Dietary Supplementation with R-(+)-Limonene Improves Growth, Metabolism, Stress, and Antioxidant Responses of Silver Catfish Uninfected and Infected with Aeromonas hydrophila
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Phytochemical
2.2. Fish and Culture Conditions
2.3. Diets
2.4. Challenge with A. hydrophila
2.5. Experimental Design
2.6. Assays
2.6.1. Growth Performance
2.6.2. Plasma Metabolic Parameters
2.6.3. Liver Metabolic Parameters
2.6.4. Lipid Profile
2.6.5. Enzymatic Markers of Liver Damage
2.6.6. Antioxidant and Oxidative Parameters in the Liver
Superoxide Anion and Antioxidant Enzymes
Glutathione S-Transferase (GST)
Oxidative Damage Biomarker
Reduced and Oxidized Glutathione
Total Antioxidant Capacity
2.6.7. Histology
2.6.8. Gene Expression
3. Statistical Analysis
4. Results
4.1. Growth Performance
4.2. Plasma Metabolic Parameters
4.3. Liver Metabolic Parameters
4.4. Lipid Profile
4.5. Liver Transaminases Activity
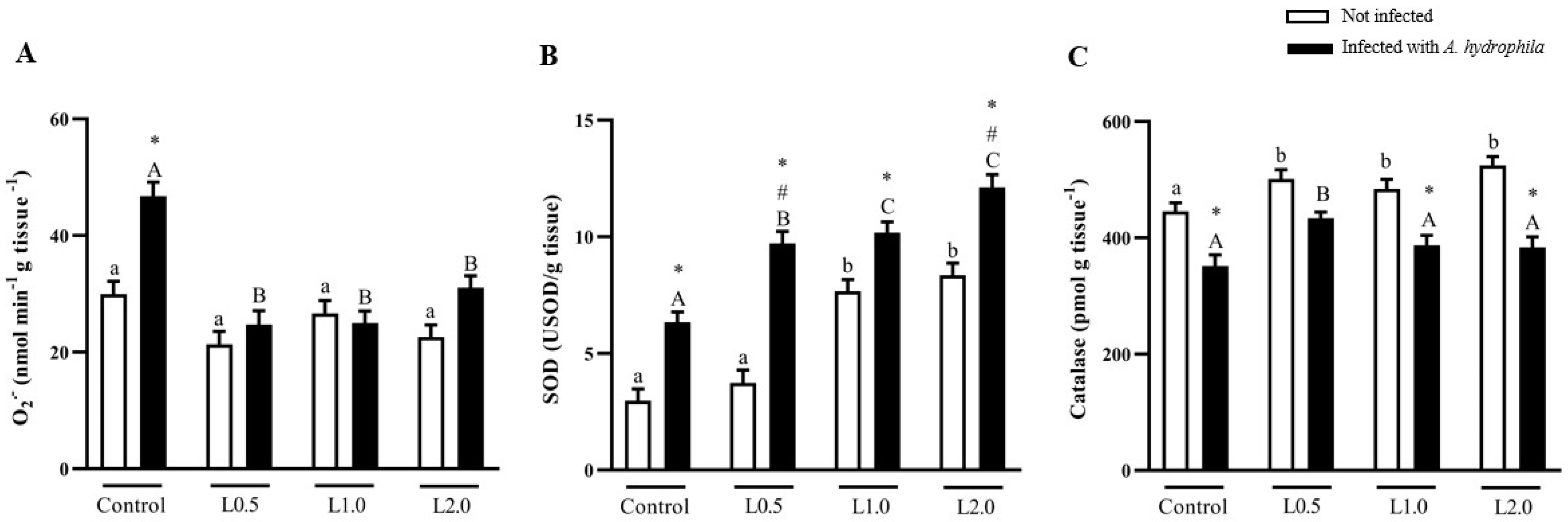
4.6. Superoxide Anion and Antioxidant Enzymes
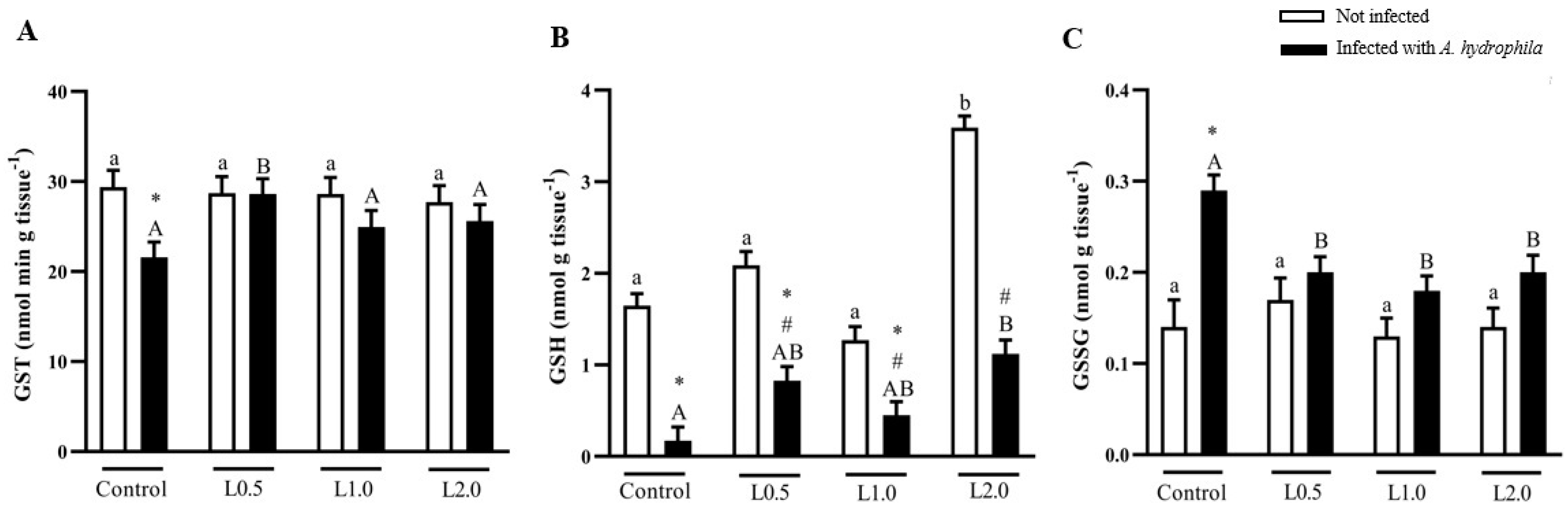
4.7. Glutathiones
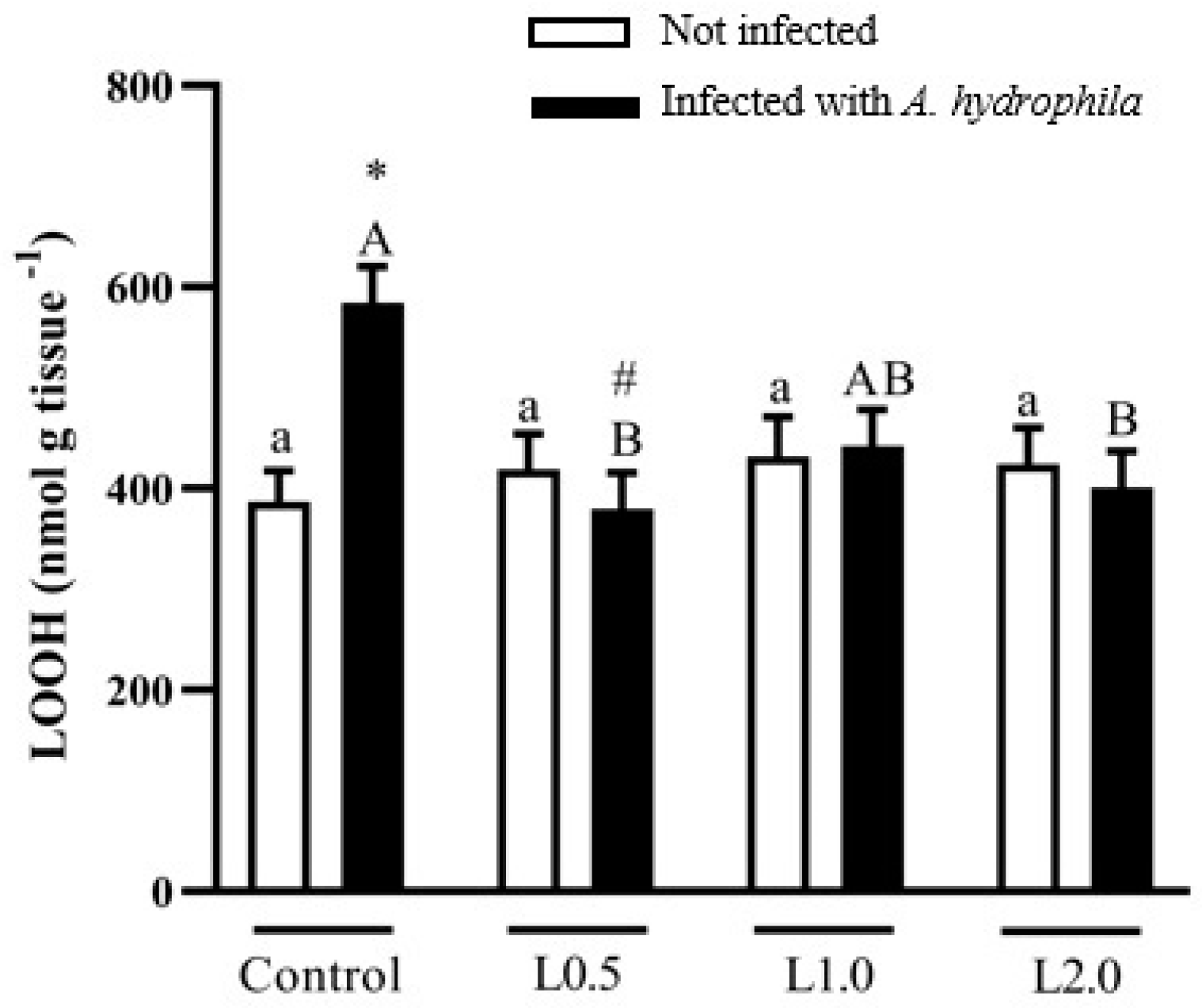
4.8. Oxidative Damage Biomarker
4.9. Total Antioxidant Capacity
4.10. Histology
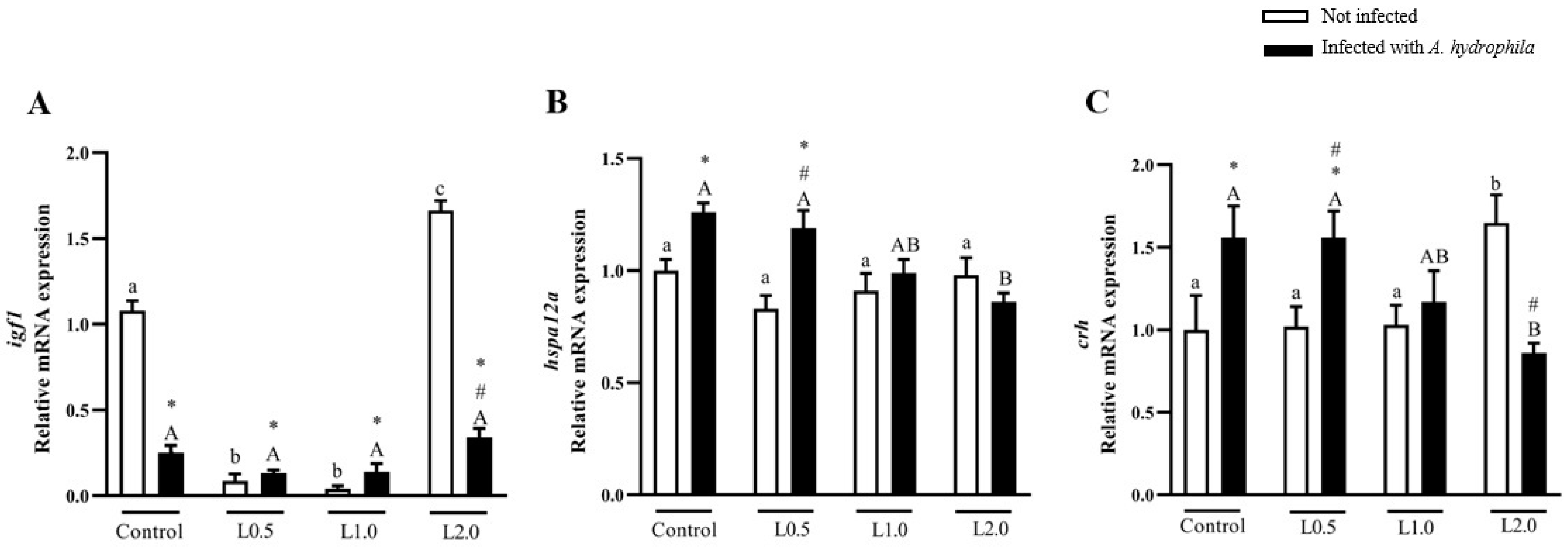
4.11. Gene Expression
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Fisheries and Aquaculture Division. Fishing and Aquaculture Statistics. Global Aquaculture Production 1950–2021 (FishStatJ). Pomegranate. 2023. Available online: https://www.fao.org/fishery/statistics-query/en/global_production/global_production_quantity (accessed on 1 October 2023).
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; Sánchez, B.; Barroso, F.G.; Toniolo, M.; Trenzado, C.E.; Rus, A.S. Effects of rearing conditions on behavioural responses, social kinetics and physiological parameters in gilthead sea bream Sparus aurata. Appl. Anim. Behav. Sci. 2017, 197, 120–128. [Google Scholar] [CrossRef]
- Ghatak, S.; Blom, J.; Das, S.; Sanjukta, R.; Puro, K.; Mawlong, M.; Shakuntala, I.; Sen, A.; Goesmann, A.; Kumar, A.; et al. Pan-genome analysis of Aeromonas hydrophila, Aeromonas veronii and Aeromonas caviae indicates phylogenomic diversity and greater pathogenic potential for Aeromonas hydrophila. Antonie Van Leeuwenhoek 2016, 109, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens. Disease of Farmed and Wild Fish, 4th ed.; Springer: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-6068-7. [Google Scholar]
- Balasch, J.C.; Tort, L. Netting the stress responses in fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.F.; Baldissera, M.D.; Baldisserotto, B.; Heinzmann, B.M.; Martos-Sitcha, J.A.; Mancera, J.M. Essential Oils as Stress Reducing Agents for Fish Aquaculture: A Review. Front Physiol. 2019, 20, 785. [Google Scholar] [CrossRef]
- Bi, B.; Yuan, Y.; Zhao, Y.; He, M.; Song, H.; Kong, L.; Gao, Y. Effect of crowding stress on growth performance, the antioxidant system and humoral immunity in hybrid sturgeon. Aquac. Rep. 2023, 28, 101468. [Google Scholar] [CrossRef]
- Quentin, P.; Séverine, J.; Allison, G.; Jessica, C.; Pascal, L.; Lisa, J. Stress responses in fishes: From molecular to evolutionary processes. Sci. Total Environ. 2019, 684, 371–380. [Google Scholar] [CrossRef]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef]
- Morshedi, V.; Bojarski, B.; Hamedi, S.; Torahi, H.; Hashemi, G.; Faggio, C. Effects of dietary bovine lactoferrin on growth performance and immuno-physiological responses of Asian sea bass (Lates calcarifer) fingerlings. Probiotics Antimicrob. Proteins 2021, 13, 1790–1797. [Google Scholar] [CrossRef]
- Khuyen, T.D.; Mandiki, S.N.M.; Cornet, V.; Douxfils, J.; Betoulle, S.; Bossier, P.; Reyes-López, F.E.; Tort, L.; Kestemont, P. Physiological and immune response of juvenile rainbow trout to dietary bovine lactoferrin. Fish Shellfish. Immunol. 2017, 71, 359–371. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Ghazanfar, S.; Abdel-Hamid, M.; Abdel-Latif, H.M.R.; Zhang, Z.; Naiel, M.A.E. Therapeutic uses and applications of bovine lactoferrin in aquatic animal medicine: An overview. Vet. Res. Commun. 2023, 47, 1015–1029. [Google Scholar] [CrossRef]
- Esteban, M.A.; Rodríguez, A.; Cuesta, A.; Meseguer, J. Effects of lactoferrin on non-specific immune responses of gilthead seabream (Sparus auratus L.). Fish Shellfish. Immunol. 2005, 18, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Kumari, J.; Swain, T.; Sahoo, P.K. Dietary bovine lactoferrin induces changes in immunity level and disease resistance in Asian catfish Clarias batrachus. Vet. Immunol. Immunopathol. 2003, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Montgomery, W.L. Algae. In Dietary Supplements for the Health and Quality of Cultured Fish; Nakagawa, H., Sato, M., Gatlin, D.M., III, Eds.; Cabi International: USA, 2007; pp. 133–167. [Google Scholar]
- Tangestani, N.; Nafisi, M.; Morshedi, V.; Bagheri, D.; Sotoudeh, E.; Ghasemi, A.; Bojarski, B. Effects of dietary macroalgae Gracilaria pygmaea on Asian sea bass (Lates calcarifer) juveniles. J. Agric. Sci. Technol. 2023, 25, 647–660. [Google Scholar]
- Acar, U.; Kesbiç, O.S.; Yılmaz, S.; Inanan, B.E.; Zemheri-Navruz, F.; Terzi, F.; Fazio, F.; Parrino, V. Effects of essential oil derived from the bitter orange (Citrus aurantium) on growth performance, histology and gene expression levels in common carp Juveniles (Cyprinus carpio). Animals 2021, 11, 1431. [Google Scholar] [CrossRef]
- Acar, Ü.; Parrino, V.; Kesbiç, O.S.; Lo Paro, G.; Saoca, C.; Abbate, F.; Fazio, F. Effects of different levels of pomegranate seed oil on some blood parameters and disease resistance against Yersinia ruckeri in rainbow trout. Front. Physiol. 2018, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Baba, E.; Acar, Ü.; Öntaş, C.; Kesbiç, O.S.; Yılmaz, S. Evaluation of Citrus limon peels essential oil on growth performance, immune response of Mozambique tilapia (Oreochromis mossambicus) challenged with Edwardsiella tarda. Aquaculture 2016, 465, 13–18. [Google Scholar] [CrossRef]
- Citarasu, M. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac. Int. 2010, 18, 403–414. [Google Scholar] [CrossRef]
- Lopes, J.M.; Marques, N.C.; Santos, M.D.M.C.; Souza, C.F.; Baldissera, M.D.; Carvalho, R.C.; Santos, L.L.; Pantoja, B.T.S.; Heinzmann, B.M.; Baldisserotto, B. Dietary limon Citrus × latifolia fruit peel essential oil improves antioxidant capacity of tambaqui (Colossoma macropomum) juveniles. Aquac. Res. 2020, 51, 4852–4862. [Google Scholar] [CrossRef]
- Lopes, J.M.; Souza, C.F.; Saccol, E.M.H.; Pavanato, M.A.; Antoniazzi, A.; Rovani, M.T.; Heinzmann, B.M.; Baldisserotto, B. Citrus × aurantium essential oil as feed additive improved growth performance, survival, metabolic, and oxidative parameters of silver catfish (Rhamdia quelen). Aquac. Nutr. 2018, 25, 310–318. [Google Scholar] [CrossRef]
- Kesbiç, O.S.; Acar, Ü.; Yilmaz, S.; Aydin, Ö.D. Effects of bergamot (Citrus bergamia) peel oil-supplemented diets on growth performance, haematology and serum biochemical parameters of Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2019, 46, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Chen, M.; Bao, X.; Yu, Y.; Shi, W.; Kumkhong, S.; Liu, Y.; Yang, Y.; Yu, H. Effects of three feed attractants on the growth performance and meat quality of the largemouth bass (Micropterus salmoides). Front. Mar. Sci. 2022, 9, 1029969. [Google Scholar] [CrossRef]
- Acar, Ü.; Kesbiç, O.S.; Yılmaz, S.; Gültepe, N.; Türker, A. Evaluation of the effects of essential oil extracted fromsweet orange peel (Citrus sinensis) on growth rate of tilapia (Oreochromis mossambicus) and possible disease resistance against Streptococcus iniae. Aquaculture 2015, 437, 282–286. [Google Scholar] [CrossRef]
- Seibel, H.; Baßmann, B.; Rebl, A. Blood will tell: What hematological analyses can reveal about fish welfare. Front. Vet. Sci. 2021, 8, 616955. [Google Scholar] [CrossRef] [PubMed]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological methods in fish-not only for beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- Figueiredo-Silva, A.; Rocha, E.; Dias, J.; Silva, P.; Rema, P.; Gomes, E.; Valente, L.M.P. Partial replacement of fish oil by soybean oil on lipid distribution and liver histology in European sea bass (Dicentrarchus labrax) and rainbow trout (Oncorhynchus mykiss) juveniles. Aquac. Nutr. 2005, 11, 147–155. [Google Scholar] [CrossRef]
- Rašković, B.; Stanković, M.; Marković, Z.; Poleksić, V. Histological methods in the assessment of different feed effects on liver and intestine of fish. J. Agric. Sci. 2011, 56, 87–100. [Google Scholar] [CrossRef]
- Lozano, A.R.; Borges, P.; Robaina, L.; Betancor, M.; Hernández-Cruz, C.M.; García, J.R.; Caballero, M.J.; Vergara, J.M.; Izquierdo, M. Effect of different dietary vitamin E levels on growth, fish composition, fillet quality and liver histology of meagre (Argyrosomus regius). Aquaculture 2017, 468, 175–183. [Google Scholar] [CrossRef]
- Bojarski, B.; Osikowski, A.; Hofman, S.; Szała, L.; Szczygieł, J.; Rombel-Bryzek, A. Effects of exposure to a glyphosate-based herbicide on haematological parameters, plasma biochemical indices and the microstructure of selected organs of the common carp (Cyprinus carpio Linnaeus, 1758). Folia Biol. 2022, 70, 213–229. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile compounds in citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Kesbiç, O.S.; Acar, Ü.; Mohammady, E.Y.; Salem, S.M.R.; Ragaza, J.A.; El-Haroun, E.; Hassaan, M.S. The beneficial effects of citrus peel waste and its extract on fish performance and health status: A review. Aquac. Res. 2022, 53, 4217–4232. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A. Limonene—A review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Zeppenfeld, C.C.; Hernández, D.R.; Santinón, J.J.; Heinzmann, B.M.; Cunha, M.A.; Schmidt, D.; Baldisserotto, B. Essential oil of Aloysia triphylla as feed additive promotes growth of silver catfish (Rhamdia quelen). Aquac. Nutr. 2016, 22, 933–940. [Google Scholar] [CrossRef]
- Bandeira Júnior, G.; Pês, T.S.; Saccol, E.M.H.; Sutili, F.J.; Rossi, W.; Murari, A.L.; Heinzmann, B.M.; Pavanato, M.A.; De Vargas, A.C.; De, L.; et al. Potential uses of Ocimum gratissimum and Hesperozygis ringens essential oils in aquaculture. Ind. Crops Prod. 2017, 97, 484–491. [Google Scholar] [CrossRef]
- Santos, A.C.; Junior, B.G.; Zago, D.C.; Zeppenfeld, C.C.; Da Silva, D.T.; Heinzmann, B.M.; Baldisserotto, B.; Da Cunha, M.A. Anesthesia and anesthetic action mechanism of essential oils of Aloysia triphylla and Cymbopogon flexuosus in silver catfish (Rhamdia quelen). Vet. Anaesth. Analg. 2017, 44, 106–113. [Google Scholar] [CrossRef]
- Lugert, V.; Thaller, G.; Tetens, J.; Schulz, C.; Krieter, J. A review on fish growth calculation: Multiple functions in fish production and their specific application. Rev. Aquac. 2016, 8, 30–42. [Google Scholar] [CrossRef]
- Elvy, J.E.; Symonds, J.E.; Hilton, Z.; Walker, S.P.; Tremblay, L.A.; Herbert, N.A. The relationships between specific dynamic action, nutrient retention and feed conversion ratio in farmed freshwater Chinook salmon (Oncorhynchus tshawytscha) J. Fish Biol. 2023, 102, 605–618. [Google Scholar] [CrossRef]
- Bag, M.P.; Mahapatra, S.C.; Rao, P.S.; Chakrabarty, D. Evaluation of growth performance of tilapia (Oreochromis mossambicus) using low cost fish feed. Int. J. Biochem. Biotechnol. 2012, 1, 150–155. [Google Scholar]
- Yones, A.M.; Hussein, M.S.; Metwalli, A.A.; Lotfy, A.M. Effect of different rates on growth performance, feed utilization and body composition for mixed sex Nile Tilapia (Oreochromis Niloticus) reared in tanks. Oceanogr. Fish Open Access J. 2019, 11, 5557807. [Google Scholar] [CrossRef]
- Amend, D.F. Potency testing of fish vaccines. In Proceedings of the International Symposium on Fish Biologics: Serodiagnostics and Vaccines, Developments in Biological Standardization, Leetown, WV, USA, 26–30 April 1981; Volume 49, pp. 447–454. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K. Colorimetric method for determination of sugars and related substances. Anal. Biochem. 1956, 28, 350–358. [Google Scholar] [CrossRef]
- Harrower, J.R.; Brown, C.H. Blood lactic acid. A micromethod adapted to field collection of microliter samples. J. Appl. Physiol. 1972, 32, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, J.R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Frings, C.S.; Fendley, T.W.; Dunn, R.T.; Queen, C.A. Improved determination of total serum lipids by the sulfo-phospho-vanillin reaction. Clin. Chem. 1972, 18, 673–674. [Google Scholar] [CrossRef]
- Wang, H.D.; Pagano, P.J.; Cayatte, A.J.; Quinn, M.T.; Brecher, P.; Cohen, R.A. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ. Res. 1998, 82, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Willmore, W.G.; Storey, K.B. Quantification of lipid peroxidation in tissue extracts based on Fe (III) xylenol orange complex formation. Free Radic. Biol. Med. 1995, 19, 271–280. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Fanti, P.; Rossi, R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 2013, 8, 1660–1669. [Google Scholar] [CrossRef]
- Campos, A.M.; Lissi, E. Kinetics of the reaction between 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) derived radical cations and phenols. Int. J. Chem. Kinet. 1997, 29, 219–224. [Google Scholar] [CrossRef]
- Souza, C.D.F.; Descovi, S.; Baldissera, M.D.; Bertolin, K.; Bianchini, A.E.; Mourão, R.H.V.; Schmidt, D.; Heinzmann, B.M.; Antoniazzi, A.; Baldisserotto, B.; et al. Involvement of HPI-axis in anesthesia with Lippia alba essential oil citral and linalool chemotypes: Gene expression in the secondary responses in silver catfish. Fish Physiol. Biochem. 2019, 45, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, H.N.P.; Almeida, A.P.G.; Souza, C.F.; Mancera, J.M.; Martos-Sitcha, J.A.; Martínez-Rodríguez, G.; Baldisserotto, B. Stress response of Rhamdia quelen to the interaction stocking density—Feeding regimen. Gen. Comp. Endocrinol. 2023, 335, 114228. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Kumar Jha, N.; Nagoor Meeran, M.F.; Kumar Kesari, K.; Beiram, R.; Ojha, S. Neuroprotective potential of limonene and limonene containing natural products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.A.; Yousef, Y.M.; El-Tras, W.F.; Khalafallaa, M.M. Dietary essential oil extract from sweet orange (Citrus limon) peels improved Nile tilapia performance and health status. Aquac. Res. 2021, 52, 1463–1479. [Google Scholar] [CrossRef]
- Rahman, A.N.A.; ElHady, M.; Shalaby, S.I. Efficacy of the dehydrated lemon peels on the immunity, enzymatic antioxidant capacity and growth of Nile tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus). Aquaculture 2019, 505, 92–97. [Google Scholar] [CrossRef]
- Gültepe, N. How the use of orange (Citrus sinensis) peel essential oil affected the growth performance of rainbow trout (Oncorhynchus mykiss)? Bull. UASVM Anim. Sci. Biotechnol. 2018, 75, 16–20. [Google Scholar] [CrossRef]
- Ngugi, C.C.; Oyoo-Okoth, E.; Muchiri, M. Effects of dietary levels of essential oil (EO) extract from bitter lemon (Citrus limon) fruit peels on growth, biochemical, haemato-immunological parameters and disease resistance in juvenile Labeo victorianus fingerlings challenged with Aeromonas Hydrophila. Aquac. Res. 2017, 48, 2253–2265. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ergün, S.; Yıgıt, M. Effects of dietary FARMARIN® XP supplement on immunological responses and disease resistance of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 496, 211–220. [Google Scholar] [CrossRef]
- Yılmaz, S.; Ergun, S. Effects of garlic and ginger oils on hematological and biochemical parameters of Sea Bass, Dicentrarchus Labrax. J. Aquat. Anim. Health 2012, 24, 219–224. [Google Scholar] [CrossRef]
- Rankin, J.C. Physiology and form of fish circulation. Rev. Fish Biol. Fish. 1992, 2, 345–347. [Google Scholar] [CrossRef]
- Carlson, R.E.; Anderson, D.P.; Bodammer, J.E. In vivo cortisol administration suppreses the in vitro primary immune response of winter flounder lymphocytes. Fish Shellfish. Immunol. 1993, 3, 299–312. [Google Scholar] [CrossRef]
- Milligam, C.L.; Wood, C.M. Intracellular and extracellular acid-base status and H exchange with the environment after exhaustive exercise in the rainbow trout. J. Exp. Biol. 1982, 123, 92–121. [Google Scholar] [CrossRef]
- Weber, J.M.; Choi, K.; Gonzalez, A.; Omlin, T. Metabolic fuel kinetics in fish: Swimming, hypoxia and muscle membranes. J. Exp. Biol. 2016, 219, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Dalla Via, J.; Van den Thillart, G.; Cattani, O.; Cortesi, P. Behavioral responses and biochemical correlates in Solea solea to gradual hypoxic exposure. Can. J. Zool. 1998, 6, 2108–2113. [Google Scholar] [CrossRef]
- Kesselring, J.; Gruber, C.; Standen, B.; Wein, S. Effect of a phytogenic feed additive on the growth performance and immunity of Pacific white leg shrimp, Litopenaeus vannamei, fed a low fishmeal diet. J. World Aquac. Soc. 2021, 52, 303–315. [Google Scholar] [CrossRef]
- Gültepe, N. Protective effect of D-limonene derived from orange peel essential oil against Yersinia ruckeri in rainbow trout. Aquac. Rep. 2020, 18, 100417. [Google Scholar] [CrossRef]
- Causey, D.R.; Pohl, M.A.N.; Stead, D.A.; Martin, S.A.M.; Secombes, C.J.; Macqueen, D.J. High-throughput proteomic profiling of the fish liver following bacterial infection. BMC Genom. 2018, 19, 719. [Google Scholar] [CrossRef]
- Saccol, E.M.H.; Uczay, J.; Pês, T.; Finamor, I.A.; Ourique, G.M.; Riffel, A.P.K.; Pavanato, M.A. Addition of Lippia alba (Mill) N. E. Brown essential oil to the diet of the silver catfish: An analysis of growth, metabolic and blood parameters and the antioxidant response. Aquaculture 2013, 416–417, 244–254. [Google Scholar] [CrossRef]
- González-Silveira, D.; Cuesta, A.; Esteban, M.A. Immune defence mechanisms presented in liver homogenates and bile of gilthead seabream (Sparus aurata). J. Fish Biol. 2021, 99, 1958–1967. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, H.L.; Wang, L.Y.; Gu, B.Y.; Fan, Q.X. Changes of ammonia, urea contents and transaminase activity in the body during aerial exposure and ammonia loading in Chinese loach Paramisgurnus dabryanus. Fish Physiol. Biochem. 2017, 43, 631–640. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Chojnacki, M.; Kamaszewski, M.; Sawosz-Chwalibóg, E. Histopathological effects of silver and copper nanoparticles on the epidermis, gills, and liver of Sib. Sturgeon. Environ. Sci. Pollut. Res. 2016, 23, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol. Biochem. 2009, 35, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Triantaphyllopoulos, K.A.; Cartas, D.; Miliou, H. Factors influencing GH and IGF-I gene expression on growth in teleost fish: How can aquaculture industry benefit? Aquaculture 2020, 12, 1637–1662. [Google Scholar] [CrossRef]
- Aanyu, M.; Betancora, M.B.; Monroiga, O. Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 488, 217–226. [Google Scholar] [CrossRef]
- Beckman, B.R. Perspectives on concordant and discordant relations between insulin-like growth factor I (IGFI) and growth in fishes. Gen. Comp. Endocrinol. 2011, 170, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Gabillard, J.C.; Kamangar, B.B.; Montserrat, N. Coordinated regulation of the GH/IGF system genes during refeeding in rainbow trout (Oncorhynchus mykiss). J. Endocrinol. 2006, 191, 15–24. [Google Scholar] [CrossRef]
- Pierce, A.L.; Shimizu, M.; Beckman, B.R.; Baker, D.M.; Dickhoff, W.W. Time course of the GH/IGF axis response to fasting and increased ration in Chinook salmon (Oncorhynchus tshawytscha). Gen. Comp. Endocrinol. 2005, 140, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Luckenbach, J.A.; Murashige, R.; Daniels, H.V.; Godwin, J.; Borski, R.J. Temperature affects insulin-like growth factor I and growth of juvenile southern flounder, Paralichthys lethostigma. Comp. Biochem. Physiol. Part A 2007, 146, 95–104. [Google Scholar] [CrossRef]
- Wenger, M.; Shved, N.; Akgül, G.; Caelers, A.; Casanova, A.; Segner, H.; Eppler, E. Developmental oestrogen exposure differentially modulates IGF-I and TNF-α expression levels in immune organs of Yersinia ruckeri-challenged young adult rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2014, 205, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.C.; Small, B.C.; Bilodeau, L. Effects of GH on immune and endocrine responses of channel catfish challenged with Edwardsiella ictaluri. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 47–53. [Google Scholar] [CrossRef]
- Jiang, D.; Li, S.; Liang, Y.; Ma, J.; Wang, B.; Zhang, C. Protective effects of the fructooligosaccharide on the growth performance, biochemical indexes, and intestinal morphology of blunt snout bream (Megalobrama amblycephala) infected by Aeromonas hydrophila. Fish Physiol. Biochem. 2023, 49, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, L.J.G.; Marqueze, A.; Trapp, M.; Quevedo, R.M.; Ferreira, D. The effects of fasting on cortisol, blood glucose and liver and muscle glycogen in adult jundiá Rhamdia quelen. Aquaculture 2010, 300, 231–236. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.; Stryer, L. Biochemistry, 5th ed.; W.H. Freeman: New York, NY, USA, 2002; ISBN 978-0716746843. [Google Scholar]
- Mazeaud, M.M.; Mazeaud, F.; Donaldson, E.M. Primary and Secondary Effects of Stress in Fish, Some New Data with a General Review. Trans. Am. Fish Soc. 1977, 106, 201–212. [Google Scholar] [CrossRef]
- Becker, A.G.; Parodi, T.V.; Zeppenfeld, C.C.; Salbego, J.; Cunha, M.A.; Heldwein, C.G.; Loro, A.; Heinzmann, B.M.; Baldisserotto, B. Pre-sedation and transport of Rhamdia quelen in water containing essential oil of Lippia alba: Metabolic and physiological responses. Fish Physiol. Biochem. 2016, 42, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Salbego, J.; Becker, A.G.; Parodi, T.V.; Zeppenfeld, C.C.; Gonçalves, J.F.; Loro, V.L.; Morsch, V.M.M.; Schetinger, M.R.C.; Maldaner, G.; Morel, A.F.; et al. Methanolic extract of Condalia buxifolia added to transport water alters biochemical parameters of the silver catfish Rhamdia quelen. Aquaculture 2015, 437, 46–50. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Tsai, S.-C.; Lin, H.-C.; Hsiao, C.-D.; Wu, S.M. Changes of glycogen metabolism in the gills and hepatic tissue of tilapia (Oreochromis mossambicus) during short-term Cd exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 296–304. [Google Scholar] [CrossRef]
- Matys, J.; Turska-Szewczuk, A.T.; Gieroba, B.; Kurzylewska, M.; Pękala-Safińska, A.; Sroka-Bartnicka, A. Evaluation of Proteomic and Lipidomic Changes in Aeromonas-Infected Trout Kidney Tissue with the Use of FT-IR Spectroscopy and MALDI Mass Spectrometry Imaging. Int. J. Mol. Sci. 2022, 23, 12551. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem.-Biol. Interact. 2018, 83, 97–106. [Google Scholar] [CrossRef]
- Ahmad, S.; Beg, Z.H. Hypolipidemic and antioxidant activities of thymoquinone and limonene in atherogenic suspensión fed rats. Food Chem. 2023, 138, 1116–1124. [Google Scholar] [CrossRef]
- Akbary, P.; Yarahmadi, S.S.; Jahanbakhshi, A. Hematological, hepatic enzymes activity and oxidative stress responses of gray mullet (Mugil cephalus) after sub-acute exposure to copper oxide. Environ. Sci. Pollut. Res. 2018, 25, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Ahmadifar, E.; Moghadam, M.S.; Ghiyasi, M.; Dawood, M.A.O.; Yilmaz, S. Lemon, Citrus aurantifolia, peel and Bacillus licheniformis protected common carp, Cyprinus carpio, from Aeromonas hydrophila infection by improving the humoral and skin mucosal immunity, and antioxidative responses. J. World Aquac. Soc. 2021, 52, 124–137. [Google Scholar] [CrossRef]
- Salem, M.E.-S.; Abdel-Ghany, H.M.; Sallam, A.E.; El-Feky, M.M.M..; Almisherfi, H.M. Effects of dietary orange peel on growth performance, antioxidant activity, intestinal microbiota and liver histology of Gilthead sea bream (Sparus aurata) larvae. Aquac. Nutr. 2019, 25, 1087–1097. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 10, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Al-Deriny, S.H.; Dawood, M.A.O.; Abou Zaid, A.A.; El-Tras, W.F.; Paray, B.A.; Van Doan, H.; Mohamed, R.A. The synergistic effects of Spirulina platensis and Bacillus amyloliquefaciens on the growth performance, intestinal histomorphology, and immune response of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100390. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007; ISBN 9780198568698. [Google Scholar]
- Lu, S.C. Glutathione synthesis. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Ploch, S.A.; Lee, Y.-P.; Maclean, E.; Di Giulio, R.T. Oxidative stress in liver of brown bullhead and channel catfish following exposure to ter-butyl hydroperoxide. Aquat. Toxic. 1999, 46, 231–240. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18 Pt B, 797–810. [Google Scholar] [CrossRef]
- Bandeira Junior, G.; Baldisserotto, B. Fish infections associated with the genus Aeromonas: A review of the effects on oxidative status. J. Appl. Microbiol. 2021, 131, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, H.; Kurhaluk, N.; Andriichuk, A.; Gasiuk, E.; Beschasniu, S. Oxidative stress biomarkers in liver of sea trout (Salmo trutta m. trutta L.) affected by ulcerative dermal necrosis syndrome. Turk. J. Fish. Aquat. Sci. 2014, 14, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Abrosimova, K.S.; Abrosimova, N.A.; Morozova, M.A. Processes of lipid peroxidation in carp affected by Aeromonas disease. IOP Conf. Ser. Earth Environ. Sci. 2021, 66, 042045. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Abbad, L.B.; Verdi, C.M.; Santos, R.C.V.; da Silva, A.S.; Baldisserotto, B. Dietary supplementation with caffeine increases survival rate, reduces microbial load and protects the liver against Aeromonas hydrophila-induced hepatic damage in the grass carp Ctenopharyngodon idella. Microb. Pathog. 2019, 135, 103637. [Google Scholar] [CrossRef]
- Swamy, J.M.; Naik, M.G.; Rathore, S.S.; Srinivasa, K.H.; Monica, K.S. Dietary supplementation of Nile tilapia (Oreochromis niloticus) diets with bay laurel (Laurus nobilis): Alleviation of oxidative stress and amelioration of immune response, serum biochemistry, and resistance against Aeromonas hydrophila. Fish Physiol. Biochem. 2023. [Google Scholar] [CrossRef]
- Ling, X.-D.; Dong, W.-T.; Zhang, Y.; Qian, X.; Zhang, W.-D.; He, W.-H.; Zhao, X.-X.; Liu, J.-X. Comparative transcriptomics and histopathological analysis of crucian carp infection by atypical Aeromonas salmonicida. Fish Shellfish. Immunol. 2019, 94, 294–307. [Google Scholar] [CrossRef]
- Mustahal; Sevia; Herjayanto, M.; Syamsunarno, M.B.; Putra, A.N. The effect of adding Bacillus NP5 to feed on growth, survival rate, and protection against Aeromonas hydrophila of Catfish (Clarias sp.). IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 012058. [Google Scholar] [CrossRef]
- Kalaiyarasi, T.; Jayakumar, N.; Jawahar, P.; Ahilan, B.; Subburaj, A. Histological changes in the gill and liver of marine spotted catfish, Arius maculatus from sewage disposal site, Therespuram off Thoothukudi, Southeast coast of India. J. Entomol. Zool. Stud. 2017, 5, 1710–1715. [Google Scholar] [CrossRef]
- Al Mamun, M.A.; Nasren, S.; Rathore, S.S.; Mahbub Alam, M.M. Histopathological analysis of atriped aatfish, Pangasianodon hypophthalmus (Sauvage, 1878) apontaneously anfected with Aeromonas hydrophila. Jordan J. Biol. Sci. 2022, 15, 93–100. [Google Scholar] [CrossRef]
- Afifi, S.; Al-Thobiati, S.; Hazaa, M.S. Bacteriological and histopathological studies on Aeromonas hydrophila infection of Nile tilapia (Oreochromis niloticus) from fish farms in Saudi Arabia. Assiut Vet. Med. J. 2000, 42, 195–205. [Google Scholar]
- Brum, A.; Cardoso, L.; Chagas, E.D.; Chaves, F.C.M.C.; Mouriño, J.L.P.; Martins, M.L. Histological changes in Nile tilapia fed essential oils of clove basil and ginger after challenge with Streptococcus Agalactiae. Aquaculture 2018, 490, 98–107. [Google Scholar] [CrossRef]
- Bellipanni, G.; Cappello, F.; Scalia, F.; Macario, E.S.; Macario, A.J.L.; Giordano, A. Zebrafish as a model for the study of chaperonopathies. J. Cell. Physiol. 2016, 10, 2107–2114. [Google Scholar] [CrossRef]
- Bandeira Junior, G.; Souza, C.F.; Descovi, S.N.; Antonizazi, A.; Cargnelutti, J.F.; Baldisserotto, B. Aeromonas hydrophila infection in silver catfish causes hyperlocomotion related to stress. Microb. Pathog. 2019, 132, 261–265. [Google Scholar] [CrossRef]
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 10, 789–801. [Google Scholar] [CrossRef]
- Eachus, H.; Ryu, S.; Placzek, M.; Wood, J. Zebrafish as a model to investigate the CRH axis and interactions with DISC1. Curr. Opin. Endocr. Metab. Res. 2022, 26, 100383. [Google Scholar] [CrossRef]
- Kiilerich, P.; Servili, A.; Péron, S.; Valotaire, C.; Goardon, L.; Leguen, I.; Prunet, P. Regulation of the corticosteroid signalling system in rainbow trout HPI axis during confinement stress. Gen. Comp. Endocrinol. 2018, 258, 184–193. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]


 (infiltration of erythrocytes into the blood sinusoids),
(infiltration of erythrocytes into the blood sinusoids),  (nuclear pyknosis),
(nuclear pyknosis),  (hemorrhage),
(hemorrhage),  (fibrosis),
(fibrosis),  (binucleated hepatocytes).
(binucleated hepatocytes).
 (infiltration of erythrocytes into the blood sinusoids),
(infiltration of erythrocytes into the blood sinusoids),  (nuclear pyknosis),
(nuclear pyknosis),  (hemorrhage),
(hemorrhage),  (fibrosis),
(fibrosis),  (binucleated hepatocytes).
(binucleated hepatocytes).

| Ingredients | (g/kg) |
|---|---|
| Meat meal | 350 |
| Soybean meal | 300 |
| Corn | 150 |
| Rice bran | 120 |
| Canola oil | 30 |
| Salt | 10 |
| Vitamin and mineral (pré-mix) * | 30 |
| Proximate composition | |
| Dry matter | 939.9 |
| Mineral matter | 252.3 |
| Crude protein | 303.2 |
| Ether extract | 74.9 |
| Neutral detergent fiber | 251.2 |
| Acid detergent fiber | 7.8 |
| Growth Performance | R-(+)-Limonene Concentration (mL/kg Diet) | |||
|---|---|---|---|---|
| 0 (control) | L (0.5) | L (1.0) | L (2.0) | |
| Initial | ||||
| W | 20.21 ± 0.07 | 19.21 ± 0.04 | 19.81 ± 0.02 | 20.07 ± 0.04 |
| SL | 11.39 ± 0.02 | 10.55 ± 0.05 | 11.07 ± 0.04 | 11.12 ± 0.02 |
| 30 days | ||||
| W | 31.91 ± 1.46 a | 41.78 ± 1.35 b | 32.50 ± 1.46 ab | 44.03 ± 1.40 b |
| SL | 14.28 ± 0.40 a | 14.86 ± 0.40 a | 14.70 ± 0.40 a | 15.07 ± 0.40 a |
| SGR | 1.78 ± 0.21 a | 3.58 ± 0.17 b | 3.14 ± 0.18 a | 4.29 ± 0.18 b |
| 60 days | ||||
| W | 62.24 ± 1.06 a | 88.44 ± 1.50 b | 73.33 ± 1.11 a | 91.33 ± 1.73 b |
| SL | 17.10 ± 0.41 a | 18.17 ± 0.41 a | 17.68 ± 0.40 a | 19.44 ± 0.40 a |
| FI | 66.43 ± 1.11 a | 75.77 ± 1.30 a | 68.03 ± 1.40 a | 68.84 ± 1.10 a |
| FCR | 1.79 ± 0.02 a | 1.33 ± 0.02 bc | 1.83 ± 0.04 a | 1.11 ± 0.05 c |
| SGR | 3.45 ± 0.07 a | 3.99 ± 0.05 c | 3.67 ± 0.07 b | 3.98 ± 0.07 c |
| HSI | 1.29 ± 0.10 a | 1.21 ± 0.10 a | 1.27 ± 0.11 a | 1.18 ± 0.11 a |
| Survival | 100 | 100 | 100 | 100 |
| R-(+)-Limonene Dose (mL/kg/Diet) | ||||
|---|---|---|---|---|
| Non-Infected Groups | ||||
| Metabolites | 0 (Control) | L (0.5) | L (1.0) | L (2.0) |
| Plasma | ||||
| Glucose | 72.32 ± 5.89 a | 79.80 ± 5.51 a | 75.37 ± 5.00 a | 79.55 ± 5.00 a |
| Lactate | 35.35 ± 2.38 a | 26.08 ± 2.38 a | 27.29 ± 2.55 a | 27.67 ± 2.38 a |
| Total Protein | 5.08 ± 0.34 a | 5.30 ± 0.32 a | 4.55 ± 0.32 a | 4.53 ± 0.32 a |
| LDH | 154.45 ± 10.82 a | 135.99 ± 10.82 a | 138.10 ± 10.82 a | 119.77 ± 10.82 b |
| Liver | ||||
| Glucose | 56.44 ± 2.92 a | 56.44 ± 2.92 a | 64.69 ± 3.46 ab | 72.50 ± 3.43 b |
| Lactate | 21.89 ± 3.12 a | 21.89 ± 3.12 a | 20.03 ± 3.12 a | 24.09 ± 3.12 a |
| Glycogen | 41.69 ± 2.5 a | 35.08 ± 2.50 a | 38.83 ± 2.50 a | 37.05 ± 2.50 a |
| Total Protein | 126.51 ± 3.64 a | 148.34 ± 3.76 a | 131.11 ± 3.89 a | 137.77 ± 3.48 a |
| Groups Infected with A. hydrophila | ||||
| Plasma | ||||
| Glucose | 123.88 ± 6.36 A* | 67.70 ± 6.36 B | 57.51 ± 5.00 B | 60.10 ± 6.00 B |
| Lactate | 68.20 ± 2.55 A* | 53.46 ± 2.38 B#* | 36.70 ± 2.38 C | 39.78 ± 2.38 C# |
| Total Protein | 8.10 ± 0.34 A* | 4.72 ± 0.32 B | 3.93 ± 0.32 B | 4.67 ± 0.32 B |
| LDH | 230.08 ± 9.14 A* | 131.20 ± 10.82 B | 94.36 ± 10.82 B#* | 137.03 ± 10.82 B |
| Liver | ||||
| Glucose | 112.73 ± 2.90 A* | 65.02 ± 3.43 B | 66.84 ± 3.13 B | 67.47 ± 3.13 B |
| Lactate | 68.95 ± 3.12 A* | 30.99 ± 3.12 B# | 27.25 ± 3.12 C | 29.16 ± 3.12 BC |
| Glycogen | 134.22 ± 2.50 A* | 42.91 ± 2.50 B# | 55.28 ± 2.50 C#* | 60.24 ± 2.50 C#* |
| Total Protein | 109.69 ± 4.12 A | 152.21 ± 3.26 B* | 151.16 ± 3.48 B | 148.70 ± 3.48 B |
| R-(+)-Limonene Dose (mL/kg/Diet) | ||||
|---|---|---|---|---|
| Non-Infected Groups | ||||
| Lipid Profile | 0 (Control) | L (0.5) | L (1.0) | L (2.0) |
| Plasma | ||||
| Triglycerides | 445.04 ± 28.06 a | 396.45 ± 28.06 a | 268.30 ± 30.31 b | 294.66 ± 30.31 b |
| Cholesterol | 111.36 ± 4.49 a | 113.52 ± 5.31 a | 106.09 ± 5.94 a | 89.75 ± 5.31 a |
| Liver | ||||
| Total lipid | 786 ± 25.34 a | 618.67 ± 22.66 b | 703.10 ± 22.66 a | 744.32 ± 25.34 a |
| Groups Infected with A. hydrophila | ||||
| Plasma | ||||
| Triglycerides | 918.64 ± 33.20 A* | 256.69 ± 30.31 B#* | 228.75 ± 30.31 B* | 301.37 ± 28.06 B* |
| Cholesterol | 219.85 ± 5.31 A* | 105.84 ± 4.85 B | 111.20 ± 5.31 B | 95.69 ± 4.85 B |
| Liver | ||||
| Total lipid | 909.48 ± 22.66 A* | 925.76 ± 25.34 A#* | 719.33 ± 22.66 B | 712.04 ± 25.34 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva, E.G.; Finamor, I.A.; Bressan, C.A.; Schoenau, W.; Vencato, M.D.S.; Pavanato, M.A.; Cargnelutti, J.F.; Da Costa, S.T.; Antoniazzi, A.Q.; Baldisserotto, B. Dietary Supplementation with R-(+)-Limonene Improves Growth, Metabolism, Stress, and Antioxidant Responses of Silver Catfish Uninfected and Infected with Aeromonas hydrophila. Animals 2023, 13, 3307. https://doi.org/10.3390/ani13213307
Da Silva EG, Finamor IA, Bressan CA, Schoenau W, Vencato MDS, Pavanato MA, Cargnelutti JF, Da Costa ST, Antoniazzi AQ, Baldisserotto B. Dietary Supplementation with R-(+)-Limonene Improves Growth, Metabolism, Stress, and Antioxidant Responses of Silver Catfish Uninfected and Infected with Aeromonas hydrophila. Animals. 2023; 13(21):3307. https://doi.org/10.3390/ani13213307
Chicago/Turabian StyleDa Silva, Elisia Gomes, Isabela Andres Finamor, Caroline Azzolin Bressan, William Schoenau, Marina De Souza Vencato, Maria Amália Pavanato, Juliana Felipetto Cargnelutti, Sílvio Teixeira Da Costa, Alfredo Quites Antoniazzi, and Bernardo Baldisserotto. 2023. "Dietary Supplementation with R-(+)-Limonene Improves Growth, Metabolism, Stress, and Antioxidant Responses of Silver Catfish Uninfected and Infected with Aeromonas hydrophila" Animals 13, no. 21: 3307. https://doi.org/10.3390/ani13213307