Genomic Selection Using Single-Step Genomic BLUP on the Number of Services per Conception Trait in Thai–Holstein Crossbreeds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical and Genetic Analysis
2.3. Estimation of the Genetic Parameters
2.4. Estimation of the Breeding Values
3. Results
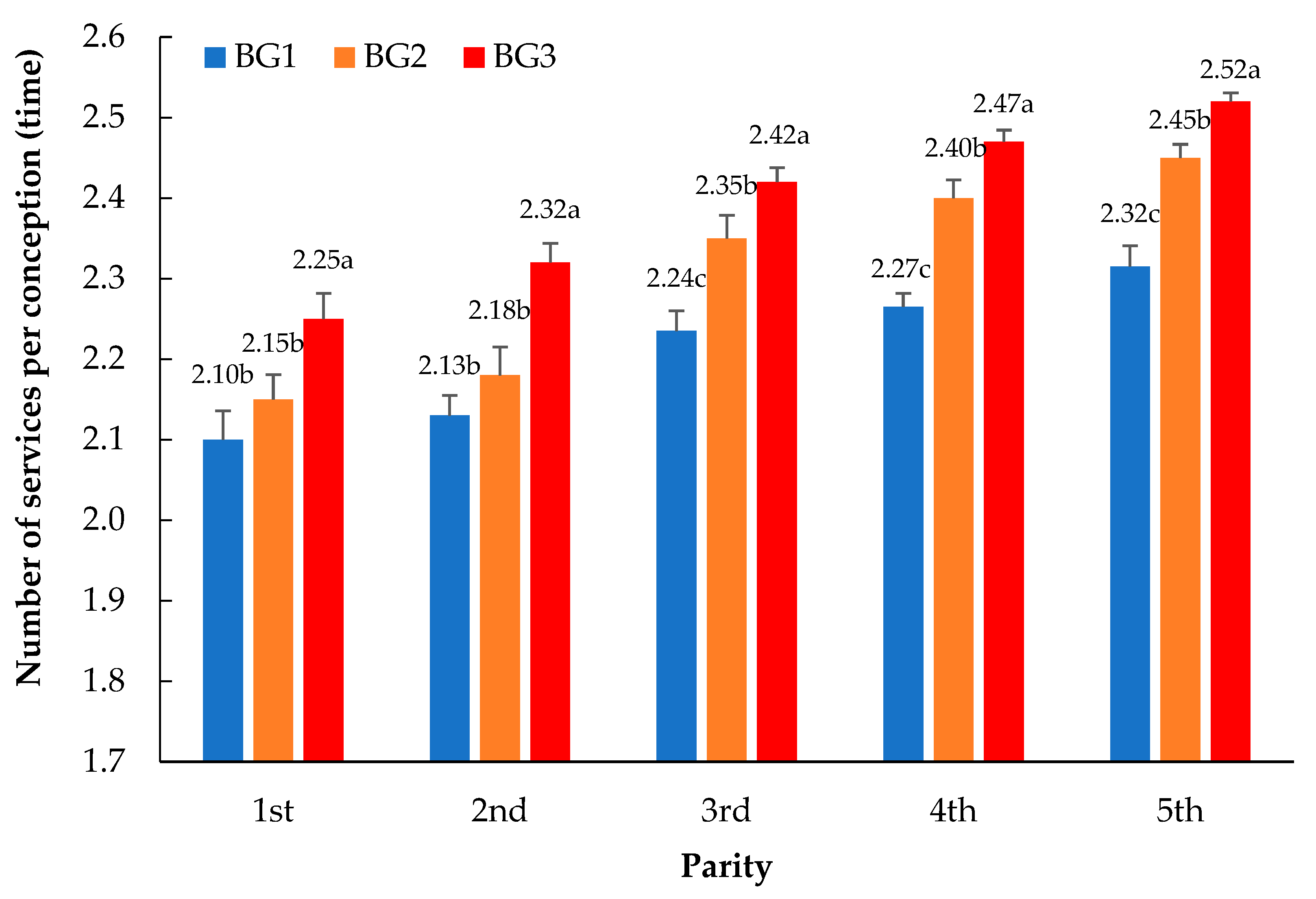
3.1. Comparison of the Number of Services per Conception between the Breed Groups within Parity
3.2. Variance Components and Genetic Parameters
3.3. Comparison of the Accuracy between the EBVs and GEBVs
3.4. Difference between the EBVs and GEBVs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abe, H.; Masuda, Y.; Susuki, M. Relationship between reproductive traits of heifers and cows and yield traits for Holsteins in Japan. J. Dairy Sci. 2009, 92, 4055–4062. [Google Scholar] [CrossRef]
- Minozzi, G.; Nicolazzi, E.L.; Stella, A.; Biffani, S.; Negrini, R.; Lazzari, B.; Ajmone-Marsan, P.; Williams, J.L. Genome wide analysis of fertility and production traits in Italian Holstein cattle. PLoS ONE 2013, 8, e80219. [Google Scholar] [CrossRef]
- Buaban, S.; Duangjinda, M.; Suzuki, M.; Masuda, Y.; Sanpote, J.; Kuchida, K. Genetic relationships of fertility traits with test-day milk yield and fat-to-protein ratio in tropical smallholder dairy farms. Anim. Sci. J. 2016, 87, 627–637. [Google Scholar] [CrossRef]
- Lucy, M.C. Stress, strain, and pregnancy outcome in postpartum cows. Anim. Reprod. 2019, 16, 455–464. [Google Scholar] [CrossRef]
- Dash, S.; Chakravarty, A.K.; Singh, A.; Upadhyay, A.; Singh, M.; Yousuf, S. Effect of heat stress on reproductive performances of dairy cattle and buffaloes: A review. Vet. World. 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Senarath, N.; Sungkhapreecha, P.; Buaban, S.; Chankitisakul, V.; Boonkum, W. Integrating genomic selection for rapid improvement of milk yield in small-scale dairy farms. Appl. Anim. Sci. 2022, 38, 246–251. [Google Scholar] [CrossRef]
- Sungkhapreecha, P.; Chankitisakul, V.; Duangjinda, M.; Buaban, S.; Boonkum, W. Determining heat stress effects of multiple genetic traits in tropical dairy cattle using single-step genomic BLUP. Vet Sci. 2022, 9, 66. [Google Scholar] [CrossRef]
- Ratchamak, R.; Ratsiri, T.; Chumchai, R.; Boonkum, W.; Chankitisakul, V. Relationship of the temperature-humidity index (THI) with ovarian responses and embryo production in superovulated Thai-Holstein crossbreds under tropical climate conditions. Vet. Sci. 2021, 8, 270. [Google Scholar] [CrossRef]
- Sun, C.; Madsen, P.; Nielsen, U.S.; Zhang, Y.; Lund, M.S.; Su, G. Comparison between a sire model for genetic evaluation of fertility traits in Danish Holstein population. J. Dairy Sci. 2009, 92, 4063–4071. [Google Scholar] [CrossRef]
- Buaban, S.; Duangjinda, M.; Suzuki, M.; Masuda, Y.; Sanpote, J.; Kuchida, K. Short communication: Genetic analysis for fertility traits of heifers and cows from smallholder dairy farms in a tropical environment. J. Dairy Sci. 2015, 98, 4990–4998. [Google Scholar] [CrossRef]
- Yusuf, M.; Nakao, T.; Ranasinghe, R.M.S.B.K.; Gautam, G.; Long, S.T.; Yoshida, C.; Koike, K.; Hayashi, A. Reproductive performance of repeat breeders in dairy herds. Theriogenology 2010, 73, 1220–1229. [Google Scholar] [CrossRef]
- Gojam, Y.; Tadesse, M.; Efffa, K.; Hunde, D. Performance of crossbred dairy cows suitable for smallholder production systems at Holetta Agricultural Research Centre. Ethiop. J. Agric. Sci. 2017, 27, 121–131. [Google Scholar]
- Getachew, Y.; Lemma, A.; Fesseha, H. Assessment on reproductive performance of crossbred dairy cows selected as recipient for embryo transfer in urban set up bishoftu, central Ethiopia. Int. J. Vet. Sci. Res. 2020, 6, 080–086. [Google Scholar]
- Bagnato, A.; Oltenacu, P.A. Genetic study of fertility traits and production in different parities in Italian Friesian cattle. J. Anim. Breed. Genet. 1993, 110, 126–134. [Google Scholar] [CrossRef]
- Pryce, J.E.; Veerkamp, R.F. The incorporation of fertility indices in genetic improvement programmes. BSAP Occas. Publ. 2001, 26, 237–249. [Google Scholar] [CrossRef]
- Rahbar, R.; Aminafshar, M.; Abdullahpour, R.; Chamani, M. Genetic analysis of fertility traits of Holstein dairy cattle in warm and temperate climate. Acta Sci. Anim. Sci. 2016, 38, 333–340. [Google Scholar] [CrossRef]
- Nishida, A.; Aziz, M.A.; Nishida, S.; Suzuki, K. Modelling number of services per conception of Japanese Black cattle by random regression. J. Anim. Breed. Genet. 2006, 123, 56–63. [Google Scholar] [CrossRef]
- Berry, D.P.; Buckley, F.; Dillon, P.; Evans, R.D.; Rath, M.; Veerkamp, R.F. Genetic relationships among body condition score, body weight, milk yield, and fertility in dairy cows. J. Dairy Sci. 2003, 86, 2193–2204. [Google Scholar] [CrossRef]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Misztal, I.; Lourenco, D.A.L.; Legarra, A. Current status of genomic evaluation. J. Anim. Sci. 2020, 98, 1–14. [Google Scholar] [CrossRef]
- Aguilar, I.; Misztal, I.; Johnson, D.L.; Legarra, A.; Tsuruta, S.; Lawlor, T.J. Hot topic: A unified approach to utilize phenotypic, full pedigree, and genomic information for genetic evaluation of Holstein final score. J. Dairy Sci. 2010, 93, 743–752. [Google Scholar] [CrossRef]
- Tsuruta, S.; Misztal, I.; Lourenco, D.A.L.; Lawlor, T.J. Assigning unknown parent groups to reduce bias in genomic evaluations of final score in US Holsteins. J. Dairy Sci. 2014, 97, 5814–5821. [Google Scholar] [CrossRef]
- Lourenco, D.A.L.; Fragomeni, B.O.; Tsuruta, S.; Aguilar, I.; Zumbach, B.; Hawken, R.J.; Legarra, A.; Misztal, I. Accuracy of estimated breeding values with genomic information on males, females, or both: An example on broiler chicken. Genet. Sel. Evol. 2015, 47, 56. [Google Scholar] [CrossRef]
- Hayes, B.J.; Bowman, P.J.; Chamberlain, A.C.; Verbyla, K.; Goddard, M.E. Accuracy of genomic breeding values in multi-breed dairy cattle populations. Genet. Sel. Evol. 2009, 41, 51. [Google Scholar] [CrossRef]
- Hayes, B.J.; Bowman, P.J.; Chamberlain, A.J.; Goddard, M.E. Invited review: Genomic selection in dairy cattle: Progress and challenges. J. Dairy Sci. 2009, 92, 433–443. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Gao, X.; Song, W.; Chen, C.; Yao, D.; Ma, J.; Xu, L.; Ma, Y. Genome-wide association study of milk components in Chinese Holstein cows using single nucleotide polymorphism. Livest. Sci. 2020, 233, 103951. [Google Scholar] [CrossRef]
- Tsuruta, S.; Misztal, I. THRGIBBS1F90 for estimation of variance components with threshold and linear models. In Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, MG, Brazil, 13–18 August 2006; pp. 27–31. [Google Scholar]
- Henderson, C.R. Use of all relatives in intraherd prediction of breeding values and producing abilities. J. Dairy Sci. 1975, 58, 1910–1916. [Google Scholar] [CrossRef]
- VanRaden, P.M. Efficient methods to compute genomic predictions. J. Dairy Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef]
- Misztal, I.; Legarra, A.; Aguilar, I. Using recursion to compute the inverse of the genomic relationship matrix. J. Dairy Sci. 2014, 97, 3943–3952. [Google Scholar] [CrossRef]
- Kadarmideen, H.N.; Thompson, R.; Coffey, M.P.; Kossaibati, M.A. Genetic parameters and evaluations from single- and multiple-trait analysis of dairy cow fertility and milk production. Livest. Prod. Sci. 2003, 81, 183–195. [Google Scholar] [CrossRef]
- Sharko, F.S.; Khatib, A.; Prokhortchouk, E.B. Genomic estimated breeding value of milk performance and fertility traits in the Russian black-and-white cattle population. Acta Naturae 2022, 14, 109–122. [Google Scholar] [CrossRef]
- Wattiaux, M.A. Reproduction Is a Multifaceted Subject, Reproduction and Genetics; The Babcock Institute, University of Wisconsin: Madison, WI, USA, 1996; pp. 102–134. [Google Scholar]
- Haile, B.; Makasha, Y. Reproductive performance of Holstein Friesian dairy cows at Alage dairy farm Ethiopia. J. Dairy Vet. Sci. 2018, 7, 555713. [Google Scholar] [CrossRef]
- Adisu, A.; Zewdu, W. Reproductive performance of indigenous cow breeds of Ethiopia: A review. J. Anim. Health. Behav. Sci. 2021, 5, 10–13. [Google Scholar]
- González-Recio, O.; Chang, Y.M.; Gianola, D.; Weigel, K.A. Number of inseminations to conception in Holstein cows using censored records and time-dependent covariates. J. Dairy Sci. 2005, 88, 3655–3662. [Google Scholar] [CrossRef]
- Tiezzi, F.; Maltecca, C.; Cecchinato, A.; Penasa, M.; Bittante, G. Thin and fat cows, and the nonlinear genetic relationship between body condition score and fertility. J. Dairy Sci. 2013, 96, 6730–6741. [Google Scholar] [CrossRef]
- Zambrano, J.C.; Echeverri, J. Genetic and environmental variance and covariance parameters for some reproductive traits of Holstein and Jersey cattle in Antioquia (Colombia). R. Bras. Zootec. 2014, 43, 132–139. [Google Scholar] [CrossRef]
- Miglior, F.; Fleming, A.; Malchiodi, F.; Brito, L.F.; Martin, P.; Baes, C.F. A 100-Year Review: Identification and genetic selection of economically important traits in dairy cattle. J. Dairy Sci. 2017, 100, 10251–10271. [Google Scholar] [CrossRef]
- Matilainen, K.; Strandén, I.; Aamand, G.P.; Mäntysaari, E.A. Single step genomic evaluation for female fertility in Nordic Red dairy cattle. J. Anim. Breed. Genet. 2018, 135, 337–348. [Google Scholar] [CrossRef]
- Habier, D.; Tetens, J.; Seefried, F.-R.; Lichtner, P.; Thaller, G. The impact of genetic relationship information on genomic breeding values in German Holstein cattle. Genet. Sel. Evol. 2010, 42, 5. [Google Scholar] [CrossRef]
- Daetwyler, H.D.; Hickey, J.M.; Henshall, J.M.; Dominik, S.; Gredler, B.; van der Werf, J.H.J.; Hayes, B.J. Accuracy of estimated genomic breeding values for wool and meat traits in a multi-breed sheep population. Anim. Prod. Sci. 2010, 50, 1004–1010. [Google Scholar] [CrossRef]
- Sungkhapreecha, P.; Misztal, I.; Hidalgo, J.; Lourenco, D.; Buaban, S.; Chankitisakul, V.; Boonkum, W. Validation of single-step genomic predictions using the linear regression method for milk yield and heat tolerance in a Thai-Holstein population. Vet. World. 2021, 14, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.J.C.; Cloete, S.W.P.; Botha, J.A. Fertility in dairy cows and ways to improve it. S. Afr. J. Anim. Sci. 2018, 48, 858–868. [Google Scholar] [CrossRef]
- Viana, J.M.S.; Piepho, H.-P.; Silva, F.F. Quantitative genetics theory for genomic selection and efficiency of breeding value prediction in open-pollinated populations. Sci. Agric. 2016, 73, 243–251. [Google Scholar] [CrossRef]
| Items | Data Records | |||
|---|---|---|---|---|
| Number of fixed effects | ||||
| Herd-year of insemination | 1755 | |||
| Age group | 7 | |||
| Breed group | 3 | |||
| Parity | 5 | |||
| Number of service sires (n) | 995 | |||
| Number of animals with records (n) | 54,027 | |||
| Number of animals in the pedigree (n) | 109,233 | |||
| Number of animals with genotypes (n) | 770 | |||
| Breed groups (BG) BG1 = less than 87.5%, BG2 = 87.5 to 93.6%, and BG3 = greater than 93.7% | Average | BG1 | BG2 | BG3 |
| Number of services per conception | ||||
| Minimum (time) | 1 | 1 | 1 | 1 |
| Maximum (time) | 10 | 10 | 10 | 10 |
| Mean (time) | 2.30 | 2.21 | 2.31 | 2.40 |
| SD (time) | 1.59 | 1.53 | 1.53 | 1.65 |
| Number of records (n) | 101,331 | 28,794 | 46,484 | 26,053 |
| Parameters | Methods | |
|---|---|---|
| Traditional BLUP | The ssGBLUP | |
| 0.16 (0.006) | 0.16 (0.005) | |
| 0.09 (0.010) | 0.12 (0.012) | |
| 0.26 (0.071) | 0.25 (0.068) | |
| 0.02 (0.002) | 0.02 (0.002) | |
| 1.82 (0.025) | 1.80 (0.022) | |
| 0.038 (0.002) | 0.051 (0.002) | |
| 0.149 (0.014) | 0.157 (0.015) | |
| DIC | 365,732.34 | 365,723.01 |
| −2logL | 50,000 | 48,852 |
| Dataset | Traditional BLUP Method | The ssGBLUP Method | % Increase in Accuracy |
|---|---|---|---|
| All animals dataset | 0.462 | 0.490 | 6.05 |
| Dam dataset | 0.465 | 0.498 | 7.10 |
| Bull dataset | 0.468 | 0.521 | 11.32 |
| Top 20% of the all animals dataset | 0.504 | 0.560 | 11.11 |
| Top 20% of the dam dataset | 0.508 | 0.572 | 12.60 |
| Top 20% of the bull dataset | 0.520 | 0.612 | 17.69 |
| Dataset | Breed Group | Traditional BLUP Method | The ssGBLUP Method |
|---|---|---|---|
| All animals dataset | BG1 | −0.008 | −0.009 |
| BG2 | −0.008 | −0.012 | |
| BG3 | −0.013 | −0.018 | |
| Dam dataset | BG1 | −0.008 | −0.008 |
| BG2 | −0.012 | −0.019 | |
| BG3 | −0.022 | −0.025 | |
| Bull dataset | BG1 | −0.112 | −0.118 |
| BG2 | −0.116 | −0.124 | |
| BG3 | −0.130 | −0.136 | |
| Top 20% of the all animals dataset | BG1 | −0.141 | −0.149 |
| BG2 | −0.150 | −0.156 | |
| BG3 | −0.152 | −0.159 | |
| Top 20% of the dam dataset | BG1 | −0.157 | −0.160 |
| BG2 | −0.163 | −0.166 | |
| BG3 | −0.169 | −0.172 | |
| Top 20% of the bull dataset | BG1 | −0.160 | −0.165 |
| BG2 | −0.172 | −0.176 | |
| BG3 | −0.180 | −0.185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonkum, W.; Chankitisakul, V.; Duangjinda, M.; Buaban, S.; Sumreddee, P.; Sungkhapreecha, P. Genomic Selection Using Single-Step Genomic BLUP on the Number of Services per Conception Trait in Thai–Holstein Crossbreeds. Animals 2023, 13, 3609. https://doi.org/10.3390/ani13233609
Boonkum W, Chankitisakul V, Duangjinda M, Buaban S, Sumreddee P, Sungkhapreecha P. Genomic Selection Using Single-Step Genomic BLUP on the Number of Services per Conception Trait in Thai–Holstein Crossbreeds. Animals. 2023; 13(23):3609. https://doi.org/10.3390/ani13233609
Chicago/Turabian StyleBoonkum, Wuttigrai, Vibuntita Chankitisakul, Monchai Duangjinda, Sayan Buaban, Pattarapol Sumreddee, and Piriyaporn Sungkhapreecha. 2023. "Genomic Selection Using Single-Step Genomic BLUP on the Number of Services per Conception Trait in Thai–Holstein Crossbreeds" Animals 13, no. 23: 3609. https://doi.org/10.3390/ani13233609
APA StyleBoonkum, W., Chankitisakul, V., Duangjinda, M., Buaban, S., Sumreddee, P., & Sungkhapreecha, P. (2023). Genomic Selection Using Single-Step Genomic BLUP on the Number of Services per Conception Trait in Thai–Holstein Crossbreeds. Animals, 13(23), 3609. https://doi.org/10.3390/ani13233609






