Effects of the Use of Rice Grain on Growth Performances, Blood Metabolites, Rumen Fermentation, and Rumen Microbial Community in Fattening Hanwoo Steers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of Experimental Diets and Chemical Analysis
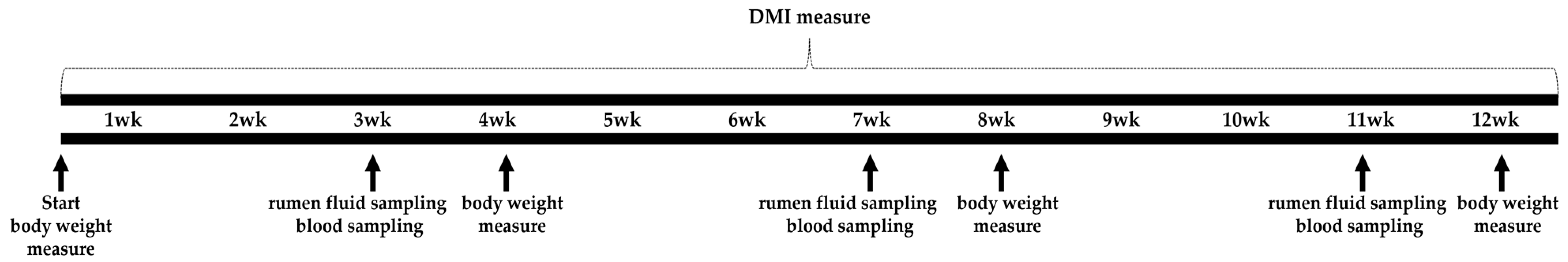
2.2. In Vivo Experimental Design
2.3. Rumen Fermentation Characteristics
2.4. Analysis of Blood Metabolites
2.5. Partial Least Square–Discriminant Analysis (PLS–DA)
2.6. Total DNA Extraction and Real-Time Quantitative PCR
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Rumen Fermentation Characteristics
3.3. Blood Metabolites
3.4. PLS−DA
3.5. Microorganism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.J.; Beak, S.-H.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; Baik, M. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 1043. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Chung, K.Y.; Park, B.H.; Kim, U.H.; Jang, S.S.; Smith, Z.K.; Kim, J. Effects of feeding high-energy diet on growth performance, blood parameters, and carcass traits in Hanwoo steers. Anim. Biosci. 2022, 35, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, B.; Kim, H.; Moon, J.; Yoo, D.; Baek, Y.-C.; Lee, S.; Seo, J. Replacement of corn with rice grains did not alter growth performance and rumen fermentation in growing Hanwoo steers. Asian-Australas. J. Anim. Sci. 2020, 33, 230. [Google Scholar] [CrossRef]
- Miyaji, M.; Matsuyama, H.; Hosoda, K.; Nonaka, K. Effect of replacing corn with brown rice grain in a total mixed ration silage on milk production, ruminal fermentation and nitrogen balance in lactating dairy cows. Anim. Sci. J. 2012, 83, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, M.; Matsuyama, H.; Hosoda, K. Effect of substituting brown rice for corn on lactation and digestion in dairy cows fed diets with a high proportion of grain. J. Dairy Sci. 2014, 97, 952–960. [Google Scholar] [CrossRef]
- NASEM. Nutrient Requirements of Beef Cattle, 8th ed.; National Academy Press: Washington, DC, USA, 2016. [Google Scholar]
- Shreve, B.; Thiex, N.; Wolf, M. National forage testing association reference method: Dry matter by oven drying for 3 hours at 105 C. In NFTA Reference Methods; National Forage Testing Association: Omaha, NE, USA, 2006. [Google Scholar]
- AOAC. Official methods of analysis of the Association of Analytical Chemists International. In Official Methods; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Yoo, D.; Hamid, M.M.A.; Kim, H.; Moon, J.; Song, J.; Lee, S.; Seo, J. Substitution effects of rice for corn grain in total mixed ration on rumen fermentation characteristics and microbial community in vitro. J. Anim. Sci. Techol. 2020, 62, 638. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, H.; Moon, J.; Kim, J.; Kim, H.; Seo, J. Effects of Red Ginseng Byproducts on Rumen Fermentation, Growth Performance, Blood Metabolites, and mRNA Expression of Heat Shock Proteins in Heat-Stressed Fattening Hanwoo Steers. Vet. Sci. 2022, 9, 220. [Google Scholar] [CrossRef]
- Eom, J.S.; Kim, E.T.; Kim, H.S.; Choi, Y.Y.; Lee, S.J.; Lee, S.S.; Kim, S.H.; Lee, S.S. Metabolomics comparison of rumen fluid and milk in dairy cattle using proton nuclear magnetic resonance spectroscopy. Anim. Biosci. 2021, 34, 213. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Whelan, J.A.; Russell, N.B.; Whelan, M.A. A method for the absolute quantification of cDNA using real-time PCR. J. Immunol. Methods 2003, 278, 261–269. [Google Scholar] [CrossRef]
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Karnati, S.K.; Yu, Z.; Morrison, M.; Firkins, J.L. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J. Nutr. 2004, 134, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-F.; Cao, W.-W.; Cerniglia, C.E. PCR detection ofRuminococcusspp. in human and animal faecal samples. Mol. Cell. Probes 1997, 11, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.M.; Weimer, P.J. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl. Microbiol. Biotechnol. 2007, 75, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef] [PubMed]
- Scheibler, R.; Schafhäuser, J.; Rizzo, F.; Nörnberg, J.; Vargas, D.; Silva, J.; Fluck, A.; Fioreze, V. Replacement of corn grain by brown rice grain in dairy cow rations: Nutritional and productive effects. Anim. Feed Sci. Technol. 2015, 208, 214–219. [Google Scholar] [CrossRef]
- White, T.; Sticker, L.; Hembry, F.; Saxton, A. Influence of fish meal level in rice-or corn-based diets for finishing steers. Prof. Anim. Sci. 1991, 7, 42–46. [Google Scholar] [CrossRef]
- Zenobi, M.; Lardner, H.; Jefferson, P.; McKinnon, J. Effect of feeding strategically blended feed pellets on rumen fermentation and nutrient digestion. Can. J. Anim. Sci. 2015, 95, 243–254. [Google Scholar] [CrossRef]
- Ha, J.; Seo, S.; Kim, C.; Moon, Y.; Baik, M.; Lee, S.; Lee, S.Y.; Lee, Y.; Jang, J.; Choi, N. Ruminant Nutrition and Physiology; Seoul National University Press: Seoul, Republic of Korea, 2013. [Google Scholar]
- Kwon, E.; Hong, S.; Seong, H.; Yun, S.; Park, B.; Cho, Y.; Cho, W.; Chang, S.; Shin, K.; Paek, B. Effects of ad libitum and restricted feeding of concentrates on body weight gain, feed intake and blood metabolites of Hanwoo steers at various growth stages. J. Anim. Sci. Techol. 2005, 47, 745–758. [Google Scholar]
- Ishiwata, T.; Uetake, K.; Abe, N.; Eguchi, Y.; Tanaka, T. Effects of an environmental enrichment using a drum can on behavioral, physiological and productive characteristics in fattening beef cattle. Anim. Sci. J. 2006, 77, 352–362. [Google Scholar] [CrossRef]
- Huntington, G.B.; Zetina, E.J.; Whitt, J.M.; Potts, W. Effects of dietary concentrate level on nutrient absorption, liver metabolism, and urea kinetics of beef steers fed isonitrogenous and isoenergetic diets. J. Anim. Sci. 1996, 74, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Z.; Leng, J.; Heng, W.; Shen, R.; Gong, X.; Chen, C.; Wang, Y.; Zhang, R.; Li, W. 20 (R)-ginsenoside Rg3, a product of high-efficiency thermal deglycosylation of ginsenoside Rd, exerts protective effects against scrotal heat-induced spermatogenic damage in mice. Biocell 2020, 44, 655. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, K.H.; Nam, I.S.; Kim, W.Y.; Yeo, J.M.; Lee, S.S.; Ju, J.C.; Oh, Y.K. Comparison of blood metabolites and enzyme activities at different slaughter ages of Hanwoo cattle. J. Anim. Sci. Techol. 2012, 54, 443–448. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.; Nam, I.; Lee, S.; Choi, C.; Kim, W.; Kwon, E.; Lee, K.; Lee, M.; Oh, Y. Effect of indigenous herbs on growth, blood metabolites and carcass characteristics in the late fattening period of Hanwoo steers. Asian-Australas. J. Anim. Sci. 2013, 26, 1562. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ramos, S.C.; Jeong, C.D.; Mamuad, L.L.; Park, K.K.; Cho, Y.I.; Son, A.; Lee, S.-S. Growth performance and blood profiles of Hanwoo steers at fattening stage fed Korean rice wine residue. J. Anim. Sci. Techol. 2020, 62, 812. [Google Scholar] [CrossRef]
- Cotta, M.A. Amylolytic activity of selected species of ruminal bacteria. Appl. Environ. Microbiol. 1988, 54, 772–776. [Google Scholar] [CrossRef]
- Forsberg, C.W.; Cheng, K.-J.; White, B.A. Polysaccharide degradation in the rumen and large intestine. In Gastrointestinal Microbiology: Volume 1 Gastrointestinal Ecosystems and Fermentations; Springer: Boston, MA, USA, 1997; pp. 319–379. [Google Scholar]
- Kopečný, J.; Zorec, M.; Mrazek, J.; Kobayashi, Y.; Marinšek-Logar, R. Butyrivibrio hungatei sp. nov. and Pseudobutyrivibrio xylanivorans sp. nov., butyrate-producing bacteria from the rumen. Int. J. Syst. Evol. Microbio. 2003, 53, 201–209. [Google Scholar] [CrossRef]

| Items | Corn TMR | Rice TMR |
|---|---|---|
| Ingredients (% DM) | ||
| Commercial concentrate mix 1 | 36.0 | 36.0 |
| Corn flake | 33.0 | 0 |
| Rice grain | 0 | 33.0 |
| Timothy | 23.5 | 23.5 |
| Alfalfa | 7.0 | 730 |
| Vitamin and mineral mix 2 | 0.5 | 0.5 |
| Chemical composition 3 | ||
| DM (% as fed) | 65.0 | 65.0 |
| CP (% DM) | 14.9 | 14.8 |
| aNDF (% DM) | 30.6 | 29.5 |
| ADF (% DM) | 19.5 | 18.5 |
| Lignin (% DM) | 4.45 | 4.17 |
| EE (% DM) | 3.50 | 3.19 |
| Ash (% DM) | 5.22 | 5.42 |
| TDN (% DM) | 72.4 | 73.8 |
| NEm (Mcal/kg of DM) | 1.67 | 1.71 |
| Target Species | Primer | Sequence (5′ → 3′) | Size (bp) | Efficiency 1 | References |
|---|---|---|---|---|---|
| General bacteria | F | CGGCAACGAGCGCAACCC | 130 | 1.992 | [16] |
| R | CCATTGTAGCACGTGTGTAGCC | ||||
| Ciliate protozoa | F | GCTTTCGWTGGTAGTGTATT | 223 | 1.947 | [17] |
| R | CTTGCCCTCYAATCGTWCT | ||||
| Fungi | F | GAGGAAGTAAAAGTCGTAACAAGGTTTC | 120 | 2.079 | [16] |
| R | CAAATTCACAAAGGGTAGGATGATT | ||||
| Fibrobacter succinogenes | F | CCCTAAAAGCAGTCTTAGTTCG | 121 | 1.925 | [16] |
| R | CCTCCTTGCGGTTAGAACA | ||||
| Ruminococcus albus | F | CCCTAAAAGCAGTCTTAGTTCG | 176 | 2.067 | [18] |
| R | CCTCCTTGCGGTTAGAACA | ||||
| Ruminococcus flavefaciens | F | CGAACGGAGATAATTTGAGTTTACTTAGG | 132 | 2.029 | [16] |
| R | CGGTCTCTGTATGTTATGAGGTATTACC | ||||
| Butyrivibrio fibrisolvens | F | ACCGCATAAGCGCACGGA | 65 | 2.006 | [19] |
| R | CGGGTCCATCTTGTACCGATAAAT | ||||
| Streptococcus bovis | F | TTCCTAGAGATAGGAAGTTTCTTCGG | 127 | 2.005 | [20] |
| R | ATGATGGCAACTAACAATAGGGGT | ||||
| Selenomonas ruminantium | F | GGCGGGAAGGCAAGTCAGTC | 83 | 2.188 | [20] |
| R | CCTCTCCTGCACTCAAGAAAGACAG | ||||
| Prevotella ruminicola | F | GCGAAAGTCGGATTAATGCTCTATG | 78 | 1.946 | [20] |
| R | CCCATCCTATAGCGGTAAACCTTTG |
| Items | Corn TMR | Rice TMR | SEM | p-Value |
|---|---|---|---|---|
| Initial BW (kg) | 488 | 506 | 5.8 | 0.13 |
| Final BW (kg) | 559 | 583 | 8.0 | <0.01 |
| ADG (g/d) | 786 | 867 | 62.2 | 0.21 |
| DMI (kg/d) | 8.00 | 8.99 | 0.059 | <0.01 |
| FCR | 10.6 | 10.7 | 0.73 | 0.99 |
| Items | Corn TMR | Rice TMR | SEM | p-Value |
|---|---|---|---|---|
| TVFA (mM) | 54 | 59 | 5.5 | 0.32 |
| Acetate (mmol/mol) | 633 | 622 | 15.9 | 0.50 |
| Propionate (mmol/mol) | 181 | 168 | 8.0 | 0.11 |
| Butyrate (mmol/mol) | 121 | 149 | 6.4 | <0.01 |
| A:P ratio | 3.55 | 3.88 | 0.201 | 0.11 |
| NH3-N (mg/dL) | 7.12 | 7.33 | 0.668 | 0.76 |
| pH | 7.13 | 7.01 | 0.100 | 0.22 |
| Items | Corn TMR | Rice TMR | SEM | p-Value |
|---|---|---|---|---|
| ALT (U/L) | 22.7 | 22.3 | 0.83 | 0.66 |
| Alb (g/dL) | 3.56 | 3.57 | 0.028 | 0.72 |
| AST (U/L) | 70.7 | 79.5 | 3.86 | <0.05 |
| BUN (mg/dL) | 15.5 | 17.9 | 0.43 | <0.01 |
| Ca (mg/dL) | 10.3 | 10.3 | 0.08 | 0.29 |
| Crea (mg/dL) | 1.35 | 1.32 | 0.003 | 0.39 |
| Glu (mg/dL) | 72.1 | 75.8 | 2.70 | 0.18 |
| IP (mg/dL) | 7.45 | 7.32 | 0.186 | 0.50 |
| Mg (mg/dL) | 2.33 | 2.28 | 0.041 | 0.19 |
| T-Chol (mg/dL) | 130.4 | 118.7 | 5.95 | 0.54 |
| TG (mg/dL) | 18.6 | 18.9 | 1.46 | 0.82 |
| TP (g/dL) | 6.94 | 7.10 | 0.084 | 0.07 |
| Items | Corn TMR | Rice TMR | SEM | p-Value |
|---|---|---|---|---|
| Absolute abundance 1 | ||||
| General bacteria | 6.24 | 6.38 | 0.910 | 0.88 |
| Ciliate protozoa | 3.35 | 3.74 | 0.958 | 0.69 |
| Fungi | 5.40 | 5.96 | 1.059 | 0.60 |
| Relative abundance (% General bacteria) | ||||
| Ruminococcus albus | 0.35 | 0.33 | 0.044 | 0.76 |
| Fibrobacter succinogenes | 3.14 | 3.75 | 0.575 | 0.29 |
| Ruminococcus flavefaciens | 0.14 | 0.10 | 0.032 | 0.17 |
| Butyrivibrio fibrisolvens | 0.065 | 0.033 | 0.0074 | <0.01 |
| Streptococcus bovis | 0.018 | 0.022 | 0.0024 | 0.09 |
| Selenomonas ruminantium | 2.35 | 2.09 | 0.260 | 0.34 |
| Prevotella rumnicola | 6.75 | 7.58 | 0.707 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, D.; Yang, S.; Kim, H.; Moon, J.; Seo, J. Effects of the Use of Rice Grain on Growth Performances, Blood Metabolites, Rumen Fermentation, and Rumen Microbial Community in Fattening Hanwoo Steers. Animals 2023, 13, 2988. https://doi.org/10.3390/ani13182988
Yoo D, Yang S, Kim H, Moon J, Seo J. Effects of the Use of Rice Grain on Growth Performances, Blood Metabolites, Rumen Fermentation, and Rumen Microbial Community in Fattening Hanwoo Steers. Animals. 2023; 13(18):2988. https://doi.org/10.3390/ani13182988
Chicago/Turabian StyleYoo, Daekyum, Sungjae Yang, Hanbeen Kim, Joonbeom Moon, and Jakyeom Seo. 2023. "Effects of the Use of Rice Grain on Growth Performances, Blood Metabolites, Rumen Fermentation, and Rumen Microbial Community in Fattening Hanwoo Steers" Animals 13, no. 18: 2988. https://doi.org/10.3390/ani13182988
APA StyleYoo, D., Yang, S., Kim, H., Moon, J., & Seo, J. (2023). Effects of the Use of Rice Grain on Growth Performances, Blood Metabolites, Rumen Fermentation, and Rumen Microbial Community in Fattening Hanwoo Steers. Animals, 13(18), 2988. https://doi.org/10.3390/ani13182988






