Spatial Ecology of an Arboreal Iguana (Oplurus cyclurus) in a Treeless Landscape
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Period
2.2. Radio-Tracking, Distance Measurements, and Environmental Variables
2.3. Home Range Estimates
2.4. Activity Pattern
2.5. Burrow Use
2.6. Basking Behaviour
2.7. Modelling Procedure
3. Results
3.1. Home Range Estimates
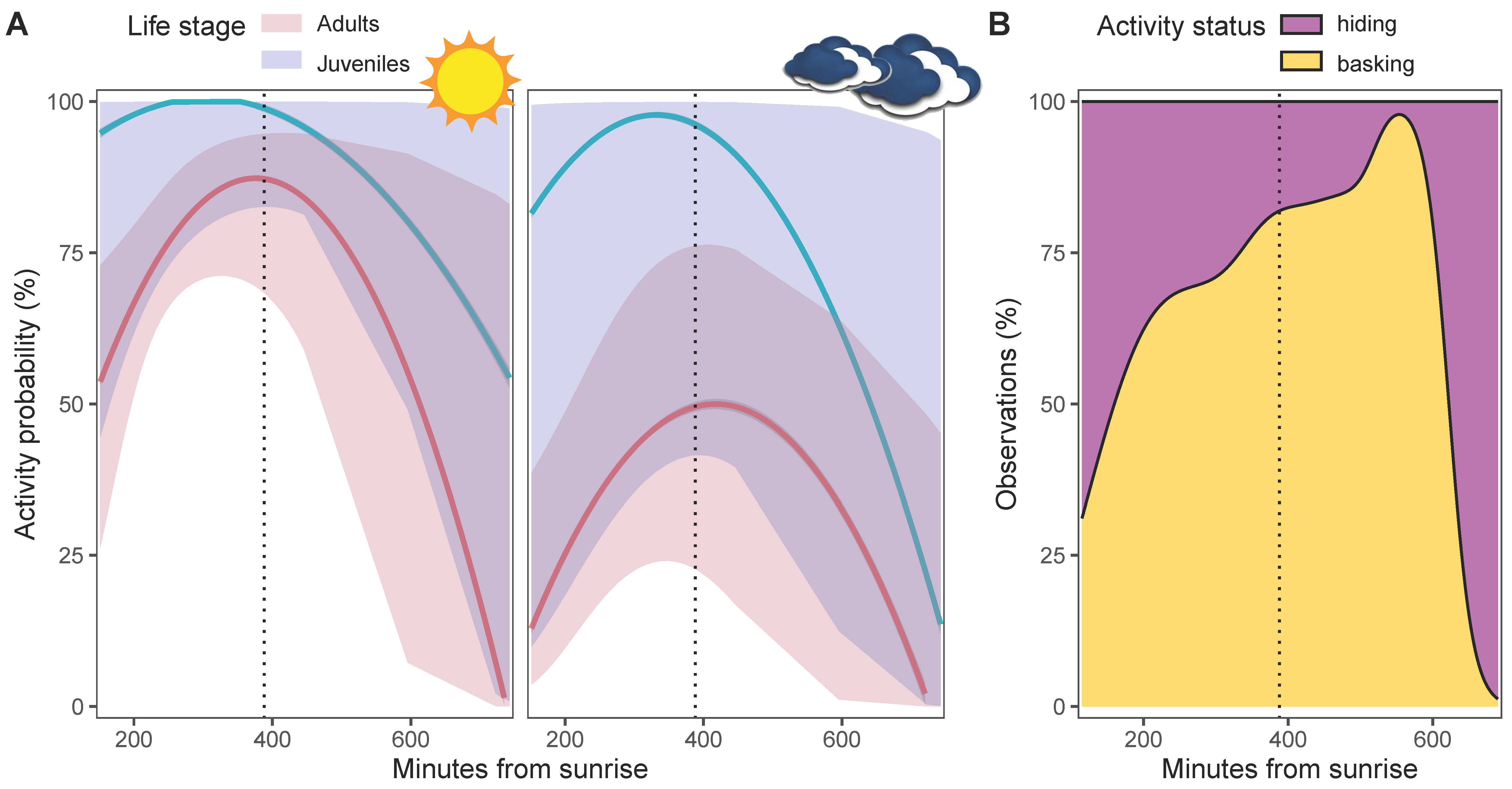
3.2. Activity Status
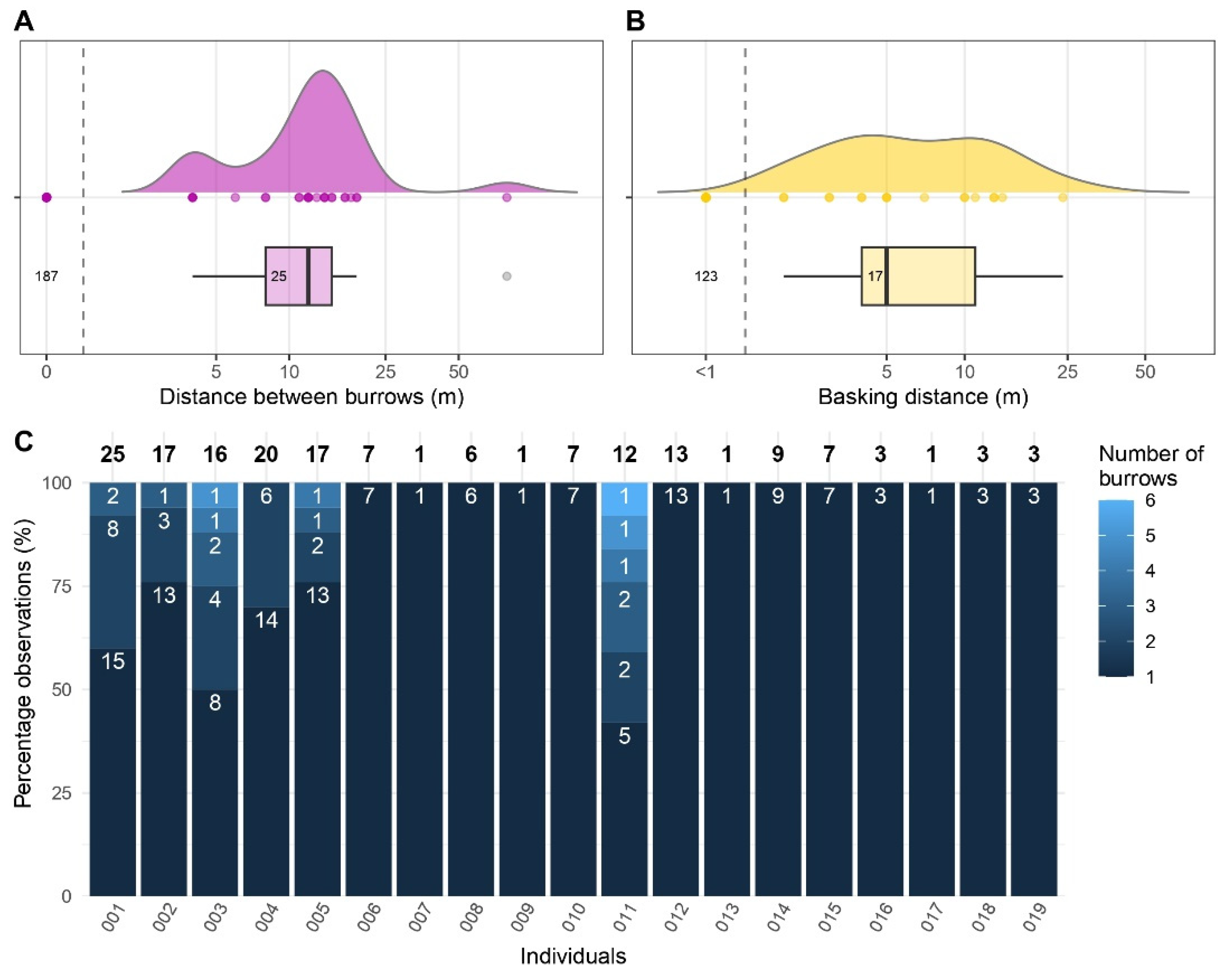
3.3. Burrow Use
3.4. Basking Behaviour
4. Discussion
4.1. Space Use
4.2. Activity Pattern and Burrow Use
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and Trends of Amphibian Declines and Extinctions Worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Andreone, F.; Cadle, J.E.; Cox, N.; Glaw, F.; Nussbaum, R.A.; Raxworthy, C.J.; Stuart, S.N.; Vallan, D.; Vences, M. Species Review of Amphibian Extinction Risks in Madagascar: Conclusions from the Global Amphibian Assessment. Conserv. Biol. 2005, 19, 1790–1802. [Google Scholar] [CrossRef]
- Andreone, F.; Carpenter, A.I.; Cox, N.; du Preez, L.; Freeman, K.; Furrer, S.; Garcia, G.; Glaw, F.; Glos, J.; Knox, D.; et al. The Challenge of Conserving Amphibian Megadiversity in Madagascar. PLoS Biol. 2008, 6, e118. [Google Scholar] [CrossRef] [PubMed]
- Gehring, P.-S.; Lutzmann, N.; Furrer, S.; Sossinka, R. Habitat Preferences and Activity Patterns of Furcifer pardalis (CUVIER, 1829) in the Masoala Rain Forest Hall of the Zurich Zoo. Salamandra 2008, 44, 129–140. [Google Scholar]
- Doody, J.S.; Burghardt, G.M.; Dinets, V. Breaking the Social–Non-Social Dichotomy: A Role for Reptiles in Vertebrate Social Behavior Research? Ethology 2013, 119, 95–103. [Google Scholar] [CrossRef]
- Smith, G.R.; Ballinger, R.E. The Ecological Consequences of Habitat and Microhabitat Use in Lizards: A Review. Contemp. Herpetol. 2001, 3, 1–28. [Google Scholar] [CrossRef]
- Vitt, L.J.; Caldwell, J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles; Academic Press: Cambridge, UK, 2013. [Google Scholar]
- Cadle, J.E.; Chan, L.; Miralles, A. Opluridae, Malagasy Iguanas, Androngovato. In The New Natural History of Madagascar; Goodman, S.M., Andrianarimisa, A., Armstrong, A.H., Cooke, A., De Wit, M., Ganzhorn, J.U., Gautier, L., Eds.; Princeton University Press: Princeton, NJ, USA, 2022; pp. 1502–1505. ISBN 978-0-691-22262-2. [Google Scholar]
- Mori, A.; Randriamahazo, H.J.A.R. Foraging Mode of a Madagascan Iguanian Lizard, Oplurus cuvieri cuvieri. Afr. J. Ecol. 2002, 40, 61–64. [Google Scholar] [CrossRef]
- Rosa, G.M.; Rakotozafy, S. Opportunistic but Voracious: Madagascan Spiny-Tailed Iguana, Oplurus cuvieri (Reptilia: Opluridae) Predation upon a Small Mammal. Herpetol. Notes 2013, 6, 459–461. [Google Scholar]
- Brillet, C. Contribution à l’étude des relations entre individus chez cinq espèces d’iguanes malgaches du genre Oplurus. Rev. D’ecologie Terre Vie 1982, 36, 79–148. [Google Scholar] [CrossRef]
- Lobón-Rovira, J.; Belluardo, F.; Crottini, A.; Rosa, G.M. Territorial Behaviour in Oplurus grandidieri (Mocquard, 1900), a Rock Dwelling Lizard from Madagascar. Herpetol. Notes 2019, 12, 783–786. [Google Scholar]
- Dickinson, H.C.; Fa, J.E. Ultraviolet Light and Heat Source Selection in Captive Spiny-Tailed Iguanas (Oplurus cuvieri). Zoo Biol. 1997, 16, 391–401. [Google Scholar] [CrossRef]
- Gibson, R.C.; Buley, K.R. Captive Management and Breeding of Madagascar Spiny Iguanas Oplurus cuvieri Cuvieri Gray, 1831. Dodo 1996, 32, 137–143. [Google Scholar]
- Randriamahazo, H.J.A.R. Activity Temperatures in Oplurus cyclurus, Oplurus cuvieri and Zonosaurus laticaudatus and Resting Metabolic Rates in the Latter Two Species. Amphib.-Reptil. 1998, 19, 215–220. [Google Scholar] [CrossRef]
- Rakotonomenjanahary, O.M.; Hawkins, A.F.A. Le Projet ‘Zicoma’ Ou ‘Zones d’Importance Pour La Conservation Des Oiseaux a Madagascar’. Ostrich 2000, 71, 168–171. [Google Scholar] [CrossRef]
- Cocca, W.; Rosa, G.; Andreone, F.; Aprea, G.; Eusebio Bergò, P.; Mattioli, F.; Mercurio, M.; Randrianirina, J.; Rosado, D.; Vences, M.; et al. The Herpetofauna (Amphibia, Crocodylia, Squamata, Testudines) of the Isalo Massif, Southwest Madagascar: Combining Morphological, Molecular and Museum Data. Salamandra 2018, 54, 178–200. [Google Scholar]
- Mercurio, V.; Aprea, G.; Crottini, A.; Mattioli, F.; Randrianirina, J.E.; Razafindrabe, T.J.; Andreone, F. The Amphibians of Isalo Massif, Southern-Central Madagascar: High Frog Diversity in an Apparently Hostile Dry Habitat. Monogr. Del. Mus. Reg. Sci. Nat. Torino 2008, XLI, 5–58. [Google Scholar]
- ANGAP (Association Nationale pour la Gestion des Aires Protegées). Plan de Gestion du Réseau National des Aires Protégées de Madagascar; ANGAP and Ministère de l’Environnement: Antananarivo, Madagascar, 2003. [Google Scholar]
- Kull, C.A. The “Degraded” Tapia Woodlands of Highland Madagascar: Rural Economy, Fire Ecology, and Forest Conservation. J. Cult. Geogr. 2002, 19, 95–128. [Google Scholar] [CrossRef]
- Tramontano, R. Continuous Radiotracking of the Common Frog, Rana temporaria. In Herpetologia Bonnensis; SEH: Bonn, Germany, 1997; pp. 359–365. [Google Scholar]
- White, G.C.; Garrott, R.A. Analysis of Wildlife Radio-Tracking Data; Academic Press: Cambridge, UK, 1990; ISBN 978-0-08-092657-5. [Google Scholar]
- Maclean, I.M.D.; Mosedale, J.R.; Bennie, J.J. Microclima: An r Package for Modelling Meso- and Microclimate. Methods Ecol. Evol. 2019, 10, 280–290. [Google Scholar] [CrossRef]
- Kearney, M.R.; Porter, W.P. NicheMapR—An R Package for Biophysical Modelling: The Microclimate Model. Ecography 2017, 40, 664–674. [Google Scholar] [CrossRef]
- Kearney, M.R.; Gillingham, P.K.; Bramer, I.; Duffy, J.P.; Maclean, I.M.D. A Method for Computing Hourly, Historical, Terrain-Corrected Microclimate Anywhere on Earth. Methods Ecol. Evol. 2020, 11, 38–43. [Google Scholar] [CrossRef]
- Hollister, J.; Shah, T.; Robitaille, A.L.; Beck, M.W.; Johnson, M. Elevatr: Access Elevation Data from Various APIs. Version 0.4.5. 2023. Available online: https://CRAN.R-project.org/package=elevatr (accessed on 6 September 2023).
- Kemp, M.U.; van Loon, E.E.; Shamoun-Baranes, J.; Bouten, W. RNCEP: Obtain, Organize, and Visualize NCEP Weather Data. Version 1.0.10. 2020. Available online: https://CRAN.R-project.org/package=RNCEP (accessed on 6 September 2023).
- Kranstauber, B.; Kays, R.; LaPoint, S.D.; Wikelski, M.; Safi, K. A Dynamic Brownian Bridge Movement Model to Estimate Utilization Distributions for Heterogeneous Animal Movement. J. Anim. Ecol. 2012, 81, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Crane, M.; Marshall, B.M.; Strine, C.T. Reptiles on the Wrong Track? Moving beyond Traditional Estimators with Dynamic Brownian Bridge Movement Models. Mov. Ecol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Kranstauber, B.; Smolla, M.; Scharf, A.K. Move: Visualizing and Analyzing Animal Track Data. Version 4.2.4. 2023. Available online: https://CRAN.R-project.org/package=move (accessed on 6 September 2023).
- Calenge, C. The Package “Adehabitat” for the R Software: A Tool for the Analysis of Space and Habitat Use by Animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Fox, J.; Monette, G. Generalized Collinearity Diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95364-9. [Google Scholar]
- Richards, S.A.; Whittingham, M.J.; Stephens, P.A. Model Selection and Model Averaging in Behavioural Ecology: The Utility of the IT-AIC Framework. Behav. Ecol. Sociobiol. 2011, 65, 77–89. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. Version 1.47.5. 2022. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 6 September 2023).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; Posit, P.B.C. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. Version 3.4.3. 2023. Available online: https://CRAN.R-project.org/package=ggplot2 (accessed on 6 September 2023).
- Perry, G.; Garland, T. Lizard Home Ranges Revisited: Effects of Sex, Body Size, Diet, Habitat, and Phylogeny. Ecology 2002, 83, 1870–1885. [Google Scholar] [CrossRef]
- Schorr, R.A.; Lambert, B.A.; Freels, E. Habitat Use and Home Range of Long-Nosed Leopart Lizards (Gambelia wislizenii) in Canyons of the Ancients National Monument, Colorado. Herpetol. Conserv. Biol. 2011, 6, 312–323. [Google Scholar]
- Warrick, G.D.; Kato, T.T.; Rose, B.R. Microhabitat Use and Home Range Characteristics of Blunt-Nosed Leopard Lizards. J. Herpetol. 1998, 32, 183–191. [Google Scholar] [CrossRef]
- Ryberg, W.A.; Garrett, T.B.; Adams, C.S.; Campbell, T.A.; Walkup, D.K.; Johnson, T.E.; Hibbitts, T.J. Life in the Thornscrub: Movement, Home Range, and Territoriality of the Reticulate Collared Lizard (Crotaphytus reticulatus). J. Nat. Hist. 2019, 53, 1707–1719. [Google Scholar] [CrossRef]
- Melo, G.; Pinheiro, L.; Passos, D.; Galdino, C. Spatial Organisation of the Neotropical Lizard Tropidurus hispidus (Squamata: Tropiduridae). Salamandra 2017, 53, 435–438. [Google Scholar]
- Ferner, J.W. Home-Range Size and Overlap in Sceloporus undulatus erythrocheilus (Reptilia: Iguanidae). Copeia 1974, 1974, 332–337. [Google Scholar] [CrossRef]
- Chan, L.M.; Choi, D.; Raselimanana, A.P.; Rakotondravony, H.A.; Yoder, A.D. Defining Spatial and Temporal Patterns of Phylogeographic Structure in Madagascar’s Iguanid Lizards (Genus Oplurus). Mol. Ecol. 2012, 21, 3839–3851. [Google Scholar] [CrossRef] [PubMed]
- Randriamahazo, H.J.A.R.; Mori, A. Spatial Utilization and Social Interactions in Oplurus Cuvieri Cuvieri (Squamata, Opluridae) in Madagascar. Jpn. J. Herpetol. 1999, 18, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Glaw, F.; Vences, M. A Field Guide to the Amphibians and Reptiles of Madagascar, 3rd ed.; Vences & Glaw: Köln, Germany, 2007; ISBN 978-3-929449-03-7. [Google Scholar]
- Row, J.R.; Blouin-Demers, G. Kernels Are Not Accurate Estimators of Home-Range Size for Herpetofauna. Copeia 2006, 2006, 797–802. [Google Scholar] [CrossRef]
- Laver, P.N.; Kelly, M.J. A Critical Review of Home Range Studies. J. Wildl. Manag. 2008, 72, 290–298. [Google Scholar] [CrossRef]
- Carrascal, L.M.; López, P.; Martín, J.; Salvador, A. Basking and Antipredator Behaviour in a High Altitude Lizard: Implications of Heat-Exchange Rate. Ethology 1992, 92, 143–154. [Google Scholar] [CrossRef]
- Burke, R.L.; Ner, S.E. Seasonal and Diel Activity Patterns of Italian Wall Lizards, Podarcis sicula campestris, in New York. Nena 2005, 12, 349–360. [Google Scholar] [CrossRef]
- Paulissen, M.A. Ontogenetic and Seasonal Comparisons of Daily Activity Patterns of the Six-Lined Racerunner, Cnemidophorus sexlineatus (Sauria: Teiidae). Am. Midl. Nat. 1988, 120, 355–361. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral Decisions Made under the Risk of Predation: A Review and Prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Faria, R.G. Predation Risk Is a Function of Seasonality Rather than Habitat Complexity in a Tropical Semiarid Forest. Sci. Rep. 2021, 11, 16670. [Google Scholar] [CrossRef]
- Shepard, D.B. Habitat but Not Body Shape Affects Predator Attack Frequency on Lizard Models in the Brazilian Cerrado. Herpetologica 2007, 63, 193–202. [Google Scholar] [CrossRef]
- Fenner, A.L.; Godfrey, S.S.; Michael Bull, C. Using Social Networks to Deduce Whether Residents or Dispersers Spread Parasites in a Lizard Population. J. Anim. Ecol. 2011, 80, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Gracceva, G.; Bombi, P.; Luiselli, L.; Bologna, M. Do Demographic Aspects of Neighbouring Lizard Populations Differ? A Case Study with the Common Wall Lizard, Podarcis muralis. Amphib.-Reptil. 2008, 29, 443–448. [Google Scholar] [CrossRef]
- Vences, M. Oplurus Cyclurus. IUCN Red List. Threat. Species 2011: e.T172831A6926304. Available online: https://doi.org/10.2305/IUCN.UK.2011-2.RLTS.T172831A6926304.en (accessed on 6 September 2023).
- Sherwin, C.M.; Christiansen, S.B.; Duncan, I.J.; Erhard, H.W.; Lay, D.C.; Mench, J.A.; O’Connor, C.E.; Petherick, J.C. Guidelines for the Ethical Use of Animals in Applied Ethology Studies. Appl. Anim. Behav. Sci. 2003, 81, 291–305. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licata, F.; Eusebio Bergò, P.; Edmonds, D.; Andreone, F.; Rosa, G.M. Spatial Ecology of an Arboreal Iguana (Oplurus cyclurus) in a Treeless Landscape. Animals 2023, 13, 3198. https://doi.org/10.3390/ani13203198
Licata F, Eusebio Bergò P, Edmonds D, Andreone F, Rosa GM. Spatial Ecology of an Arboreal Iguana (Oplurus cyclurus) in a Treeless Landscape. Animals. 2023; 13(20):3198. https://doi.org/10.3390/ani13203198
Chicago/Turabian StyleLicata, Fulvio, Paolo Eusebio Bergò, Devin Edmonds, Franco Andreone, and Gonçalo M. Rosa. 2023. "Spatial Ecology of an Arboreal Iguana (Oplurus cyclurus) in a Treeless Landscape" Animals 13, no. 20: 3198. https://doi.org/10.3390/ani13203198
APA StyleLicata, F., Eusebio Bergò, P., Edmonds, D., Andreone, F., & Rosa, G. M. (2023). Spatial Ecology of an Arboreal Iguana (Oplurus cyclurus) in a Treeless Landscape. Animals, 13(20), 3198. https://doi.org/10.3390/ani13203198






