Exencephaly–Anencephaly Sequence Associated with Maxillary Brachygnathia, Spinal Defects, and Palatoschisis in a Male Domestic Cat
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
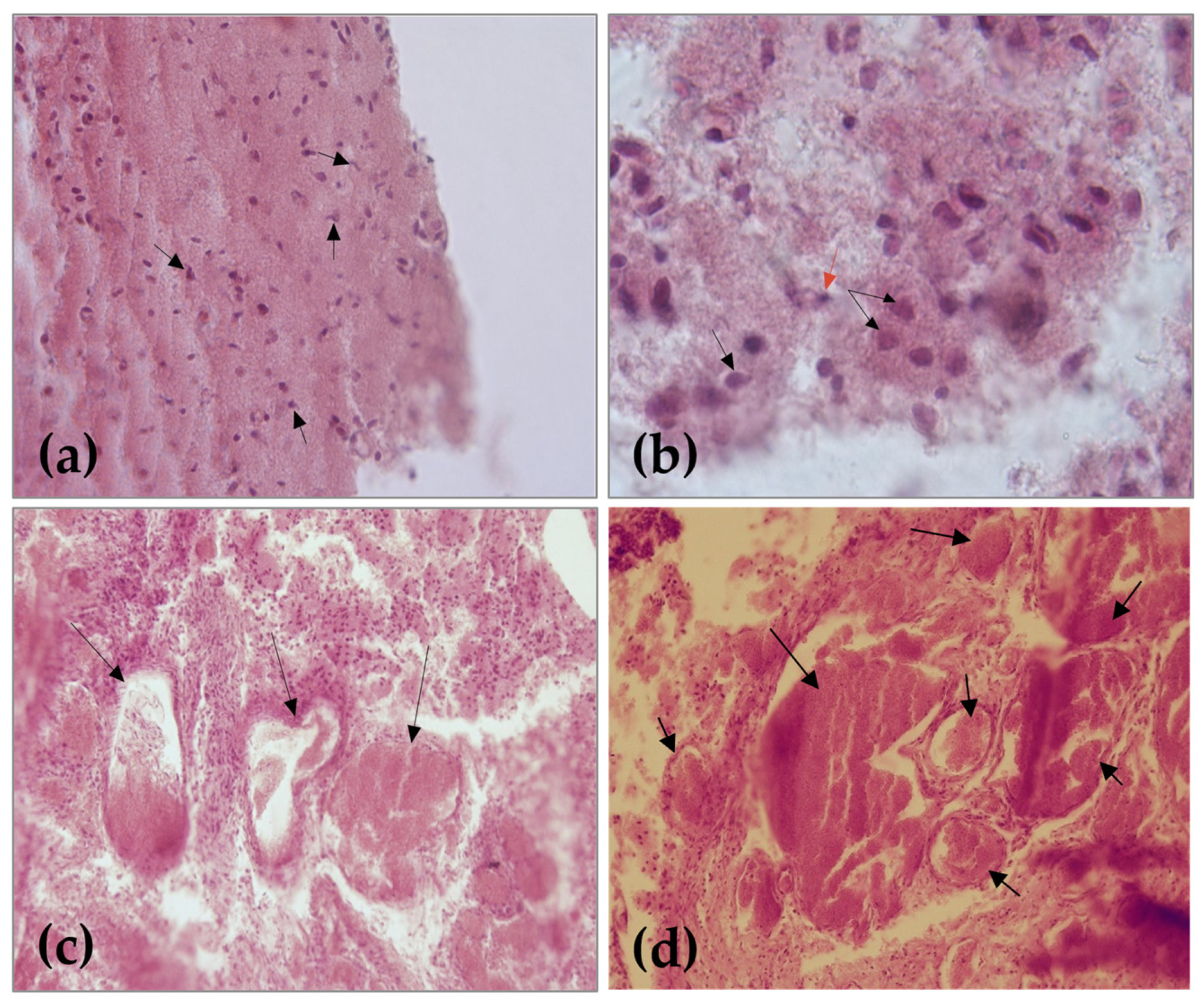
2.2. Cytohistological Investigation
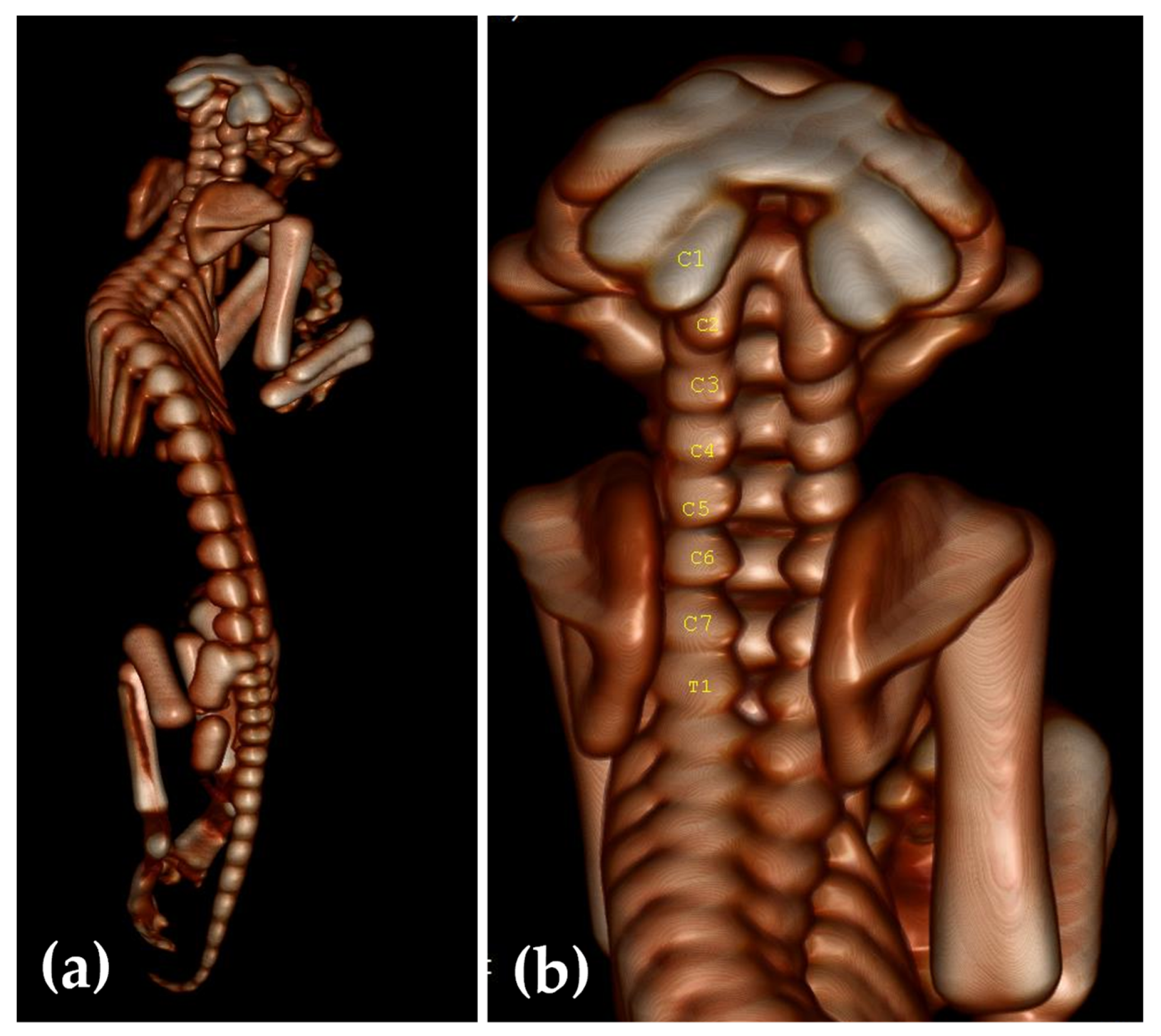
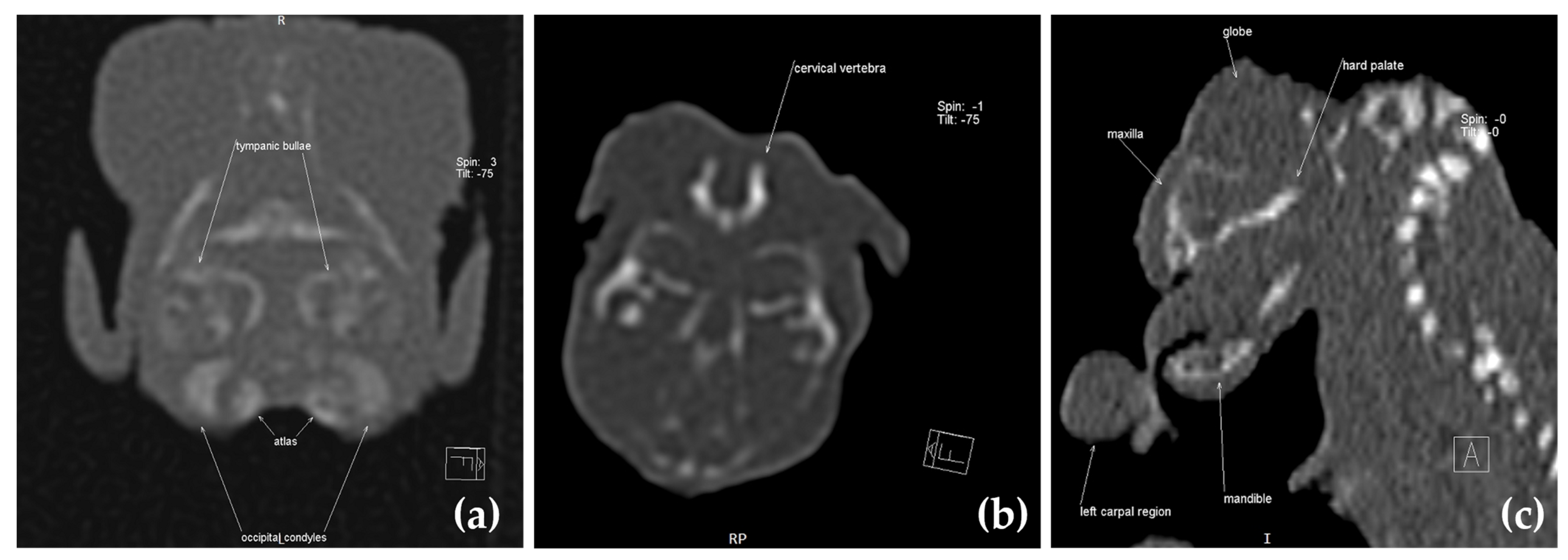
2.3. Computed Tomography Investigation
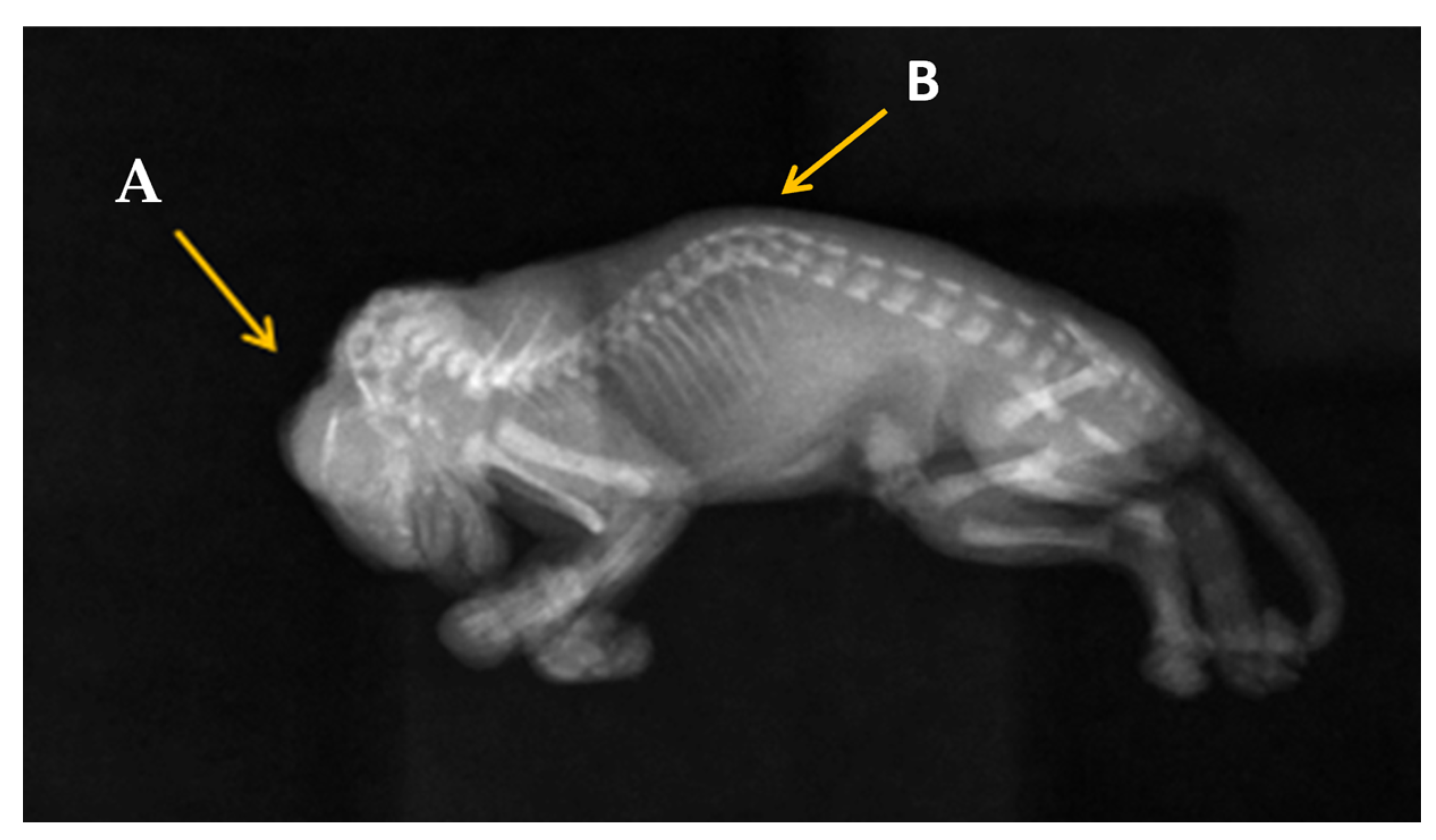
2.4. Radiography Investigation
2.5. Dissection Investigation
3. Results
3.1. Clinical Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zarzycki, A.; Thomas, Z.M.; Mazrier, H. Comparison of inherited neural tube defects in companion animals and livestock. Birth Defects Res. 2021, 113, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Hyttel, P.; Sinowatz, F.; Vejlsted, M. Essentials of Domestic Animal Embryology; Keith, B., Ed.; Saunders Elsevier: London, UK, 2010. [Google Scholar]
- Harrington, M.J.; Hong, E.; Brewster, R. Comparative analysis of neurulation: First impressions do not count. Mol. Reprod. Dev. 2009, 76, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Massa, V.; George, T.M.; Qureshy, S.; Bulfamante, G.P.; Finnell, R.H. Overview on neural tube defects: From development to physical characteristics. Birth Defects Res. 2019, 111, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.A.; Murshid, W.R.; Seidahmed, M.Z. Classification, clinical features, and genetics of neural tube defects. Saudi Med. J. 2014, 35, S5–S14. [Google Scholar] [PubMed]
- Golden, J.A.; Chernoff, G.F. Multiple sites of anterior neural tube closure in humans: Evidence from anterior neural tube defects (anencephaly). Pediatrics 1995, 95, 506–510. [Google Scholar]
- Monteagudo, A. Exencephaly-anencephaly Sequence. Am. J. Obstet. Gynecol. 2020, 223, B5–B8. [Google Scholar] [CrossRef]
- Cho, D.Y.; Leipold, H.W. Anencephaly in calves. Cornell Vet. 1978, 68, 60–69. [Google Scholar]
- Washburn, K.E.; Streeter, R.N. Congenital defects of the ruminant nervous system. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 413–434. [Google Scholar] [CrossRef]
- Hiraga, T.; Abe, M. Anencephaly and Other Congenital Defects in a Calf. Jpn. J. Vet. Sci. 1986, 48, 595–598. [Google Scholar] [CrossRef]
- Dennis, S.M.; Leipold, H.W. Anencephaly in sheep. Cornell Vet. 1972, 62, 273–281. [Google Scholar]
- Brook, F. Ultrasound Diagnosis of Anencephaly in the Fetus of a Bottlenose Dolphin (Tursiops aduncas). J. Zoo Wildl. Med. 1994, 25, 569–574. [Google Scholar]
- Balseiro, A.; Polledo, L.; Tuñón, J.; García Marín, J.F. Anencephaly and Severe Myelodysplasia in a Stillborn Brown Bear (Ursus arctos arctos). Animals 2022, 12, 2345. [Google Scholar] [CrossRef]
- Huisinga, M.; Reinacher, M.; Nagel, S.; Herden, C. Anencephaly in a German Shepherd Dog. Vet. Pathol. 2010, 47, 948–951. [Google Scholar] [CrossRef]
- Luz, M.; de Mello Bobány, D.; Monteiro Silva, M.E.; Mouura Carvalho, C.F. Anencephaly associated with cleft palate in a Bull Terrier litter: Case report. IOSR J. Agric. Vet. Sci. 2016, 9, 18–20. [Google Scholar] [CrossRef]
- Thomas, Z.M.; Podadera, J.M.; Donahoe, S.L.; Foo, T.S.Y.; Weerakoon, L.; Mazrier, H. Neural tube defects in four Shetland sheepdog puppies: Clinical characterisation and computed tomography investigation. Aust. Vet. J. 2020, 98, 312–318. [Google Scholar] [CrossRef]
- Ortega-Pacheco, A.; Lezama-García, M.A.; Colín-Flores, R.; Jiménez-Coello, M.; Acevedo-Arcique, C.; Gutiérrez-Blanco, E. Presence of congenital anomalies in three dog litters. Reprod. Domest. Anim. 2020, 55, 652–655. [Google Scholar] [CrossRef]
- Nonato, I.d.A.; Teixeira, M.R.; de Miranda, J.L.; Bressan Braz, H.M.; Machado, J.P. Cranioschisis and Anencephaly in a Dog—Challenging etiology. Acta Sci. Vet. 2019, 47. [Google Scholar] [CrossRef]
- Yavaş, Ö.; Yavaş, S.E.; BaşAr, D.; Avci, Z.; Sariçetin, A.; Yildiz, E.R.; Ersoy, S.; Özyiğit, Ö. Anencephaly, Bifid Tongue, and Cleft Palate in a Pomeranian Dog: GFAP and NeuN Immunoreactivities. Ank. Üniv. Vet. Fakültesi Derg. 2023, 1–5. [Google Scholar] [CrossRef]
- Schaftenaar, W.; Fernandes, T.; Fritsch, G.; Frey, R.; Szentiks, C.A.; Wegner, R.D.; Hildebrandt, T.B.; Hermes, R. Dystocia and Fetotomy Associated with Cerebral Aplasia in a Greater One-horned Rhinoceros (Rhinoceros unicornis). Reprod. Domest. Anim. 2011, 46, e97–e101. [Google Scholar] [CrossRef]
- Greene, N.D.E.; Copp, A.J. Neural Tube Defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef]
- Franco, G.G.; Siqueira, E.G.M.; Souza, J.A.L.; Prado, L.O.C.; Rahal, S.C.; Mamprim, M.J.; Minto, B.W.; Brandao, C.V.S.; Costa, J.S., Jr. Closed spinal dysraphism in a 6-month-old mixed breed dog. Vet. Med. 2021, 66, 219–224. [Google Scholar] [CrossRef]
- Westworth, D.R.; Sturges, B.K. Congenital Spinal Malformations in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 951–981. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.; Klionsky, N.B.; Nadarajah, U.; Chaturvedi, A.; Meyers, S.P. Malformed vertebrae: A clinical and imaging review. Insights Imaging 2018, 9, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Eckalbar, W.L.; Fisher, R.E.; Rawls, A.; Kusumi, K. Scoliosis and segmentation defects of the vertebrae. WIREs Dev. Biol. 2012, 1, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Pourquié, O. Vertebrate Segmentation: From Cyclic Gene Networks to Scoliosis. Cell 2011, 145, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, S.; Hellige, M.; Feige, K.; Wenning, P.; Distl, O. Phenotypic classification of variability of non-syndromic congenital cleft lip and jaw in Vorderwald × Montbéliarde cattle. Acta Vet. Scand. 2015, 57, 87. [Google Scholar] [CrossRef] [PubMed]
- Lupp, B.; Reinhardt, M.; Maus, F.; Hellige, M.; Feige, K.; Distl, O. Right-sided cleft lip and jaw in a family of Vorderwald × Montbéliarde cattle. Vet. J. 2012, 192, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.D.; Norman, T.E.; Arnold, C.E.; Coleman, M.C. Clinical characteristics of horses and foals diagnosed with cleft palate in a referral population: 28 cases (1988–2011). Can. Vet. J. 2015, 56, 756–760. [Google Scholar]
- Garnier, P.; Viateau, V.; Manassero, M.; Maurice, E. Surgically treated congenital cleft palate in a 4-month-old kitten: Medium-term clinical and CT assessment. J. Feline Med. Surg. Open Rep. 2022, 8, 20551169221082556. [Google Scholar] [CrossRef]
- Henschele, W.P. Cleft palate in lions of one litter. J. Amer. Vet. Med. Assoc. 1959, 134, 8365–8366. [Google Scholar]
- Scott, J.H.; Prophett, A.S. Histologic investigation of cleft palate in tiger, dog and man. J. Dent. Res. 1955, 34, 785. [Google Scholar]
- McMichael, L.; McLean, J.; Taylor, J.; Martinez, Y.; Meers, J. Cleft Palate Syndrome in the Endangered Spectacled Flying Fox (Pteropus conspicillatus): Implications for Conservation and Comparative Research. Vet. Sci. 2023, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Pahi, J.; Pradhan, M.; Pandey, A.; Gujral, R.; Agarwal, S. Iniencephaly: A Report of 19 Cases. Indian Pediatr. 1998, 35, 891–896. [Google Scholar] [PubMed]
- Moore, L. Anencephaly. J. Diagn. Med. Sonogr. 2010, 26, 286–289. [Google Scholar] [CrossRef]
- Farré Mariné, A.; Pumarola, M.; Luján Feliu-Pascual, A. Polysulfone tailor-made implant for the surgical correction of a frontoparietal meningoencephalocoele in a cat. J. Feline Med. Surg. Open Rep. 2022, 8, 20551169221098940. [Google Scholar] [CrossRef]
- Sponenberg, D.P.; Graf-Webster, E. Hereditary meningoencephalocele in Burmese cats. J. Hered. 1986, 77, 60–60. [Google Scholar] [CrossRef]
- Butterfield, S.; Garcia-Gonzalez, B.; Driver, C.J.; Rusbridge, C. Limited dorsal myeloschisis in three cats: A distinctive form of neural tube defect. J. Feline Med. Surg. Open Rep. 2020, 6, 2055116920924307. [Google Scholar] [CrossRef]
- Lyons, L.A.; Erdman, C.A.; Grahn, R.A.; Hamilton, M.J.; Carter, M.J.; Helps, C.R.; Alhaddad, H.; Gandolfi, B. Aristaless-Like Homeobox protein 1 (ALX1) variant associated with craniofacial structure and frontonasal dysplasia in Burmese cats. Dev. Biol. 2016, 409, 451–458. [Google Scholar] [CrossRef]
- Muennich, A. Congenital and hereditary diseases to be diagnosed in the kitten. In Proceedings of the XIII Congreso de Especialidades Veterinarias, Bilbao, Spain, 2–4 February 2014. [Google Scholar]
- Padmanabhan, R. Etiology, pathogenesis and prevention of neural tube defects. Congenit. Anom. 2006, 46, 55–67. [Google Scholar] [CrossRef]
- Burren, K.A.; Savery, D.; Massa, V.; Kok, R.M.; Scott, J.M.; Blom, H.J.; Copp, A.J.; Greene, N.D.E. Gene–environment interactions in the causation of neural tube defects: Folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum. Mol. Genet. 2008, 17, 3675–3685. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, F.; Bao, Y.; Lu, X.; Quan, L.; Lu, P. Association between MTHFR gene polymorphism and NTDs in Chinese Han population. Int. J. Clin. Exp. Med. 2014, 7, 6. [Google Scholar]
- Stover, P.J.; MacFarlane, A.J.; Field, M.S. Bringing clarity to the role of MTHFR variants in neural tube defect prevention2. Am. J. Clin. Nutr. 2015, 101, 1111–1112. [Google Scholar] [CrossRef] [PubMed]
- Momb, J.; Lewandowski, J.P.; Bryant, J.D.; Fitch, R.; Surman, D.R.; Vokes, S.A.; Appling, D.R. Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, E.; Sirois, J.; Tremblay, M.L.; MacKenzie, R.E. Mitochondrial NAD-Dependent Methylenetetrahydrofolate Dehydrogenase-Methenyltetrahydrofolate Cyclohydrolase Is Essential for Embryonic Development. Mol. Cell. Biol. 2002, 22, 4158–4166. [Google Scholar] [CrossRef] [PubMed]
- Narisawa, A.; Komatsuzaki, S.; Kikuchi, A.; Niihori, T.; Aoki, Y.; Fujiwara, K.; Tanemura, M.; Hata, A.; Suzuki, Y.; Relton, C.L.; et al. Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans. Hum. Mol. Genet. 2012, 21, 1496–1503. [Google Scholar] [CrossRef]
- Pangilinan, F.; Molloy, A.M.; Mills, J.L.; Troendle, J.F.; Parle-McDermott, A.; Signore, C.; O’Leary, V.B.; Chines, P.; Seay, J.M.; Geiler-Samerotte, K.; et al. Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects. BMC Med. Genet. 2012, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita, J.A.; Oetama, B.; Bennett, G.D.; van Waes, J.; Kamen, B.A.; Richardson, J.; Lacey, S.W.; Anderson, R.G.W.; Finnell, R.H. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat. Genet. 1999, 23, 228–232. [Google Scholar] [CrossRef]
- Shaw, G.M.; Lu, W.; Zhu, H.; Yang, W.; Briggs, F.B.S.; Carmichael, S.L.; Barcellos, L.F.; Lammer, E.J.; Finnell, R.H. 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med. Genet. 2009, 10, 49. [Google Scholar] [CrossRef]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA Methyltransferases Dnmt3a and Dnmt3b Are Essential for De Novo Methylation and Mammalian Development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Fukuda, T.; Tokunaga, A.; Sakamoto, R.; Yoshida, N. Fbxl10/Kdm2b deficiency accelerates neural progenitor cell death and leads to exencephaly. Mol. Cell. Neurosci. 2011, 46, 614–624. [Google Scholar] [CrossRef]
- Vega, R.B.; Matsuda, K.; Oh, J.; Barbosa, A.C.; Yang, X.; Meadows, E.; McAnally, J.; Pomajzl, C.; Shelton, J.M.; Richardson, J.A.; et al. Histone Deacetylase 4 Controls Chondrocyte Hypertrophy during Skeletogenesis. Cell 2004, 119, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Bijok, J.; Dąbkowska, S.; Kucińska-Chahwan, A.; Massalska, D.; Nowakowska, B.; Gawlik-Zawiślak, S.; Panek, G.; Roszkowski, T. Prenatal diagnosis of acrania/exencephaly/anencephaly sequence (AEAS): Additional structural and genetic anomalies. Arch. Gynecol. Obstet. 2023, 307, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Rull, R.P.; Ritz, B.; Shaw, G.M. Neural Tube Defects and Maternal Residential Proximity to Agricultural Pesticide Applications. Am. J. Epidemiol. 2006, 163, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Baldo, C.; Campaña, H.; Gili, J.; Poletta, F.; Lopez Camelo, J. Anencephaly and residence near textile industries: An epidemiological case-control study in South America. BAG J. Basic Appl. Genet. 2008, 19, 9–14. [Google Scholar]
- Tong, M.; Yu, J.; Liu, M.; Li, Z.; Wang, L.; Yin, C.; Ren, A.; Chen, L.; Jin, L. Total mercury concentration in placental tissue, a good biomarker of prenatal mercury exposure, is associated with risk for neural tube defects in offspring. Environ. Int. 2021, 150, 106425. [Google Scholar] [CrossRef] [PubMed]
- DeSesso, J.; Jacobson, C.; Scialli, A.; Farr, C.; Holson, J. An assessment of the developmental toxicity of inorganic arsenic 11Armand Lione, Ph.D., served as guest editor for this submission. Reprod. Toxicol. 1998, 12, 385–433. [Google Scholar] [CrossRef] [PubMed]
- Willhite, C.C. Arsenic-induced axial skeletal (dysraphic) disorders. Exp. Mol. Pathol. 1981, 34, 145–158. [Google Scholar] [CrossRef]
- Stånge, L.; Carlström, K.; Eriksson, M. Hypervitaminosis a in early human pregnancy and malformations of the central nervous system. Acta Obstet. Gynecol. Scand. 1978, 57, 289–291. [Google Scholar] [CrossRef]
- Graham, J.M.; Ferm, V.H. Heat- and Alcohol-Induced Neural Tube Defects: Interactions with Folate in a Golden Hamster Model. Pediatr. Res. 1985, 19, 247–251. [Google Scholar] [CrossRef]
- Cagnotti, G.; Sammartano, F.; Bertone, I.; Capucchio, M.T.; Nicola, I.; Sacchi, P.; Bellino, C.; D’Angelo, A. Imaging and genetic investigations of neural tube defect in a calf: Case report and review of the literature. J. Vet. Diagn. Investig. 2019, 31, 228–234. [Google Scholar] [CrossRef]
- Copp, A.J.; Stanier, P.; Greene, N.D.E. Neural tube defects: Recent advances, unsolved questions, and controversies. Lancet Neurol. 2013, 12, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.L.; Famula, T.R.; Feng, L.C.; Power, H.M.N.; Bullis, J.M. Folic acid supplementation does not decrease stillbirths and congenital malformations in a guide dog colony. J. Small Anim. Pract. 2021, 62, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Domosławska, A.; Jurczak, A.; Janowski, T.E. Oral folic acid supplementation decreases palate and/or lip cleft occurrence in Pug and Chihuahua puppies and elevates folic acid blood levels in pregnant bitches. Pol. J. Vet. Sci. 2013, 16, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Zaganjor, I.; Sekkarie, A.; Tsang, B.L.; Williams, J.; Razzaghi, H.; Mulinare, J.; Sniezek, J.E.; Cannon, M.J.; Rosenthal, J. Describing the Prevalence of Neural Tube Defects Worldwide: A Systematic Literature Review. PLoS ONE 2016, 11, e0151586. [Google Scholar] [CrossRef] [PubMed]
- Kıymaz, N.; Yılmaz, N.; Demir, İ.; Keskin, S. Prognostic Factors in Patients with Occipital Encephalocele. Pediatr. Neurosurg. 2010, 46, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Zada, G.; Lopes, M.B.S.; Mukundan, S.; Laws, E. Meningoceles and Encephaloceles. In Atlas of Sellar and Parasellar Lesions: Clinical, Radiologic, and Pathologic Correlations; Zada, G., Lopes, M.B.S., Mukundan, S., Jr., Laws, E.R., Jr., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 483–488. [Google Scholar]
- Coulibaly, O.; Sogoba, Y.; Kanikomo, D.; Dama, M.; Camara, M.A.; Diallo, O. Giant occipital meningohydroencephalocele in an adult: Another historical case in neural tube defects. Neurochirurgie 2016, 62, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Kopke, M.A.; Jack, M.W.; Baltzer, W.I.; Wightman, P.F.; Gal, A. Dermoid sinus type VI associated with spina bifida and tethered cord syndrome in a French Bulldog. J. Vet. Diagn. Investig. 2019, 31, 294–297. [Google Scholar] [CrossRef]
- Kiviranta, A.M.; Lappalainen, A.K.; Hagner, K.; Jokinen, T. Dermoid sinus and spina bifida in three dogs and a cat. J. Small Anim. Pract. 2011, 52, 319–324. [Google Scholar] [CrossRef]
- Takahashi, K.; Kimura, S.; Chambers, J.K.; Nakano, Y.; Ishikawa, T.; Maeda, S.; Kamishina, H. Case Report: Surgical Treatment of Type IV Spinal Dermoid Sinus in a Shiba Inu. Front. Vet. Sci. 2022, 9, 849025. [Google Scholar] [CrossRef]
- Wolf, Z.T.; Brand, H.A.; Shaffer, J.R.; Leslie, E.J.; Arzi, B.; Willet, C.E.; Cox, T.C.; McHenry, T.; Narayan, N.; Feingold, E.; et al. Genome-Wide Association Studies in Dogs and Humans Identify ADAMTS20 as a Risk Variant for Cleft Lip and Palate. PLoS Genet. 2015, 11, e1005059. [Google Scholar] [CrossRef]
- Juriloff, D.M.; Harris, M.J. Mouse genetic models of cleft lip with or without cleft palate. Birth Defects Res. Part A Clin. Mol. Teratol. 2008, 82, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Gebuijs, I.G.E.; Raterman, S.T.; Metz, J.R.; Swanenberg, L.; Zethof, J.; Van den Bos, R.; Carels, C.E.L.; Wagener, F.A.D.T.G.; Von den Hoff, J.W. Fgf8a mutation affects craniofacial development and skeletal gene expression in zebrafish larvae. Biol. Open 2019, 8, bio039834. [Google Scholar] [CrossRef] [PubMed]
- Moura, E.; Pimpão, C.T. Cleft Lip and Palate in the Dog: Medical and Genetic Aspects; InTech Open: London, UK, 2017. [Google Scholar]
- Lobodzinska, A.; Gruszczynska, J.; Max, A.; Jan Bartyzel, B.; Mikula, M.; Mikula, I., Jr.; Grzegrzolka, B. Cleft palate in the domestic dog Canis Lupus Familiaris—Etiology, pathophtsiology, diagnosis, prevention and treatment. Acta Sci. Pol. Zootech. 2014, 13, 5–28. [Google Scholar]
- Mahajan, T.; Ganguly, S.; Saroj. Embryological development of gastrointestinal tract in animals. Indian J. Sci. Res. Technol. 2015, 3, 22–24. [Google Scholar]
- Bhatia, A.; Shatanof, R.A.; Bordoni, B. Embryology, Gastrointestinal; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lejeune, B.; Miclard, J.; Stoffel, M.H.; Meylan, M. Intestinal Atresia and Ectopia in a Bovine Fetus. Vet. Pathol. 2010, 48, 830–833. [Google Scholar] [CrossRef]
- Jaskwhich, D.; Ali, R.M.; Patel, T.C.; Green, D.W. Congenital scoliosis. Curr. Opin. Pediatr. 2000, 12, 61–66. [Google Scholar] [CrossRef]
- Eid, T.; Ghostine, B.; Kreichaty, G.; Daher, P.; Ghanem, I. Congenital costo-vertebral fibrous band and congenital kyphoscoliosis: A previously unreported combination. Eur. Spine J. 2013, 22, 424–428. [Google Scholar] [CrossRef][Green Version]
- Ryan, R.; Gutierrez-Quintana, R.; ter Haar, G.; De Decker, S. Prevalence of thoracic vertebral malformations in French bulldogs, Pugs and English bulldogs with and without associated neurological deficits. Vet. J. 2017, 221, 25–29. [Google Scholar] [CrossRef]
- Havlicek, M.; Mathis, K.R.; Beck, J.A.; Allan, G.S. Surgical management of vertebral malformation in a Manx cat. J. Feline Med. Surg. 2009, 11, 514–517. [Google Scholar] [CrossRef]
- Inglez de Souza, M.C.C.M.; Ryan, R.; ter Haar, G.; Packer, R.M.A.; Volk, H.A.; De Decker, S. Evaluation of the influence of kyphosis and scoliosis on intervertebral disc extrusion in French bulldogs. BMC Vet. Res. 2018, 14, 5. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marc, S.; Savici, J.; Sicoe, B.; Boldura, O.M.; Paul, C.; Otavă, G. Exencephaly–Anencephaly Sequence Associated with Maxillary Brachygnathia, Spinal Defects, and Palatoschisis in a Male Domestic Cat. Animals 2023, 13, 3882. https://doi.org/10.3390/ani13243882
Marc S, Savici J, Sicoe B, Boldura OM, Paul C, Otavă G. Exencephaly–Anencephaly Sequence Associated with Maxillary Brachygnathia, Spinal Defects, and Palatoschisis in a Male Domestic Cat. Animals. 2023; 13(24):3882. https://doi.org/10.3390/ani13243882
Chicago/Turabian StyleMarc, Simona, Jelena Savici, Bogdan Sicoe, Oana Maria Boldura, Cristina Paul, and Gabriel Otavă. 2023. "Exencephaly–Anencephaly Sequence Associated with Maxillary Brachygnathia, Spinal Defects, and Palatoschisis in a Male Domestic Cat" Animals 13, no. 24: 3882. https://doi.org/10.3390/ani13243882
APA StyleMarc, S., Savici, J., Sicoe, B., Boldura, O. M., Paul, C., & Otavă, G. (2023). Exencephaly–Anencephaly Sequence Associated with Maxillary Brachygnathia, Spinal Defects, and Palatoschisis in a Male Domestic Cat. Animals, 13(24), 3882. https://doi.org/10.3390/ani13243882






