0.11 | 0.00 | Down | 2.07 | 0.11 | 0.04 | Down | - | - | - | - | |
| 7-Oxostigmasterol | 2.59 | 0.10 | 0.00 | Up | 2.18 | 0.03 | 0.01 | Down | 1.96 | 0.10 | 0.01 | Up | |
| 8Z,14Z-Eicosadienoic acid | 2.01 | 0.36 | 0.01 | Up | - | - | - | - | - | - | - | - | |
| 9,10-Dihydroxy-12Z-octadecenoic acid | - | - | - | - | 3.10 | 0.07 | 0.00 | Up | 1.84 | 0.07 | 0.02 | Down | |
| Camellenodiol | 2.66 | 0.06 | 0.00 | Up | 1.93 | 0.06 | 0.00 | Up | - | - | - | - | |
| Cortisone | 1.69 | 0.30 | 0.03 | Up | - | - | - | - | 2.19 | 0.30 | 0.00 | Up | |
| Hydrocortisone | 1.56 | 0.04 | 0.00 | Down | 3.10 | 0.04 | 0.00 | Down | 1.46 | 0.04 | 0.00 | Up | |
| Isopropyl tiglate | 2.41 | 0.03 | 0.05 | Down | - | - | - | - | 2.67 | 0.03 | 0.04 | Down | |
| Linoleic acid methyl ester | 2.64 | 0.10 | 0.00 | Down | 2.50 | 0.10 | 0.02 | Down | - | - | - | - | |
| LysoPE 15:0 | 2.18 | 0.28 | 0.00 | Down | 2.11 | 0.28 | 0.02 | Down | - | - | - | - | |
| LysoPE 16:0 | 2.28 | 0.04 | 0.00 | Down | 2.80 | 0.04 | 0.00 | Down | - | - | - | - | |
| LysoPE 18:0 | 2.60 | 0.13 | 0.00 | Down | 1.65 | 0.13 | 0.02 | Down | - | - | - | - | |
| Oleyl alcohol | 1.89 | 0.05 | 0.01 | Down | 2.51 | 0.05 | 0.00 | Down | - | - | - | - | |
| Traumatin | 2.60 | 0.07 | 0.01 | Up | - | - | - | - | 2.62 | 0.07 | 0.00 | Up | |
| Zymosterol | 2.07 | 0.08 | 0.00 | Up | 1.85 | 0.17 | 0.00 | Up | - | - | - | - | |
| Organic acids and derivatives | Aspartic acid | 2.70 | 0.10 | 0.00 | Up | 3.29 | 0.10 | 0.00 | Up | - | - | - | - |
| Methionine sulfoxide | 2.30 | 0.17 | 0.00 | Up | - | - | - | - | - | - | - | - | |
| N-Acetyl-dl-valine | 1.94 | 0.16 | 0.00 | Up | - | - | - | - | 2.31 | 0.16 | 0.00 | Up | |
| N-Acetyl-d-norleucine | 2.01 | 0.07 | 0.02 | Up | - | - | - | - | 1.81 | 0.07 | 0.03 | Up | |
| Proline | - | - | - | - | - | - | - | - | 1.38 | 0.16 | 0.04 | Up | |
| Tiglylglycine | 2.67 | 0.08 | 0.01 | Up | 1.88 | 0.08 | 0.02 | Up | |||||
| Tyrosine | - | - | - | - | 2.42 | 0.07 | 0.03 | Up | 2.42 | 0.07 | 0.03 | Down | |
| 2,2,6,7-Tetramethylbicyclo[4.3.0]nona-1(9),4-diene-7,8-diol | - | - | - | - | - | - | - | - | 2.40 | 0.24 | 0.04 | Down | |
| Trehalose | 1.93 | 0.34 | 0.01 | Down | 3.09 | 0.34 | 0.00 | Down | - | - | - | - | |
| Organoheterocyclic compounds | Tryptophan | 2.22 | 0.07 | 0.02 | Up | - | - | - | - | - | - | - | - |
| Indole-3-pyruvic acid | 2.28 | 0.07 | 0.00 | Up | - | - | - | - | 2.43 | 0.07 | 0.00 | Up | |
| Indoleacetic acid | 3.04 | 0.07 | 0.00 | Up | - | - | - | - | - | - | - | - | |
| Isonicotinic acid | 2.21 | 0.22 | 0.00 | Up | - | - | - | - | 2.08 | 0.22 | 0.00 | Up | |
| Methylimidazoleacetic acid | 2.53 | 0.18 | 0.00 | Up | - | - | - | - | 2.27 | 0.18 | 0.00 | Up | |
| Pergolide | 2.38 | 0.08 | 0.00 | Down | - | - | - | - | - | - | - | - | |
| Phaeophorbide b | 2.24 | 0.20 | 0.00 | Down | 2.54 | 0.20 | 0.00 | Down | - | - | - | - | |
| Protoporphyrin IX | 1.92 | 0.14 | 0.03 | Down | 2.13 | 0.14 | 0.02 | Down | - | - | - | - | |
| Superclass | Metabolite | Pairwise Comparison | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D_h and D_I | D_h and D_m | D_I and D_m | |||||||||||
| VIP | CV | p-Value | RG | VIP | CV | p-Value | RG | VIP | CV | p-Value | RG | ||
| Benzenoids | 3-Ethylphenol | - | - | - | - | - | - | - | - | 2.10 | 0.13 | 0.01 | Down |
| 3-Methylphenylacetic acid | - | - | - | - | - | - | - | - | 1.93 | 0.11 | 0.00 | Up | |
| 4-Ethyl-2-methoxyphenol | - | - | - | - | - | - | - | - | 1.86 | 0.05 | 0.02 | Up | |
| 4-Methylhippuric acid | - | - | - | - | - | - | - | - | 2.56 | 0.34 | 0.00 | Up | |
| D8’-Merulinic acid A | - | - | - | - | - | - | - | - | 2.04 | 0.11 | 0.00 | Up | |
| Lipids and lipid-like molecules | 12-hydroxyheptadecanoic acid | 1.96 | 0.41 | 0.03 | Up | 2.72 | 0.04 | 0.01 | Up | ||||
| 2(R)-hydroxy icosanoic acid | 2.52 | 0.11 | 0.00 | Up | - | - | - | - | - | - | - | - | |
| 2-Hydroxy-2-methylbutyric acid | 1.82 | 0.35 | 0.00 | Down | - | - | - | - | 2.46 | 0.35 | 0.01 | Down | |
| 2-Hydroxymyristic acid | 1.58 | 0.03 | 0.00 | Up | - | - | - | - | 2.39 | 0.03 | 0.00 | Up | |
| 3a,7b,12a-Trihydroxyoxocholanyl-Glycine | 1.74 | 0.03 | 0.03 | Down | 1.81 | 0.03 | 0.04 | Down | - | - | - | - | |
| Corosolic acid | 2.21 | 0.08 | 0.00 | Up | - | - | - | - | - | - | - | - | |
| Dodecanedioic acid | 2.59 | 0.07 | 0.01 | Up | 2.76 | 0.07 | 0.01 | Up | |||||
| Furfuryl isovalerate | 1.63 | 0.11 | 0.01 | Up | - | - | - | - | - | - | - | - | |
| Heptadecanoic acid | - | - | - | - | - | - | - | - | 2.02 | 0.13 | 0.01 | Up | |
| Lucidenic acid L | 1.83 | 0.20 | 0.03 | Up | - | - | - | - | - | - | - | - | |
| Lucidenic acid N | 1.57 | 0.01 | 0.00 | Up | 2.84 | 0.01 | 0.00 | Down | 4.32 | 0.01 | 0.00 | Up | |
| LysoPC 18:0 | 1.68 | 0.20 | 0.00 | Down | 2.08 | 0.20 | 0.02 | Down | - | - | - | - | |
| LysoPC 18:1 | 1.75 | 0.38 | 0.01 | Down | - | - | - | - | - | - | - | - | |
| LysoPE 14:0 | 1.52 | 0.31 | 0.02 | Down | 2.72 | 0.31 | 0.00 | Down | - | - | - | - | |
| LysoPE 15:0 | 2.62 | 0.05 | 0.00 | Down | 2.28 | 0.05 | 0.00 | Down | - | - | - | - | |
| LysoPE 16:0 | 2.10 | 0.05 | 0.00 | Down | 2.79 | 0.05 | 0.00 | Down | - | - | - | - | |
| LysoPE 18:0 | 2.64 | 0.10 | 0.00 | Down | 2.19 | 0.10 | 0.00 | Down | - | - | - | - | |
| LysoPE 18:1 | 2.28 | 0.02 | 0.00 | Down | 1.87 | 0.02 | 0.01 | Down | - | - | - | - | |
| LysoPG 14:0; LysoPG 14:0 | 1.65 | 0.07 | 0.04 | Down | 2.27 | 0.07 | 0.05 | Down | - | - | - | - | |
| LysoPG 15:0; LysoPG 15:0 | 1.45 | 0.32 | 0.02 | Up | 1.98 | 0.06 | 0.02 | Down | 2.29 | 0.32 | 0.00 | Up | |
| LysoPS 16:0 | 1.72 | 0.14 | 0.01 | Down | 2.76 | 0.14 | 0.00 | Down | - | - | - | - | |
| Mevalonic acid | 2.00 | 0.04 | 0.00 | Up | - | - | - | - | - | - | - | - | |
| Myristic acid | 1.84 | 0.06 | 0.01 | Up | 2.86 | 0.06 | 0.00 | Up | |||||
| Palmitic acid | - | - | - | - | - | - | - | - | 1.86 | 0.04 | 0.02 | Up | |
| Polyethylene, oxidized | 2.12 | 0.11 | 0.00 | Up | - | - | - | - | - | - | - | - | |
| Ursocholic acid | 2.37 | 0.02 | 0.01 | Up | - | - | - | - | - | - | - | - | |
| Organic acids and derivatives | 2-Hydroxy-2-(2-oxopropyl)butanedioic acid | 3.18 | 0.04 | 0.00 | Down | 3.49 | 0.04 | 0.03 | Down | - | - | - | - |
| d-(+)-Pantothenic acid | 2.01 | 0.04 | 0.00 | Down | - | - | - | - | - | - | - | - | |
| l-Aspartic acid | 3.13 | 0.07 | 0.00 | Up | 3.07 | 0.07 | 0.00 | Up | - | - | - | - | |
| l-Tyrosine | 2.27 | 0.26 | 0.02 | Up | 2.27 | 0.26 | 0.02 | Up | 2.08 | 0.26 | 0.02 | Down | |
| N-Acetyl-d-norleucine | 2.12 | 0.00 | 0.00 | Up | - | - | - | - | 1.82 | 0.00 | 0.00 | Up | |
| N-Acetyl-l-phenylalanine | 1.67 | 0.02 | 0.02 | Up | - | - | - | - | 1.85 | 0.02 | 0.02 | Up | |
| N-Palmitoyl phenylalanine | 1.43 | 0.06 | 0.00 | Up | - | - | - | - | 2.66 | 0.06 | 0.00 | Up | |
| N-Phenylacetylglutamic acid | - | - | - | - | - | - | - | - | 1.89 | 0.12 | 0.00 | Up | |
| Organic oxygen compounds | 1,5-Anhydro-d-sorbitol | 2.19 | 0.16 | 0.01 | Down | - | - | - | - | 2.35 | 0.16 | 0.00 | Down |
| Maltotriose | 1.49 | 0.24 | 0.01 | Down | 2.76 | 0.24 | 0.00 | Down | - | - | - | - | |
| Organoheterocyclic compounds | Kynurenic acid | 2.15 | 0.17 | 0.00 | Up | 2.09 | 0.17 | 0.00 | Up | - | - | - | - |
| Paramethadione | 2.83 | 0.11 | 0.01 | Up | - | - | - | - | 2.07 | 0.11 | 0.02 | Up | |
| Tryptophan | 2.04 | 0.05 | 0.00 | Up | - | - | - | - | - | - | - | - | |
| Xanthine | 2.49 | 0.05 | 0.05 | Up | - | - | - | - | - | - | - | - | |
| Phenylpropanoids and polyketides | Hydroxygaleon | - | - | - | - | - | - | - | - | 2.05 | 0.26 | 0.02 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Ni, X.; Khan, M.; Zhao, X.; Yang, H.; Danzeng, B.; Raja, I.H.; Quan, G. Effects of Dietary Protein Levels on Sheep Gut Metabolite Profiles during the Lactating Stage. Animals 2024, 14, 121. https://doi.org/10.3390/ani14010121
Ali S, Ni X, Khan M, Zhao X, Yang H, Danzeng B, Raja IH, Quan G. Effects of Dietary Protein Levels on Sheep Gut Metabolite Profiles during the Lactating Stage. Animals. 2024; 14(1):121. https://doi.org/10.3390/ani14010121
Chicago/Turabian StyleAli, Sikandar, Xiaojun Ni, Muhammad Khan, Xiaoqi Zhao, Hongyuan Yang, Baiji Danzeng, Imtiaz Hussain Raja, and Guobo Quan. 2024. "Effects of Dietary Protein Levels on Sheep Gut Metabolite Profiles during the Lactating Stage" Animals 14, no. 1: 121. https://doi.org/10.3390/ani14010121
APA StyleAli, S., Ni, X., Khan, M., Zhao, X., Yang, H., Danzeng, B., Raja, I. H., & Quan, G. (2024). Effects of Dietary Protein Levels on Sheep Gut Metabolite Profiles during the Lactating Stage. Animals, 14(1), 121. https://doi.org/10.3390/ani14010121







