African Elephant Milk Short Saccharide and Metabolite Composition and Their Changes over Lactation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Sample Preparation of Milk for NMR Analysis
2.3. H-NMR Analysis
2.4. H-NMR Processing
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCullagh, K.G.; Widdowson, E.M. The milk of the African elephant. Br. J. Nutr. 1970, 24, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kobeni, S.; Osthoff, G.; Madende, M.; Hugo, A.; Marabini, L. The dynamic changes of African elephant milk composition over lactation. Animals 2020, 10, 948. [Google Scholar] [CrossRef]
- Abbondanza, F.N.; Power, M.L.; Dickson, M.A.; Brown, J.; Oftedal, O.T. Variation in the composition of milk of Asian elephants (Elephas maximus) throughout lactation. Zoo Biol. 2013, 32, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Dierenfeld, E.S.; Han, Y.A.M.; Mar, K.U.; Aung, A.; Soe, A.T.; Lummaa, V.; Lahdenperä, M. Milk Composition of Asian Elephants (Elephas maximus) in a Natural Environment in Myanmar during Late Lactation. Animals 2020, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Uemura, Y.; Asakuma, S.; Yon, L.; Saito, T.; Fukuda, K.; Arai, I.; Urashima, T. Structural determination of the oligosaccharides in the milk of an Asian elephant (Elephas maximus). Comp. Biochem. Physiol. A 2006, 145, 468–478. [Google Scholar] [CrossRef]
- Osthoff, G.; Dickens, L.; Urashima, T.; Bonnet, S.L.; Uemura, Y.; Van der Westhuizen, J.H. Structural characterization of oligosaccharides in the milk of an African elephant (Loxodonta africana africana). Comp. Biochem. Physiol. B 2008, 150, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Arita-Merino, N.; Yener, S.; Van Valenberg, H.J.F.; Hugo, A.; Osthoff, G. Triacylglycerol profile and crystallization behavior of African elephant milk fat. Eur. J. Lipid Sci. Technol. 2020, 122, e2000119. [Google Scholar] [CrossRef]
- Madende, M.; Osthoff, G.; Patterton, H.; Martin, P.; Opperman, D.J. Characterization of casein and alpha lactalbumin of African elephant (Loxodonta africana) milk. J. Dairy Sci. 2015, 98, 8308–8318. [Google Scholar] [CrossRef]
- Madende, M.; Kemp, G.; Stoychev, S.; Osthoff, G. Characterization of African elephant beta casein and its relevance to the chemistry of caseins and casein micelles. Int. Dairy J. 2015, 85, 112–120. [Google Scholar] [CrossRef]
- Osthoff, G.; Madende, M.; Hugo, A.; Butler, H.J.B. Milk evolution with emphasis on the Atlantogenata. Afr. Zool. 2020, 55, 257–266. [Google Scholar] [CrossRef]
- Dessì, A.; Briana, D.; Corbu, S.; Gavrili, S.; Marincola, F.C.; Georgantzi, S.; Pintus, R.; Fanos, V.; Malamitsi-Puchner, A. Metabolomics of Breast Milk: The Importance of Phenotypes. Metabolites 2018, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.S.; Lewis, A.N.; Gong, Q.; Sollome, J.J.; DeWitt, O.N.; Williams, R.D.; Good, M. Untargeted Metabolomic Analysis of Human Milk from Mothers of Preterm Infants. Nutrients 2021, 13, 3604. [Google Scholar] [CrossRef] [PubMed]
- Kortesniemi, M.; Jafari, T.; Zhang, Y.; Yang, B. 1H NMR Metabolomics of Chinese Human Milk at Different Stages of Lactation among Secretors and Non-Secretors. Molecules 2022, 27, 5526. [Google Scholar] [CrossRef]
- Enjalbert, F.; Nicot, M.C.; Bayourthe, C.; Moncoulon, R. Ketone bodies in milk and blood of dairy cows: Relationship between concentrations and utilization for detection of subclinical ketosis. J. Dairy Sci. 2001, 84, 583–589. [Google Scholar] [CrossRef]
- Tenori, L.; Santucci, C.; Meoni, G.; Morrocchi, V.; Matteucci, G.; Luchinat, C. NMR metabolomic fingerprinting distinguishes milk from different farms. Food Res. Int. 2018, 113, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Scano, P.; Murgia, A.; Pirisi, F.M.; Caboni, P. A gas chromatography-mass spectrometry-based metabolomic approach for the characterization of goat milk compared with cow milk. J. Dairy Sci. 2014, 97, 6057–6066. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Zhang, J.; Miao, J.; Chen, G.; Dong, J.; Wu, L.; Yue, H.; Yang, J. Untargeted metabolomics allows to discriminate raw camel milk, heated camel milk, and camel milk powder. Int. Dairy J. 2022, 124, 105140. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, N.; Zhao, X.; Zhang, Y.; Han, R.; Yang, J.; Zhao, S.; Li, S.; Guo, T.; Zang, C.; et al. Metabolomic biomarkers identify differences in milk produced by Holstein cows and other minor dairy animals. J. Proteom. 2016, 136, 174–182. [Google Scholar] [CrossRef]
- Xu, W.; Van Knegsel, A.; Saccenti, E.; Van Hoeij, R.; Kemp, B.; Vervoort, J. Metabolomics of milk reflects a negative energy balance in cows. J. Proteome Res. 2020, 19, 2942–2949. [Google Scholar] [CrossRef]
- Rocchetti, G.; O’Callaghan, T.F. Application of metabolomics to assess milk quality and traceability. Curr. Opin. Food Sci. 2021, 40, 168–178. [Google Scholar] [CrossRef]
- Li, M.; Kang, S.; Zheng, Y.; Shao, J.; Zhao, H.; An, Y.; Cao, G.; Li, Q.; Yue, X.; Yang, M. Comparative metabolomics analysis of donkey colostrum and mature milk using ultra-high-performance liquid tandem chromatography quadrupole time-of-flight mass spectrometry. J. Dairy Sci. 2020, 103, 992–1001. [Google Scholar]
- Li, M.; Chen, J.; Shen, X.; Abdlla, R.; Liu, L.; Yue, X.; Li, Q. Metabolomics-based comparative study of breast colostrum and mature breast milk. Food Chem. 2022, 384, 132491. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.J.; Al-Khalidi, A.; Jaiteh, M.; Clarke, E.; Hyde, M.J.; Modi, N.; Holmes, E.; Kampmann, B.; Le Doare, K.M. Role of human milk oligosaccharides in Group B Streptococcus colonization. Clin. Transl. Immunol. 2016, 5, e99. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Chapter Four—Human Milk Oligosaccharides (HMOS): Structure, Function, and Enzyme-Catalyze Synthesis. Adv. Carboh. Chem. Biochem. 2015, 72, 113–190. [Google Scholar]
- Urashima, T.; Messer, M.; Oftedal, O.T. Comparative Biochemistry and Evolution of Milk Oligosaccharides of Monotremes, Marsupials and Eutherians. In Evolutionary Biology: Genome Evolution, Speciation, Coevolution and Origin of Life; Pontarotti, P., Ed.; Springer: Cham, Switzerland, 2014; pp. 3–33. [Google Scholar]
- Urashima, T.; Messer, M.; Oftedal, O.T. Oligosaccharides in the Milk of Other Mammals. In Prebiotics and Probiotics in Human Milk: Origins and Functions of Milk-Borne Oligosaccharides and Bacteria; Pontarotti, P., Ed.; Springer: Cham, Switzerland, 2014; pp. 45–139. [Google Scholar]
- Sumiyoshi, W.; Urashima, T.; Nakamura, T.; Arai, I.; Saito, T.; Tsumura, N.; Wang, B.; Brand-Miller, J.; Watanabe, Y.; Kimura, K. Determination of each neutral oligosaccharide in the milk of Japanese women during the course of lactation. Br. J. Nutr. 2003, 89, 61–69. [Google Scholar] [CrossRef]
- Sumiyoshi, W.; Urashima, T.; Nakamura, T.; Arai, I.; Nagasawa, T.; Saito, T.; Tsumura, N.; Wang, B.; Brand-Miller, J.; Watanabe, Y.; et al. Sialyl Oligosaccharides in the Milk of Japanese Women: Changes in Concentration during the Course of Lactation. J. Appl. Glycosci. 2003, 50, 461–467. [Google Scholar] [CrossRef]
- Osthoff, G.; Hugo, A.; DeWaal, H.O.; Botes, P. The composition of African elephant (Loxodonta africana) milk collected a few days postpartum. Comp. Biochem. Physiol. A 2005, 141, 223–229. [Google Scholar]
- Erasmus, E.; Mason, S.; van Reenen, M.; Steffens, F.E.; Vorster, B.C.; Reinecke, C.J. A laboratory approach for characterizing chronic fatigue: What does metabolomics tell us? Metabolomics 2019, 15, 158. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar]
- Addinsoft, 2020. XLSTAT Statistical and Data Analysis Solution. NCCS 11 Statistical Software; NCSS, LLC: Kaysville, UT, USA, 2018. [Google Scholar]
- Coppa, G.V.; Pierani, P.; Zampini, L.; Carloni, I.; Gabrielli, O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr. 1999, 430, 89–94. [Google Scholar]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef]
- Kunz, C.; Rudloff, S.; Shad, W.; Braun, D. Lactose-derived oligosaccharides in the milk of elephants: Comparison with human milk. Br. J. Nutr. 1999, 82, 391–399. [Google Scholar] [CrossRef]
- Ridgway, N.D. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Menard, O. Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids Surf. B Biointerfaces 2011, 83, 29–41. [Google Scholar] [CrossRef]
- Nurjhan, N.; Bucci, A.; Perriello, G.; Stumvoll, M.; Dailey, G.; Bier, D.M.; Toft, I.; Jenssen, T.G.; Gerich, J.E. Glutamine: A major gluconeogenic precursor and vehicle for interorgan carbon transport in man. J. Clin. Investig. 1995, 95, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Wondisford, F.E. Tracking the carbons supplying gluconeogenesis. J. Biol. Chem. 2020, 295, 14419–14429. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Umewaki, M.; Taufik, E.; Ohshima, T.; Fukuda, K.; Saito, T.; Whitehouse-Tedd, K.; Budd, J.A.; Oftedal, O.T. Chemical structures of oligosaccharides in milks of the American black bear (Ursus americanus americanus) and cheetah (Acinonyx jubatus). Glycoconj. J. 2020, 37, 57–76. [Google Scholar] [CrossRef]
- Sikes, R.S. The Animal Care and Use Committee of the American Society of Mammalogists. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]



| Peak for Metabolite or Unknown Compound | Assignment |
|---|---|
| Formate | 8.46 (s) |
| Unknown 1 | 8.39 (d) |
| Unknown 2 | 8.33 (d) |
| Unknown 3 | 8.27 (d) |
| Unknown 4 | 8.22 (d) |
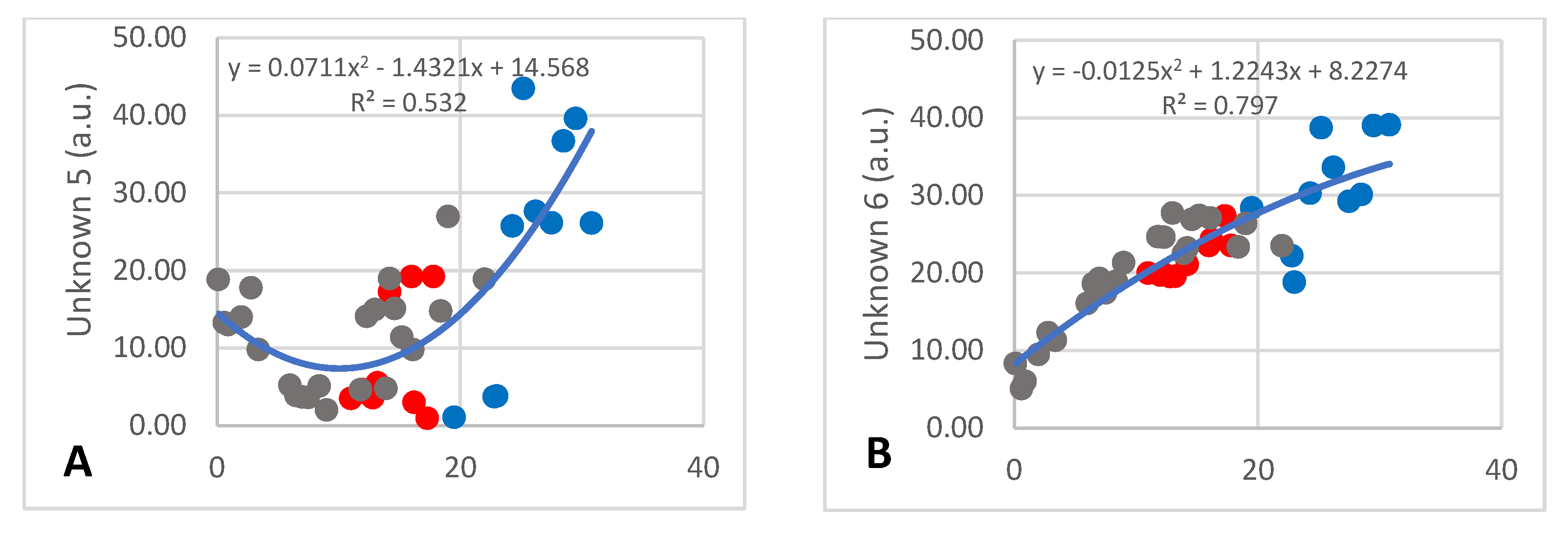
| Unknown 5 | 8.12 (d) |
| Unknown 6 | 8.05 (d) |
| Hippurate | 8.52 (s); 7,84 (s); 7.66 (m); 7.64 (m); 7.63 (m) |
| Phenylalanine derivate | 8.47 (s); 7.44 (s); 7.41 (s); 7.38 (m); 7.36 (m); 7.30 (m); 7.11 (m) |
| Fumarate | 6.52 (s) |
| Oligosaccharide 1 | 5.5 (q) |
| Oligosaccharide 2 | 5.48 (d) |
| Difucosyllactose | 5.46 (d) |
| Lacto-N-difucohexaose II | 5.43 (d); 2.05 (s) |
| 3-Fucosyllactose | 5.44 (d); 5.39 (d) |
| Oligosaccharide 3 | 5.36 (q) |
| 2′-Fucosyllactose | 5.32 (m); 4.53 (d) |
| Oligosaccharide 4 | 5.27 (d) |
| Lactose | 5.24 (d); 4.46 (d) |
| Lacto-N-difucohexaose I | 5.15 (d); 2.07 (s) |
| Glycerophosphocholine | 3.23 (s) |
| Phosphocholine | 3.22 (s) |
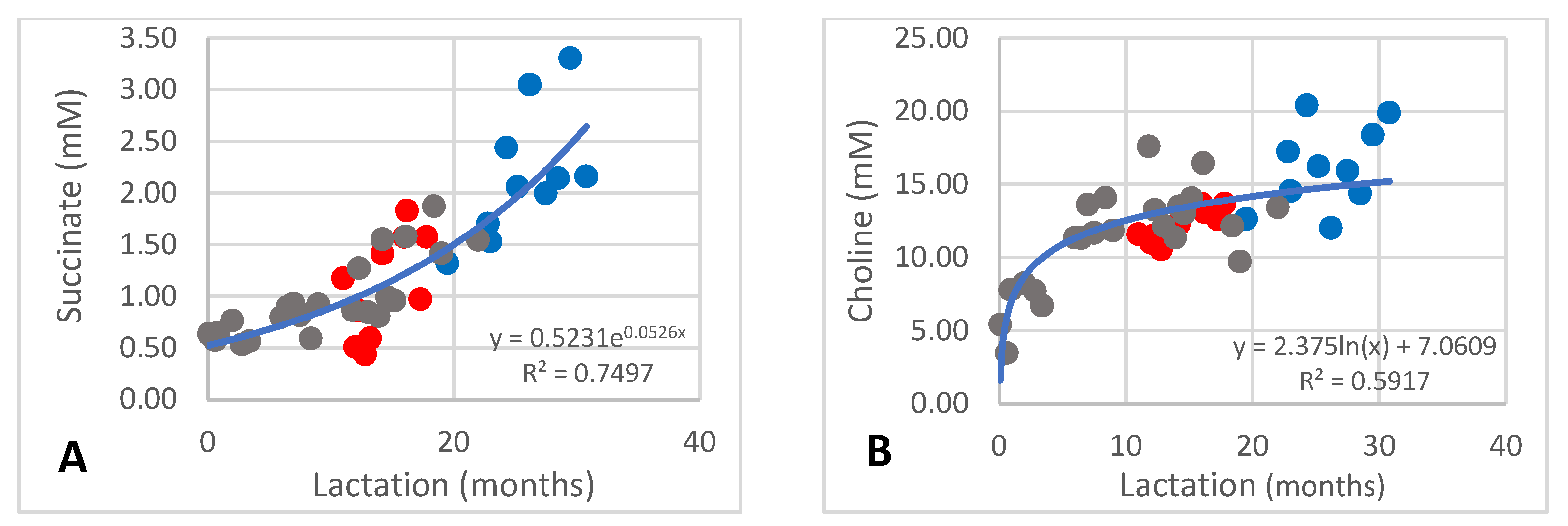
| Choline | 3.20 (s) |
| Acetylcarnitine | 3.20 (s) |
| Unknown 8 | 3.16 (s) |
| Unknown 9 | 3.05 (s) |
| Unknown 10 | 3.05 (s) |
| Creatine | 3.04 (s) |
| 3′-Sialyllactose | 2.78 (d); 2.75 (d) |
| Unknown 11 | 2.44 (s) |
| Succinate | 2.41 (s) |
| Glutamate | 2.37 (s) |
| Caprate | 2.17 (t) |
| Unknown 12 | 2.06 (s) |
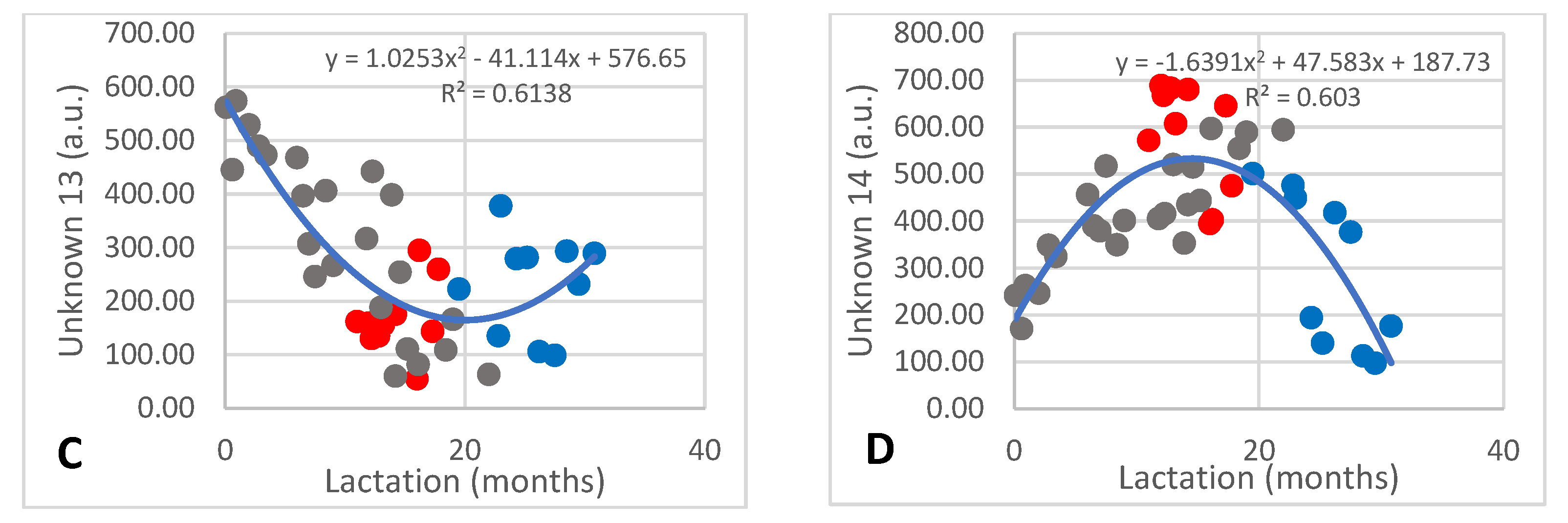
| Unknown 13 | 2.05 (s) |
| Unknown 14 | 2.03 (s) |
| Acetate | 1.92 (s) |
| Up to 12 Months | After 12 Months | |||
|---|---|---|---|---|
| Metabolites | Average ± STD | Range | Average ± STD | Range |
| Acetate (mM) | 7.07 ± 2.33 | 3.02–10.87 | 6.34 ± 1.41 | 4.08–11.06 |
| Acetylcarnitine (mM) | 0.15 ± 0.07 | 0.05–0.31 | 0.25 ± 0.09 | 0.04–0.49 |
| Caprate (mM) | 117.21 ± 83.84 | 28.34–315.41 | 132.64 ± 93.08 | 32.76–340.82 |
| Choline (mM) | 10.23 ± 3.66 | 3.47–17.60 | 13.91 ± 2.66 | 9.72–20.40 |
| Creatine (mM) | 16.98 ± 3.08 | 12.00–23.44 | 19.90 ± 3.83 | 15.10–28.48 |
| Formate (mM) | 1.48 ± 0.37 | 1.00–2.30 | 1.34 ± 0.36 | 0.69–2.20 |
| Fumarate (mM) | 0.09 ± 0.02 | 0.06–0.13 | 0.10 ± 0.02 | 0.06–0.15 |
| Glutamate (mM) | 277.55 ± 95.28 | 125.04–457.21 | 251.06 ± 69.16 | 79.59–370.60 |
| Glycerophosphocholine (mM) | 24.83 ± 6.32 | 13.52–38.06 | 23.37 ± 10.12 | 6.45–46.28 |
| Hippurate (mM) | 3.43 ± 1.90 | 0.39–7.00 | 3.80 ± 1.58 | 1.37–6.56 |
| Lactose (mM) | 949.70 ± 437.65 | 400.7–2018.53 | 335.10 ± 177.93 | 110.31–816.01 |
| Phenylalanine derivate (mM) | 5.56 ± 3.87 | 1.26–13.19 | 4.58 ± 3.79 | 0.44–13.46 |
| Phosphocholine (mM) | 52.90 ± 30.44 | 3.01–121.19 | 44.44 ± 35.78 | 5.99–116.92 |
| Succinate (mM) | 0.75 ± 0.19 | 0.51–1.17 | 1.56 ± 0.68 | 0.44–3.31 |
| Unknown 1 (a.u.) | 35.07 ± 4.05 | 25.62–40.98 | 33.25 ± 6.88 | 15.47–41.55 |
| Unknown 2 (a.u.) | 21.33 ± 2.53 | 16.76–26.14 | 21.97 ± 3.73 | 13.31–28.81 |
| Unknown 3 (a.u.) | 35.32 ± 4.41 | 26.36–42.05 | 30.48 ± 6.66 | 14.92–42.40 |
| Unknown 4 (a.u.) | 33.35 ± 9.85 | 18.17–61.27 | 24.21 ± 7.48 | 9.88–35.56 |
| Unknown 5 (a.u.) | 8.19 ± 5.69 | 2.03–18.82 | 16.31 ± 11.93 | 0.96–43.47 |
| Unknown 6 (a.u.) | 15.23 ± 6.03 | 5.06–24.65 | 26.46 ± 5.69 | 18.80–39.08 |
| Unknown 8 (a.u.) | 12.02 ± 6.51 | 0.29–22.88 | 5.21 ± 3.70 | 0.49–14.07 |
| Unknown 9 (a.u.) | 10.36 ± 2.35 | 6.04–14.75 | 16.13 ± 9.35 | 6.36–41.52 |
| Unknown 10 (a.u.) | 10.01 ± 4.94 | 2.35–18.40 | 12.58 ± 6.43 | 2.34–25.76 |
| Unknown 11 (a.u.) | 5.59 ± 1.72 | 3.02–8.76 | 9.83 ± 2.51 | 4.52–15.96 |
| Unknown 12 (a.u.) | 1017.41 ± 131.31 | 814.04–14.47 | 811.58 ± 121.47 | 562.40–986.88 |
| Unknown 13 (a.u.) | 386.50 ± 136.89 | 158.88–573.39 | 197.64 ±106.28 | 54.82–442.13 |
| Unknown 14 (a.u.) | 383.43 ± 135.64 | 170.39–688.69 | 429.02 ± 183.51 | 97.17–682.69 |
| Difucosyllactose (mM) | 6.77 ± 4.15 | 3.35–17.07 | 12.65 ± 4.47 | 4.17–20.81 |
| 2′-Fucosyllactose (mM) | 2.02 ± 0.49 | 1.20–2.62 | 1.06 ± 0.54 | 0.27–1.90 |
| 3-Fucosyllactose (mM) | 44.66 ± 29.17 | 11.70–102.89 | 75.15 ± 22.19 | 40.37–130.30 |
| 3′-Sialyllactose (mM) | 104.15 ± 36.82 | 48.27–155.25 | 112.82 ± 32.37 | 67.87–187.05 |
| Lacto-N-difucohexaose I (mM) | 886.72 ± 188.61 | 534.12–1147.00 | 400.77 ± 244.59 | 98.89–934.54 |
| Lacto-N-difucohexaose II (mM) | 7.52 ± 2.91 | 4.37–13.69 | 10.00 ± 2.42 | 5.73–15.12 |
| Total identified oligosaccharides (mM) | 1025.10 ± 186.76 | 659.53–1285.40 | 651.99 ± 335.71 | 292.34–1227.70 |
| Oligosaccharide 1 | 23.63 ± 10.45 | 7.01–41.67 | 43.30 ± 13.3 | 16.48–64.96 |
| Oligosaccharide 2 | 6.58 ± 9.76 | 1.13–33.07 | 7.68 ± 7.14 | 1.06–30.27 |
| Oligosaccharide 3 | 15.49 ± 11.53 | 1.04–39.05 | 62.88 ± 18.96 | 11.97–96.54 |
| Oligosaccharide 4 | 8.65 ± 6.47 | 2.73–24.58 | 2.62 ± 1.32 | 1.42–7.73 |
| Total unidentified oligosaccharides (a.u.) | 49.84 ± 16.82 | 24.26–76.16 | 199.73 ± 17.88 | 86.78–150.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osthoff, G.; Wiese, I.; Deacon, F. African Elephant Milk Short Saccharide and Metabolite Composition and Their Changes over Lactation. Animals 2023, 13, 544. https://doi.org/10.3390/ani13030544
Osthoff G, Wiese I, Deacon F. African Elephant Milk Short Saccharide and Metabolite Composition and Their Changes over Lactation. Animals. 2023; 13(3):544. https://doi.org/10.3390/ani13030544
Chicago/Turabian StyleOsthoff, Gernot, Irenie Wiese, and Francois Deacon. 2023. "African Elephant Milk Short Saccharide and Metabolite Composition and Their Changes over Lactation" Animals 13, no. 3: 544. https://doi.org/10.3390/ani13030544