Simple Summary
Normal feed intake and efficient nutrient absorption are prerequisites for achieving rapid growth, high body weight and dimensions, high carcass and meat yield, and feed efficiency in chickens. Gut hormones released from enteroendocrine cells (EECs) have been recognized as regulators for appetite as well as nutrient absorption in other species. However, the underlying regulation mechanisms of appetite control and nutrient absorption by gut hormones are not fully understood in chickens. This review suggests that gut hormones released from EECs play important roles in appetite and nutrient absorption, and these hormones are considered to be able to influence the reduction of feed intake by infection (e.g., Eimeria spp. and Salmonella spp.) and environmental stresses (e.g., heat stress and high stock density).
Abstract
This review focuses on the role of hormones derived from enteroendocrine cells (EECs) on appetite and nutrient absorption in chickens. In response to nutrient intake, EECs release hormones that act on many organs and body systems, including the brain, gallbladder, and pancreas. Gut hormones released from EECs play a critical role in the regulation of feed intake and the absorption of nutrients such as glucose, protein, and fat following feed ingestion. We could hypothesize that EECs are essential for the regulation of appetite and nutrient absorption because the malfunction of EECs causes severe diarrhea and digestion problems. The importance of EEC hormones has been recognized, and many studies have been carried out to elucidate their mechanisms for many years in other species. However, there is a lack of research on the regulation of appetite and nutrient absorption by EEC hormones in chickens. This review suggests the potential significance of EEC hormones on growth and health in chickens under stress conditions induced by diseases and high temperature, etc., by providing in-depth knowledge of EEC hormones and mechanisms on how these hormones regulate appetite and nutrient absorption in other species.
1. Introduction
One of the many functions of gut hormones secreted before and after feeding is to give signals to the brain that regulate feed intake [1,2]. Numerous studies have reported that gut hormones (e.g., ghrelin, cholecystokinin, CCK, peptide YY, PYY, glucagon-like peptide-1, and GLP-1) induce substantial change in feed intake [3,4]. In addition, certain gut hormones help to digest feed and absorb nutrients by acting on the endothelial cells and gastrointestinal epithelium [5]. Although the absorption of carbohydrates, protein, and fat has been persistently investigated in many studies, it is not clearly shown how the gut hormones work for nutrient absorption [6,7,8]. Mellitzer et al. [9] and Beucher et al. [10] reported that the absence of enteroendocrine cells (EECs) in mice resulted in failed fat absorption, thereby affecting body weight and survival rate. Likewise, McCauley [5] indicated that loss of all EECs can cause chronic malabsorptive diarrhea. These findings suggest that gut hormones are closely involved in nutrient absorption [11,12]. The understanding of the effects of gut hormones on feed intake regulation and nutrient absorption has been established mostly in humans, rodents, and/or other mammals such as pigs and cattle. However, the physiological roles of gut hormones in poultry differ from those of other species [13]. Therefore, in the current review, we discuss the overall mechanisms of these hormones in controlling feed intake and sensing nutrients in broilers. Additionally, this review provides an overview of the process of the reduction of feed intake and weight gain by several infections (e.g., Salmonella, Eimeria spp., and Clostridium perfringens).
2. Structure of Enteroendocrine Cells Secreting Gut Hormone
Enteroendocrine Cells
Enteroendocrine cells (EECs) are dispersed in villi and crypts throughout the intestinal tract [14,15]. They are located with nonendocrine cells such as absorptive enterocytes, goblet cells, stem cells, and paneth cells (Figure 1) [16] and are also considered the largest endocrine cells relative to the total number of cells, although they occupy less than 1% of the epithelial cell population [16,17]. EECs have been identified and classified by hormone contents (e.g., I cell, K cell, L cell, N cell, S cell, etc.) [18,19]. An overview of the different types of EECs, hormones, and their brief functions are presented in Table 1. The primary function of hormones derived from EECs is to regulate various metabolic responses including interaction with nutrient transporters in brush border and different receptors in the central nervous system (CNS) following feed ingestion [20,21,22]. Therefore, this review provides the mechanisms and effects of gut hormones on feed intake and nutrient absorption according to the hormones released from EECs.
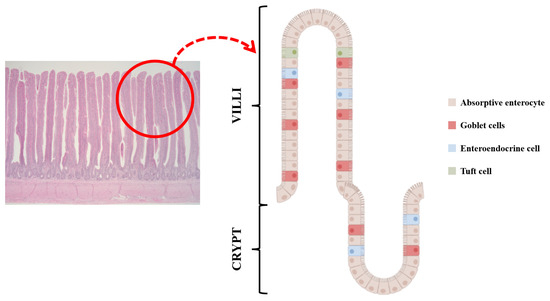
Figure 1.
Enteroendocrine cells (EECs) are embedded with other cells such as absorptive enterocytes, goblet cells, stem cells, paneth cells, and tuft cells in intestinal epithelial cells. Along with released hormones, their cell type changes and they are also associated with appetite and nutrient absorption.

Table 1.
Overview of the different types of enteroendocrine cells, secreted peptides, and their brief function.
3. Feed Intake Control: Mode of Action
3.1. Orexogenic Effects
The mechanism of appetite can mainly be divided into two kinds of effects: orexigenic and anorexigenic effects (Figure 2). The orexigenic effect is related to the increased feed intake and is mitigated by the central and peripheral nervous systems. The arcuate nucleus (ARC) and the lateral hypothalamus (LH) play an important role in appetite regulation. Neuropeptide Y (NPY) and agouti-related protein (AgRP) neurons released from the ARC are called orexigenic neurons [23,24]. The secretion of gamma-aminobutyric acid (GABA) as a neurotransmitter is stimulated by NPY and AgRP, and GABA regulates orexigenic effects by inhibiting anorexigenic neurons [23,25]. Moreover, orexin and melanin-concentrating hormones (MCH) are involved in appetite regulation, although the effect of orexin is inconsistent according to the results of previous studies [26,27] reporting that orexin did not affect the feed intake in broilers.
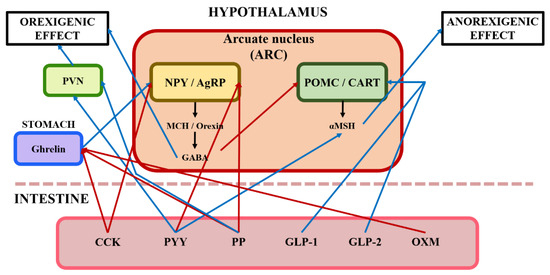
Figure 2.
Illustration of the central nervous system's response to gut hormones from enteroendocrine cells in the intestine. Blue arrows represent that it stimulates pathways of orexigenic effect/anorexigenic effect; Red arrows represent that it inhibits pathways of orexigenic effect/anorexigenic effect; CCK, cholecystokinin; PYY, peptide YY; PP, pancreatic polypeptide; GLP, pancreatic polypeptide; OXM, oxyntomodulin; PVN, paraventricular nucleus; NPY, neuropeptide Y; AgRP, agouti-related protein; POMC, pro-opiomelanocortin; CART, cocaine- and amphetamine-regulated transcript; MCH, melanin-concentrating hormone; GABA, gamma-aminobutyric acid; α-MSH, alpha-melanocyte-stimulating hormone.
3.2. Anorexogenic Effects
The anorexigenic effect causes feed intake reduction mitigated by central and peripheral nervous systems (Figure 2). Pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) neurons produced from the ARC are called anorexigenic neurons [24,27,28]. Anorexigenic effects have two distinct mechanisms. Firstly, GABA released by NPY and AgRP neurons inhibits the generation of POMC and CART neurons. Secondly, POMC and CART neurons release the alpha-melanocyte-stimulating hormone (α-MSH), leading to feed intake reduction [27].
4. Hormones from Enteroendocrine Cells on Feed Intake Control
4.1. Ghrelin
Ghrelin is the only known orexigenic gut hormone and hunger hormone [29]. It is an amino acid peptide hormone produced by the EECs in the oxyntic glands in the stomach fundus [30]. Ghrelin binds to G protein-coupled receptors (e.g., growth hormone secretagogue receptor) that are located in areas of the brainstem, the pituitary gland, and the hypothalamus [31,32,33]. During fasting, the ghrelin levels increase nearly two times just prior to meals and then decrease to the lowest value after meals; in addition, ghrelin intravenous injections increase appetite and food intake in humans [34,35]. In rodents, ghrelin injections via intracerebroventricular (ICV) and intraperitoneal (IP) routes also stimulated feed intake and appetite and increased weight gain (Table 2) [29,36]. The possible orectic mechanism of ghrelin is as follows: Feed intake is tightly regulated by the ARC of the hypothalamus [37]. In response to ghrelin binding to growth-hormone secretagogue (GHS) receptors, protein kinase A (PKA) triggers the AMP-activated protein kinase (AMPK). By phosphorylating AMPK, ghrelin stimulates orexigenic NPY and AgRP secretion in the ARC of the hypothalamus [38,39]. As a result, ghrelin increases AMPK and NPY/AgRP and thereby feed intake is increased in mammals. Furthermore, the brainstem, via the vagus nerve, could be a potential extrahypothalamic location to release ghrelin [33]. It has been suggested that the stomach and the nucleus of the solitary tract (NTS) of the brainstem, which has outputs to the ARC, are connected via vagal afferents [33,38,39]. However, the orexigenic effects of ghrelin in avian species have shown controversial results. For example, some studies showed that increased ghrelin levels in blood-induced anepithymia [40], whereas other studies reported that ghrelin injection into intravascular (IV) and IP routes decreased or did not alter feed intake in broilers and laying hens, respectively [41,42,43,44]. The IP injection of 0.5–1 nmol/bird of ghrelin increased feed intake, while 3 nmol/bird and ICV injection of 0.5–1.0 nmol/bird decreased feed intake in Japanese quail [45]. IV administration of acyl-ghrelin at 1 nM/100 g BW/d suppressed feed intake, whereas des-acyl-ghrelin improved feed intake [46]. These findings suggest that these inconsistent outcomes may be attributed to the differences in doses, forms (e.g., acyl- and des-acyl-ghrelin), and injection routes. In addition, different outcomes between mammals and avian species may be due to the activation of AMPK. Xu et al. [47] reported that administration of ghrelin in chickens down-regulated AMPK and decreased appetite. Although all studies mentioned above did not measure AMPK activation and feed intake at the same time, ghrelin injection in chickens showed the opposite results such as reduction of AMPK, unlike studies with other species reporting that ghrelin activates AMPK and then stimulates NPY and AgRP in ARC thereby activating feed intake. However, there is a limitation in previous studies that only estimated the effects of artificial ghrelin injection on feed intake and appetite. Therefore, further research is needed to determine whether endogenous ghrelin suppresses feed intake in avian species.

Table 2.
Effects of functions and/or effects according to dosage and location of ghrelin injection.
4.2. Peptide YY (PYY)
Peptide YY belongs to the PP fold protein family, which also includes NPY and PP [33]. It is a 36 amino acid peptide and is derived from the L cells of the gastrointestinal tract, particularly those in the colon and rectum [48]. PYY1-36 is divided at the last two amino acids of the N-terminus by DPP-IV, resulting in the truncated form PYY3-36 [49]. However, the major form of PYY is PYY3-36 in animals and humans [50,51]. During fasting, PYY levels are low but reach a peak within a few hours after a meal [48]. The effects of PYY are different depending on the injection location (Table 3). For example, ICV injection of PYY1-36 and PYY3-36 had orexigenic effects [52,53,54,55]. Interestingly, PYY via intra-arcuate (IA) injection showed anorexigenic effects in rodents and humans [51]. At the same time, PYY3-36 injection into IV decreased the feed intake in rodents [51,56]. PYY binds preferentially to the NPY Y2 receptor (Y2R) among the isoform receptors (e.g., Y1, Y2, Y4, Y5, and Y6) and is mainly found on NPY neurons of the ARC and NTS [57,58,59,60]. Feed intake was inhibited after the administration of a selective Y2R agonist as well as direct injection of PYY3-36 into the ARC [61]. The inhibitory effect of peripheral PYY3-36 is reduced in rats treated with Y2R antagonists and abolished in knockout mice for Y2R (Table 3) [51,62]. The anorectic mechanism of PYY3-36 via IV injection can be explained as follows: PYY3-36 inhibits NPY neurons with consequent disinhibition of POMC neurons in the ARC. Additionally, hypothalamic explants incubated in vitro with PYY3-36 resulted in a decrease in NPY release and an increase in α-MSH release [51]. However, orexigenic effects by ICV injection of PYY may suggest that PYY binds Y1R and Y5R, which are mainly expressed in the hypothalamic paraventricular nucleus (PVN) [63]. Similar results were observed in studies with chickens. In chickens, PYY isolated from the small intestine did not contain PYY3-36, the major form of PYY in mammals [64]. ICV injection of PYY1–36 stimulated feed intake in neonatal chickens [65], whereas PYY3-36 administration via IV injection inhibited feed intake in neonatal chickens [66]. These inconsistent results may be attributed to the differences in doses and injection routes. Moreover, the main sites where PYY mRNA is expressed are different depending on the species [67]. In broilers, sites where PYY mRNA is mainly expressed are present in the small intestine, while sites where PYY mRNA is expressed are located mainly in the large intestine in mammals [67,68]. Because most of the studies have been performed with the administration of exogenous PYY [69], further research is needed to determine how to exert the endogenous PYY in animals including avian species.

Table 3.
Effects of functions and/or effects according to dosage and location of peptide YY (PYY) injection.
4.3. Glucagon-like Peptide (GLP)
GLP is released in response to food intake from the L-cells of the small intestine and colon, the neurons in the NTS of the brainstem, and the α-cells of the Islets of Langerhans [70,71,72]. Also, pre-proglucagon, a precursor of GLP, is converted to glucagon, glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2), and oxyntomodulin (OXM), depending on the sites of syntheses by prohormone convertase 1 and 2 [73]. Especially, in mammals and chickens, GLP-1 and GLP-2 brain-gut peptides are produced by cleavage of the precursor preproglucagon [74].
4.3.1. Glucagon-like Peptide-1 (GLP-1)
Glucagon-like peptide-1 (GLP-1) is synthesized and secreted from the L-cells in the intestinal epithelium [75,76]. A key function of GLP-1 is to regulate blood glucose levels by increasing glucose-stimulated insulin secretion and decreasing glucagon secretion [77]. Several studies reported that GLP-1 had a negative effect on feed intake of experimental animals [74,78,79]. For example, feed intake of rats and mice was reduced by ICV and IP injection of GLP-1 [78,80,81], and increased feed intake was induced by ICV injection of the GLP-1 receptor antagonist (Table 4) [78,82,83]. Furthermore, a study on humans showed the anorectic effects of administrating GLP-1, thereby causing reduced feed intake [84]. Actually, obese subjects have shown reduced levels of GLP-1 and higher weight gain [85,86]. A central nervous system component plays a role in the regulation of GLP-1, and GLP-1 is especially affected by GLP-1 receptor (GLP-1R) [87,88]. GLP-1R is abundantly detected in the hypothalamus (i.e., the ARC). This fact may be supported by the result of a previous study reporting that ICV injection of GLP-1 increased the expression of c-fos in the ARC [89]. It means that GLP-1 has the potential to act directly on the ARC in the hypothalamus. GLP-1R is also synthesized by the POMC neurons and GLP-1R agonists that exert direct as well as indirect effects on these neurons [90,91]. GLP-1R expressed in POMC neurons also indirectly affects NPY neurons [88]. According to the results of Secher et al. [92], they observed that GLP-1R restricts the synapses of NPY/AgRP neurons by influencing GABAergic neurons. As a result, GLP-1 suppressed NPY and AgRP expression, which promotes appetite, and increased POMC expression, which alleviates appetite [93]. The anorexigenic effect of GLP-1 can be explained by incretin production. Incretin is a hormone originally secreted from the digestive system while nutrients are absorbed in the intestinal tract [76]. It also increases the release of insulin and inhibits the movement of the gastrointestinal tract, causing suppressed feed intake. In chickens and ducks, GLP-1 is released from L cells in the epithelium of the jejunum and ileum [94,95,96]. This result is in agreement with the results of studies on other species reporting that GLP-1 was secreted from L cells in the intestines of mammals [71,97]. Several studies demonstrated that the anorectic effects of GLP-1 were detected in poultry as well. GLP-1 regulates the emptying of the crop and strongly reduces feed intake in chickens [98,99]. ICV and IP injection of GLP-1 significantly reduced the feed intake in laying hens and Japanese quail, respectively [100]. On the other hand, the injection of GLP-1 via IP and ICV did not affect the feed intake of laying hens and broilers [98,101]. GLP-1 levels in the blood did not fluctuate for fasting and refeeding periods in broilers [102]. In chickens, the GLP-1 receptor mRNA is widely detected in the brain as well as the gastrointestinal tract [103]. Although the role of GLP-1 in the regulation of feed intake in chickens has not been elucidated yet, the anorectic effect of GLP-1 in chickens is considered to be similar to the mechanism mentioned above in other species. It is necessary to conduct further research to determine the physiological importance of GLP-1 among various gut hormones in birds.

Table 4.
Effects of functions and/or effects according to dosage and location of glucagon-like peptide-1 (GLP-1) injection.
4.3.2. Glucagon-like Peptide-2 (GLP-2)
In the L cells of the intestine, GLP-2 is co-encoded and co-stored with GLP-1 by the gene that encodes proglucagon [104]. GLP-2 has been known to play a physiological role in mammals as an intestinal growth factor [74,105]. GLP-2, like GLP-1, has various actions in distal tissues, such as regulation of appetite, inhibition of gastric emptying, and reduction of bone resorption [106,107,108]. The process of gastric emptying is critical for regulating short-term intake and may be used to modulate appetite. Previous studies reported that the anorexigenic effects of GLP-2 are closely related to gastric emptying (Table 5) [109]. Also, these actions are regulated by GLP-2 receptors that are present in the gut and brain [110]. Likewise, GLP-2 suppressed feeding behavior and activated POMC neurons in experimental animals when administered via ICV and IP [111,112,113,114]. In chickens, Honda et al. [115,116] found that central and peripheral injection of GLP-2 significantly suppressed feed intake. Moreover, Kewan et al. [117] reported that ICV injection of GLP-2 significantly improved POMC levels in the hypothalamus in layers. These findings suggest that GLP-2 exerts anorexigenic effects as GLP-1. However, another study reported that GLP-2 injection via ICV and IP did not affect feed intake and body temperature in Japanese quail [100]. GLP-2 regulation of feed intake in chickens has also not been explained yet and led to different results. Therefore, judging by these results, the anorexigenic effects of GLPs may differ depending on various factors, including animal species, dosage, and injection location of exogenous GLP-2. Further studies are required to elucidate the relationship between GLP-2 and anorexigenic effects.

Table 5.
Effects of functions and/or effects according to dosage and location of glucagon-like peptide-2 (GLP-2) injection.
4.4. Oxyntomodulin (OXM)
OXM is derived from the cleavage product of preproglucagon processed in endocrine L-cells of the intestine and CNS. After meal ingestion, OXM is co-secreted with the hormones GLP-1 and PYY3-36 [118]. Previous studies reported that the ICV and intranuclear injection of OXM reduced feed intake in rats (Table 6) [119,120]. In humans, OXM via intravenous (IV) injection caused reduced energy intake by 19.3%, and pre-prandial injection in obese subjects decreased body weight gain by 1.8 kg [121,122]. These findings supported that OXM is well established as a gut hormone having anorectic effects in rodents as well as humans. Although the anorectic mechanism of OXM has not been clearly elucidated, there are some hypotheses on the suppression of appetite as follows. The anorectic mechanism of OXM may be similar to that of GLP-1. Previous studies reported that the appetite suppression of OXM via IP injection was restricted when GLP-1 receptor antagonist exendin (9–39) was injected in the ARC and when the GLP-1 receptor was eliminated in mice [120,123]. Moreover, other studies have shown that OXM has an incretin effect, meaning that it multiplies glucose-dependent insulin secretion like GLP-1 [124,125]. Additionally, the anorectic mechanism of OXM may be explained by the reduction of ghrelin in blood. The administration of OXM showed a reduction of ghrelin by 44% and 15–20% in humans and rodents, respectively [120,121], suggesting that OXM may indirectly inhibit feed intake by reducing circulating ghrelin levels. In studies with chickens, OXM inhibited not only feed intake but also water intake in broilers and Japanese quail [126,127,128]. In laying hens, ICV injection of OXM dramatically reduced feed intake and increased plasma glucose concentration [127]. In addition, peripheral and intrahepatic glucose injection enhanced plasma glucose levels while suppressing feed intake in layer chicks and cockerels [129,130,131]. At least in the early post-hatch period, OXM and GLP-1 likely suppress feed intake through different mechanisms.

Table 6.
Effects of functions and/or effects according to dosage and location of oxyntomodulin (OXM) injection.
4.5. Cholecystokinin (CCK)
It was also discovered that cholecystokinin (CCK) affects appetite [132]. CCK is widely distributed across the gastrointestinal tract but is mainly synthesized in the I-cells of the duodenum and jejunum [133]. CCK is a local regulator in the gastrointestinal tract that stimulates gallbladder contraction and pancreatic enzyme secretion and inhibits gastric emptying (Table 7) [134,135]. Within 15 min of starting a meal, plasma CCK levels rise [134]. Studies in humans have shown that CCK reduces food intake and meal size [136]. Additionally, CCK has also been evaluated for its therapeutic potential in the treatment of obesity. CCK-A may be a more important receptor in the regulation of food intake and is found in the pancreas, vagal afferent, efferent neurons, nucleus of the solitary tract, and area postrema in the brainstem [137]. There has been evidence that abdominal or gastric vagotomy can block the satiety effect of peripherally administered CCK, suggesting that the CCK-A receptors on the vagus nerve may play an important role in the effect of CCK on food intake [138]. Actually, the administration of a CCK-A antagonist inhibited the reduction of feed intake by CCK in rats [139,140]. This finding is similar to the results of studies with chickens. For example, previous researchers reported that the administration of CCK in chickens restricted feed intake [141] and suppression of CCK-A receptors increased growth and body weight [142]. However, the difference between other species and poultry is the location where CCK is expressed. In poultry, CCK is released from the small intestines of chickens [143] and from the small and large intestines of ducks [144,145]. Despite the distal small intestine being the primary production site for CCK mRNA in poultry, CCK is abundant in mammal’s proximal small intestines [2,146]. CCK receptors are also located in the brain and peripheral tissues like in other species [147,148].

Table 7.
Effects of functions and/or effects according to dosage and location of cholecystokinin (CCK) injection.
4.6. Gastric Inhibitory Polypeptide (GIP)
GIP was first reported in the study with dogs and is a 42 amino acid hormone. It is secreted from the K cell in the proximal small intestine (duodenum and jejunum) after the uptake of nutrients, including glucose, peptide, and fat [149,150]. In addition to glucagon-like peptide, GIP is classified to act as an incretin and can regulate feed intake (Table 8) [151,152]. Previous studies showed that IP and ICV injection of GIP decreased body weight gain and feed and water intake compared with mice challenged with saline [81,153]. These results clearly suggest that GIP is reduced during the hungry stage. However, there are not enough studies to elucidate the mechanisms of GIP and reduced feed intake in avian species. Therefore, further research is needed to determine whether GIP suppresses feed intake in avian species.

Table 8.
Effects of functions and/or effects according to dosage and location of gastric inhibitory polypeptide (GIP) injection.
4.7. Serotonin (5-Hydroxytrptamine, 5-HT)
About 95% of serotonin is secreted via the enterochromaffin (EC) cells in EECs. The function of serotonin is different depending on its central and peripheral sites because it does not pass into the blood-brain barrier [154]. Central serotonin plays a role in regulating food consumption by increasing POMC and CART expression within the ARC, thereby having anorexigenic effects [155,156]. More specifically, the anorexigenic effects of central serotonin are controlled by 5-HT receptor 2C (HTR2C). Tecott et al. [157] found that HTR2C absence in mice was associated with increased feed intake. According to a previous study, HTR2C appeared within POMC neurons, and thus increased central serotonin can stimulate POMC neurons that are involved in the suppression of appetite [158]. On the other hand, peripheral serotonin produced by L-tryptophan has orexigenic effects [159]. Numerous studies have reported that high tryptophan supplementation increased the synthesis of serotonin, thereby increasing feed intake in pigs [160,161]. Moreover, peripheral serotonin increased intestinal motility and reduced plasma leptin and adiponectin concentrations, which can decrease appetite [159]. In poultry, insufficient tryptophan has been known to decrease serotonin synthesis [162]. However, most studies have focused on stress mitigation and meat quality, but not feed intake. There are only a few studies on serotonin and feed intake in avian species. Therefore, further research is needed to determine whether serotonin suppresses feed intake in avian species.
4.8. Neurotensin (NTS)
NTS is released in response to nutrient ingestion, in particular to fat, regulates GI motility and pancreatic and biliary secretion, facilitates fat translocation, and acts as an incretin [163]. NTS has been reported as a hormone having anorectic effects and is co-secreted with PYY and GLP-1, which also have anorectic effects. Actually, systemic administration of PEGylated NTS, with an increased half-life, causes a sustained reduction in food intake coupled with increased hypothalamic POMC expression [164]. Moreover, other researchers reported that peripheral injection of NTS might positively influence weight reduction [165].
5. Regulation of Nutrient Absorption by Gut Hormone
5.1. Carbohydrate Absorption by EEC Hormones
The absorption of nutrients by the gut is critical to stimulate gut hormone secretion by monosaccharides, peptides, and lipids [166]. EECs can actively respond to the rate at which nutrients are absorbed apart from sensing nutrients in the gut lumen [166]. Ingested carbohydrates are broken down into monosaccharides by a combination of amylases from saliva and the pancreas and hydrolases from enterocytes [167]. Glucose absorption is mainly regulated by glucose transporter (GLUT2) and sodium-glucose cotransporter 1 (SGLT1) (Figure 3). GLUT2 helps glucose absorption by translocating to the brush border in the membrane following high luminal sugar concentrations [168]. In addition, it transports glucose out of the cell by facilitated diffusion. SGLT1 is mainly used as a glucose transporter for mixed meal conditions and carries one glucose or galactose molecule as well as two Na+ ions to intestinal epithelial cells [169]. SGLT1 is expressed in EECs and absorbs two Na+ ions and glucose, leading to the stimulation of EEC hormones (GLP-1, GIP, and GLP-2) [167]. In addition, gut hormones secreted from EECs are also involved in the regulation of glucose absorption. GIP affects glucose absorption in the small intestine and increases intracellular cAMP, suggesting that GIP may up-regulate SGLT1, which is partly regulated by cAMP [170,171]. Moreover, as a result of GIP injection, GLUT2 is stimulated to release glucose to the basolateral membrane and GLUT2 translocation is increased [172,173]. GLP-2 plays a more important role in intestinal glucose absorption along with GIP [171]. Ogawa et al. [174] reported that GLP-2 up-regulates the gene expression of SGLT1 in enterocytes, resulting in an increase in the Na+/glucose transport activity. In addition, GLP-2 increases the basolateral export of GLUT2, as well as the translocation of GLUT2 to the apical brush border, thereby resulting in an increase in glucose absorption [172,175]. However, the effects of GLP-1 secreted with GLP-2 vary among previous studies. There was a reduction in the secretion of GIP and GLP-1 by mice lacking SGLT1, while the inhibition of SGLT1 enhanced GLP-1 release in SGLT1 knockout mice [176,177]. There is another gut hormone exerting negative effects on glucose absorption in the small intestine. Serotonin (5-HT) stimulates water and ion secretion in the intestine and inhibits Na+/dependent galactose absorption [178]. CCK also down-regulates the localization of SGLT1 to the brush border, thereby causing a reduction in glucose absorption [5,179]. However, there are no studies evaluating the relationship between gut hormones and glucose absorption in avian species. Therefore, further research is needed to determine the relationship among glucose transporters, gut hormones, and nutrient digestibility in avian species.
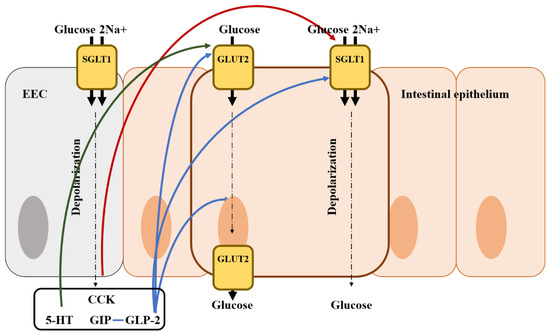
Figure 3.
Illustration of the mode of action of glucose regulation by gut hormones from an enteroendocrine cell in the intestine and the location of the glucose transporter. Blue arrows represent that it stimulates glucose transporter and absorption; Red arrows represent that it inhibits glucose transporter and absorption; Green arrows represent that it may stimulate glucose transporter and absorption; EEC, enteroendocrine cell; CCK, cholecystokinin; GIP, gastric inhibitory polypeptide; 5-HT, 5-hydroxytryptamine (serotonin).
5.2. Fat Absorption by EEC Hormones
Fat absorption is regulated by various gut hormones. Mice lacking CCK showed reduced weight gain and triglyceride absorption [180]. CCK is known to regulate the secretion of bile, bicarbonate, and pancreatic enzymes that play a role in emulsifying and hydrolyzing dietary fats (Figure 4) [181]. Incretins (e.g., GLP-1 and GIP) also regulate fat absorption, including an increase in insulin secretion in β-cells and a reduction of blood glucose [182,183].
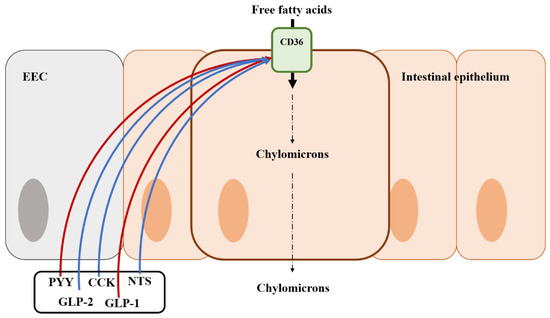
Figure 4.
Illustration of the mode of action of fat regulation by gut hormones from an enteroendocrine cell in the intestine and the location of the fat transporter. Blue arrows represent that it stimulates the fat transporter or absorption; Red arrows represent that it inhibits the fat transporter or absorption; EEC, enteroendocrine cell; CK, cholecystokinin; GLP-1, Glucagon-like peptide 1; GLP-2, Glucagon-like peptide 2; PYY, peptide YY; NTS, Neurotensin.
Lipid transport proteins are fatty acid binding proteins and CD36, expressed in EECs, has been known as a regulator of various EEC hormones in response to fat ingestion [184,185]. Mice lacking CCK showed poor triglyceride absorption although this appeared to be independent of pancreatic enzyme secretion [5]. These findings were related to CD36 expressed in EECs. CD36 deficiency remarkably caused a reduction of CCK and secretin in experiments [184]. Therefore, CD36 can be a receptor of secretin and CCK, increasing fat absorption in the small intestine [186]. Interestingly, GLP-1 restricts the expression of CD36 but can help fat digestion via activating protein kinase A (PKA) in humans and rodents [187,188]. On the other hand, GLP-2, co-secreted with GLP-1, improves fat absorption via the up-regulation of the expression of CD36 [189]. Neurotensin (NTS) is also involved in fat absorption. Previous studies reported that NTS enhanced fat absorption by GLP-2 [190,191]. Like GLP-1, PYY has negative effects on fat absorption. The treatment of exogenous PYY to intestinal cells inhibits apolipoprotein synthesis and chylomicron formation, which are important for fat absorption, in an in vitro experiment [192]. However, unlike other nutrient regulations, there are no studies to determine the exact mechanisms of fat regulation by gut hormones.
5.3. Protein Absorption by EEC Hormones
Ingested proteins are broken down into amino acids, dipeptides, and tripeptides by proteases in the GI tract [193]. The absorption of amino acids can be facilitated by the difference in H+ ion concentration, and peptide transporter 1 (PEPT1) is involved in most protein absorption [194]. In addition to the regulation of glucose absorption, amino acid absorption is also regulated via various gut hormones. Previous studies found that GIP improved dipeptide absorption via PEPT1 by increasing the activity of cAMP and phosphoinositide-3 kinase (Figure 5) [195]. GLP-2 also is known as a regulator of amino acid absorption [196,197,198]. This evidence could be supported by the results that GLP-2R knock-out mice had reduced amino acid uptake [199]. Conversely, GLP1, which is co-secreted with GLP-2, did not influence amino acid absorption via PEPT1 [200]. There are very few studies on protein absorption by EEC hormones compared with those on glucose and fat absorption. Therefore, it is considered that additional research is needed to determine the roles of EEC hormones on protein and amino acid absorption in poultry.
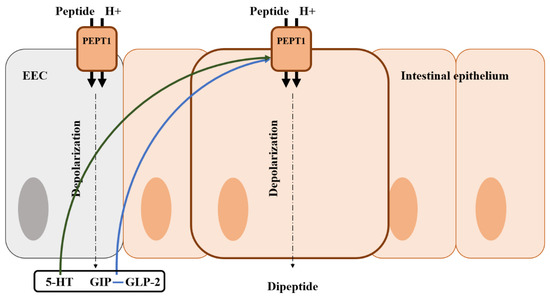
Figure 5.
Illustration of the mode of action of amino acid regulation by gut hormones from an enteroendocrine cell in the intestine and the location of the amino acid transporter. Blue arrows represent that it stimulates the peptide transporter and absorption; Green arrows represent that it may stimulate the peptide transporter and absorption; EEC, enteroendocrine cell; GIP, gastric inhibitory polypeptide; 5-HT, 5-hydroxytryptamine (serotonin).
6. Change of Feed Intake, Nutrient Absorption and Gut Environment in Chickens under Different Stress Conditions
6.1. Disease
6.1.1. Eimeria spp. Challenge
Eimeria spp. infects via the fecal-oral route and invades the epithelium of the intestine, thereby causing severe cell damage, diarrhea, impaired feed intake, and mortality [201,202,203]. E. maxima infection causes a severe reduction in feed intake due to sickness and lethargy in chickens [204]. Moreover, a meta-analysis study revealed that an Eimeria spp. infection decreased average daily feed intake by 20% [205]. Many studies showed that feed intake was decreased in broiler chickens challenged by Eimeria spp. [206,207,208]. The reduction in feed intake might be attributed to impaired nutrient absorption and intestinal cell functions by Eimeria infection. Chickens challenged by Eimeria spp. have shown severely damaged intestinal morphology, inflammation, and oxidative stress in many previous studies [209,210,211,212,213]. Chapman [202] reported that an Eimeria (e.g., E. maxima, E. acervuline, E. mitis, E. tenella) infection caused epithelial inflammation and disruption of the villi. Additionally, other researchers reported that Eimeria spp. oral inoculation resulted in epithelial damage of the small intestine by decreasing villus height (VH) and increasing crypt depth (CD) [214,215]. These results were attributed to the fact that Eimeria sporozoites and merozoites secrete proteins that can form a moving junction at the cell membrane [216,217]. By utilizing this moving junction, sporozoites and merozoites penetrate and damage intestinal epithelial cells and decrease nutrient absorption [218,219]. Previous experiments reported that Eimeria spp. increased ileal endogenous amino acid (AA), thereby reducing AA digestibility and decreasing ileal digestibility of dry matter, starch, and fat [202,220,221,222,223,224]. Also, an Eimeria infection significantly decreased the expression of amino acid and glucose transporters (e.g., APN, B°AT, b°,+ AT, EAAT3, PepT1, rBAT, GLUT2, and GLUT5) in the brush border of intestinal epithelium [223,224,225,226,227,228,229].
6.1.2. Pathogen Challenge
Several studies reported that an S. Typhimurium infection decreased FI, thereby reducing BWG and feed efficiency [230,231,232,233,234]. These results are in agreement with those of Moharreri et al. [235] who reported that other types of salmonella (e.g., S. enteritis) also reduced total feed intake and body weight in chickens. The reduced performance observed in the challenged birds is probably due to intestinal mucosal damage induced by S. Typhimurium [231]. According to the results of Jazi et al. [236] and Choi et al. [234], S. Typhimurium led to a remarkable reduction in VH and VH: CD after challenge. These results were confirmed by previous studies, which reported Salmonella spp. (e.g., S. Typhimurium and S. Enteritidis) reduced VH and the VH: CD ratio in the small intestine of broilers [235,237,238]. Birds infected with Salmonella spp. exhibited a damaged intestinal morphological structure and reduced goblet cell numbers in the jejunum, leading to impaired absorption of nutrients along with other pathogenic bacteria [235,239]. Likewise, a C. perfringens infection, which is a main factor in the outbreak of necrotic enteritis (NE), and an E. coli infection, which is a major source of Avian colibacillosis, caused the reduction of feed intake and nutrient digestibility along with impaired intestinal morphology (e.g., reduced VH, VW and the number of goblet cells) [240,241,242].
Pathogen-induced anorexia is well known and associated with most infections [243,244]. It could be caused by the high requirements of nutrient resources to restore damage and stimulate immune responses in response to infection [243]. Thus, since most of the feed intake is controlled via the brain-gut axis by appetite-related hormones, it is considered that the reduction of feed intake by infection is inevitably caused by changes in these gut hormones. However, although these infections by pathogenic bacteria and parasites undoubtedly cause the reduction of feed intake and nutrient absorption, there are not enough studies on the relationship between infection and appetite-related hormones. Therefore, it is considered that further research is needed to understand the underlying mechanism between infection and appetite-related hormones.
6.2. Environment
6.2.1. Heat Stress
Chickens exposed to heat stress showed significant reduction in body weight gain and feed intake [245]. Several studies reported that heat stress remarkably increased mortality and decreased feed intake in poultry [246,247,248]. Moreover, heat stress negatively affects nutrient digestibility, resulting in a reduction of DM and energy digestibility [249]. The impairment of nutrient digestibility by heat stress is supported by previous studies [250,251]. Heat stress also impaired nutrient digestibility as well as the nutrient transporters [252,253]. These findings were consistent with those of Orhan et al. [254] and Sun et al. [255], who reported that heat stress significantly decreased fatty acid binding protein (FABP) expression; binding fatty acids in the jejunum; SGLT1, which binds glucose; and PEPT1 and 2, which bind peptides in the ileum. These results were attributed to the damaged intestinal morphology, causing increased intestinal permeability to endotoxins regardless of animal species [256,257,258]. Moreover, many researchers detected that heat stress increased CD and decreased VH in the jejunum [246,247,250,259,260]. Goblet cells produce mucus to cover the intestinal epithelium, which protects it from pathogen attacks and environmental toxins. These cells also contribute to the healing of minor wounds and injuries to the epithelium. Liu et al. [260] reported that black-boned chickens exposed to heat stress showed significantly decreased goblet cell numbers in the jejunum and ileum. Similarly, Zhang et al. [261] showed that heat stress reduced the number of goblet cells and the mRNA level of the mucin-2 gene in the jejunum. Although we may expect that appetite-related hormones would not be secreted normally as various stresses damage the intestine, He et al. [262] and Wang et al. [263] found that heat stress increased CCK concentration in the serum and jejunum. On the other hand, He et al. [264] also found that heat stress caused increased ghrelin, which upregulates appetite along with CCK, which downregulates appetite in broiler chickens. Therefore, further research is needed because of these inconsistent results and there are not enough studies on the relationship between heat stress and appetite-related hormones.
6.2.2. Stocking Density
High stocking density resulted in a reduction in feed intake of broilers [265,266,267,268]. High stocking densities may cause reduced feed intake due to the high environmental temperature and the reduced airflow at the bird level [269]. Similar results have been reported by Uzum and Toplu [270], showing that birds housed at high stocking density were not able to effectively dissipate their body heat to the environment, resulting in feed intake reduction for maintenance of body homeostasis. Additionally, VHs in the duodenum, jejunum, and ileum were decreased when broilers were reared at high stock density [271,272]. Moreover, stress under high stock density induced the disruption of mucosal tight junction [273]. However, although high stock densities undoubtedly cause the reduction of feed intake, there are no studies on the relationship between high stocking density and feed intake hormones; thus, further research is needed.
7. Conclusions and Future Perspectives
Over the last three decades, there has been significant research achievement on the gut-brain axis, which has revealed a wealth of information about the role of gut hormones in the regulation of appetite and nutrient absorption in mammals and rodents. According to the increasing evidence of EECs, EECs have been found to directly affect the regulation of appetite and nutrient absorption. Many scientists have agreed that endocrine hormones released from EECs circulate in the blood and act on CNS targets in rodents and mammals including humans. Likewise, some studies have been conducted to evaluate the appetite regulation system of chickens in recent years. The physiological roles of PYY, CCK, GLP-1, and GLP-2 have been studied in chickens like other species. In other species, hormones released from EECs have also been reported to have significant effects on nutrient absorption, and the EEC number is changed by intestinal inflammation [274]. EEC cells were increased in some species such as fish, lamb, pigs, mice, and humans during the infection [21,275,276]. Intestinal epithelial cells damaged during infection try to recover to maintain intestinal homeostasis. In particular, GLP-1 is associated with the anorexigenic effect and GLP-2 is associated with epithelial homeostasis and barrier function, and repair following injury is increased after intestinal damage [277]. In many studies, chickens under stress conditions commonly had impaired intestinal morphology, resulting in reduced feed intake and nutrient digestibility. The structural integrity of the intestine is essential for efficient digestive function and nutrient absorption, which depends on the normal development of intestinal mucosa. Therefore, nutrient digestibility and feed intake could be negatively affected if intestines are damaged and an alternation of subsequent hormones happens. However, there have been few studies on nutrient absorption and EEC hormones in chickens. Therefore, further studies are essential to elucidate the relationship between EEC hormones, appetite regulation, and nutrient absorption in chickens. In conclusion, comprehensive studies on EEC hormones are essential to understand the regulation mechanism of appetite and nutrient absorption and to determine the physiological importance of each EEC hormone in chickens under stress conditions in the future.
Author Contributions
Conceptualization, J.L. and W.K.K.; writing—original draft preparation, J.L.; writing-review and editing, J.L. and W.K.K. All authors read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
No applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
The data presented in this study are available in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hussain, S.S.; Bloom, S.R. The regulation of food intake by the gut-brain axis: Implications for obesity. Int. J. Obes. 2013, 37, 625–633. [Google Scholar] [CrossRef]
- Honda, K.; Saneyasu, T.; Kamisoyama, H. Gut hormones and regulation of food intake in birds. J. Poult. Sci. 2016, 54, 160100. [Google Scholar] [CrossRef]
- Steinert, R.E.; Feinle-Bisset, C.; Geary, N.; Beglinger, C. Digestive physiology of the pig symposium: Secretion of gastrointestinal hormones and eating control. J. Anim. Sci. 2013, 91, 1963–1973. [Google Scholar] [CrossRef]
- Honda, K. Glucagon-related peptides and the regulation of food intake in chickens. Anim. Sci. J. 2016, 87, 1090–1098. [Google Scholar] [CrossRef]
- McCauley, H.A. Enteroendocrine regulation of nutrient absorption. J. Nutr. 2020, 150, 10–21. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Xiao, C.; Stahel, P.; Carreiro, A.L.; Buhman, K.K.; Lewis, G.F. Recent advances in triacylglycerol mobilization by the gut. Trends Endocrinol. Metab. 2018, 29, 151–163. [Google Scholar] [CrossRef]
- Auclair, N.; Melbouci, L.; St-Pierre, D.; Levy, E. Gastrointestinal factors regulating lipid droplet formation in the intestine. Exp. Cell. Res. 2018, 363, 1–14. [Google Scholar] [CrossRef]
- Mellitzer, G.; Beucher, A.; Lobstein, V.; Michel, P.; Robine, S.; Kedinger, M.; Gradwohl, G. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J. Clin. Investig. 2010, 120, 1708–1721. [Google Scholar] [CrossRef]
- Beucher, A.; Gjernes, E.; Collin, C.; Courtney, M.; Meunier, A.; Collombat, P.; Gradwohl, G. The homeodomain-containing transcription factors Arx and Pax4 control enteroendocrine subtype specification in mice. PLoS ONE 2012, 7, e36449. [Google Scholar] [CrossRef]
- Wang, J.; Cortina, G.; Wu, S.V.; Tran, R.; Cho, J.H.; Tsai, M.J.; Bailey, T.J.; Jamrich, M.; Ament, M.E.; Treem, W.R.; et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N. Engl. J. Med. 2006, 355, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Cortina, G.; Smart, C.N.; Farmer, D.G.; Bhuta, S.; Treem, W.R.; Hill, I.D.; Martín, M.G. Enteroendocrine cell dysgenesis and malabsorption, a histopathologic and immunohistochemical characterization. Hum. Pathol. 2007, 38, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Honda, K. Peripheral regulation of food intake in chickens: Adiposity signals, satiety signals and others. Worlds Poult. Sci. J. 2021, 77, 301–312. [Google Scholar] [CrossRef]
- Schonhoff, S.E.; Giel-Moloney, M.; Leiter, A.B. Minireview: Development and differentiation of gut endocrine cells. Endocrinology 2004, 145, 2639–2644. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; van Es, J.H.; Makrini, L.; Brulin, B.; Mellitzer, G.; Robine, S.; Romagnolo, B.; Shroyer, N.F.; Bourgaux, J.F.; Pignodel, C.; et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell. Biol. 2011, 192, 767–780. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Tovar, A.R.; Torres, N. Diet: Friend or foe of enteroendocrine cells: How it interacts with enteroendocrine cells. Adv. Nutr. 2012, 3, 8–20. [Google Scholar] [CrossRef]
- Jenny, M.; Uhl, C.; Roche, C.; Duluc, I.; Guillermin, V.; Guillemot, F.; Jensen, J.; Kedinger, M.; Gradwohl, G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002, 21, 6338–6347. [Google Scholar] [CrossRef]
- Rehfeld, J.F. A centenary of gastrointestinal endocrinology. Horm. Metab. Res. 2004, 36, 735–741. [Google Scholar] [CrossRef]
- Breer, H.; Eberle, J.; Frick, C.; Haid, D.; Widmayer, P. Gastrointestinal chemosensation: Chemosensory cells in the alimentary tract. Histochem. Cell. Biol. 2012, 138, 13–24. [Google Scholar] [CrossRef]
- Choi, S.; Lee, M.; Shiu, A.L.; Yo, S.J.; Halldén, G.; Aponte, G.W. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am. J. Physiol. Gastroint. Liver. Physiol. 2007, 292, G1366–G1375. [Google Scholar] [CrossRef]
- Moran, G.W.; Leslie, F.C.; Levison, S.E.; McLaughlin, J.T. Enteroendocrine cells: Neglected players in gastrointestinal disorders? Ther. Adv. Gastroenterol. 2008, 1, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.P.; Chavez, D.I.; Espero, E.; Hao, S.; Wank, S.A.; Raybould, H.E. Protein hydrolysate-induced cholecystokinin secretion from enteroendocrine cells is indirectly mediated by the intestinal oligopeptide transporter PepT1. Am. J. Physiol. Gastroint. Liver. Physiol. 2011, 300, G895–G902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, Z.; Everaert, N.; Wang, Y.; Decuypere, E.; Buyse, J. The endocrine control of energy homeostasis in chickens. Gen. Comp. Endocrinol. 2013, 190, 112–117. [Google Scholar] [CrossRef]
- Argente-Arizón, P.; Freire-Regatillo, A.; Argente, J.; Chowen, J.A. Role of non-neuronal cells in body weight and appetite control. Front. Endocrinol. 2015, 6, 42. [Google Scholar]
- Sternson, S.M.; Atasoy, D. Agouti-related protein neuron circuits that regulate appetite. Neuroendocrinology 2014, 100, 95–102. [Google Scholar] [CrossRef]
- Richards, M.P. Genetic regulation of feed intake and energy balance in poultry. Poult. Sci. 2003, 82, 907–916. [Google Scholar] [CrossRef]
- Sohn, J.W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015, 48, 229. [Google Scholar] [CrossRef]
- Becskei, C.; Riediger, T.; Hernádfalvy, N.; Arsenijevic, D.; Lutz, T.A.; Langhans, W. Inhibitory effects of lipopolysaccharide on hypothalamic nuclei implicated in the control of food intake. Brain. Behav. Immun. 2008, 22, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Small, C.J.; Ward, H.L.; Murphy, K.G.; Dakin, C.L.; Taheri, S.; Kennedy, A.R.; Roberts, G.H.; Morgan, D.G.A.; Ghatei, M.A.; et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000, 141, 4325–4328. [Google Scholar] [CrossRef]
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T.; Matsukura, S.; Kangawa, K.; Nakazato, M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261. [Google Scholar] [CrossRef]
- Guan, X.M.; Yu, H.; Palyha, O.C.; McKee, K.K.; Feighner, S.D.; Sirinathsinghji, D.J.; Smith, R.G.; van der Ploeg, L.H.T.; Howard, A.D. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Mol. Brain. Res. 1997, 48, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Englehardt, V.; von Engelhardt, N.; Leng, G.; Smith, R.G.; Dickson, S.L. Growth hormone secretagogue activation of the arcuate nucleus and brainstem occurs via a non-noradrenergic pathway. J. Neuroendocrinol. 2000, 12, 191–197. [Google Scholar]
- Hameed, S.; Dhillo, W.S.; Bloom, S.R. Gut hormones and appetite control. Oral. Dis. 2009, 15, 18–26. [Google Scholar] [CrossRef]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef]
- Druce, M.R.; Wren, A.M.; Park, A.J.; Milton, J.E.; Patterson, M.; Frost, G.; Ghatei, M.A.; Small, C.; Bloom, S.R. Ghrelin increases food intake in obese as well as lean subjects. Int. J. Obes. 2005, 29, 1130–1136. [Google Scholar] [CrossRef]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Dhillo, W.S. Appetite regulation: An overview. Thyroid 2007, 17, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Broberger, C.; Johansen, J.; Johansson, C.; Schalling, M.; Hökfelt, T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA 1998, 95, 15043–15048. [Google Scholar] [CrossRef] [PubMed]
- Kola, B.; Korbonits, M. Shedding light on the intricate puzzle of ghrelin’s effects on appetite regulation. J. Endocrinol. 2009, 202, 191–198. [Google Scholar] [CrossRef]
- Vizcarra, J.A.; Wright, H.; Vizcarra, A. The effect of passive immunization against ghrelin on feed and water intake in turkeys. Poult. Sci. 2012, 91, 2305–2309. [Google Scholar] [CrossRef]
- Geelissen, S.M.E.; Swennen, Q.; van der Geyten, S.; Kühn, E.R.; Kaiya, H.; Kangawa, K.; Decuypere, E.; Buyse, J.; Darras, V.M. Peripheral ghrelin reduces food intake and respiratory quotient in chicken. Domest. Anim. Endocrinol. 2006, 30, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kaiya, H.; Saito, E.S.; Tachibana, T.; Furuse, M.; Kangawa, K. Changes in ghrelin levels of plasma and proventriculus and ghrelin mRNA of proventriculus in fasted and refed layer chicks. Domest. Anim. Endocrinol. 2007, 32, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Buyse, J.; Janssen, S.; Geelissen, S.; Swennen, Q.; Kaiya, H.; Darras, V.M.; Dridi, S. Ghrelin modulates fatty acid synthase and related transcription factor mRNA levels in a tissue-specific manner in neonatal broiler chicks. Peptides 2009, 30, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Ocłoń, E.; Pietras, M. Peripheral ghrelin inhibits feed intake through hypothalamo-pituitary-adrenal axis-dependent mechanism in chicken. J. Anim. Feed. Sci. 2011, 20, 118–130. [Google Scholar] [CrossRef]
- Shousha, S.; Nakahara, K.; Kojima, M.; Miyazato, M.; Hosoda, H.; Kangawa, K.; Murakami, N. Different effects of peripheral and central ghrelin on regulation of food intake in the Japanese quail. Gen. Comp. Endocrinol. 2005, 141, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Taofeek, N.; Chimbetete, N.; Ceron-Romero, N.; Verghese, M.; Vizcarra, J. The Effect of Systemic Infusion of Active and Non-Active Ghrelin on Feed Intake, Weight Gain, and Corticosterone Concentrations in Male Broiler Chickens; IPPE: Atlanta, GA, USA, 2020. [Google Scholar]
- Xu, P.; Siegel, P.B.; Denbow, D.M. Genetic selection for body weight in chickens has altered responses of the brain’s AMPK system to food intake regulation effect of ghrelin, but not obestatin. Behav. Brain. Res. 2011, 221, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Adrian, T.E.; Ferri, G.L.; Bacarese-Hamilton, A.J.; Fuessl, H.S.; Polak, J.M.; Bloom, S.R. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985, 89, 1070–1077. [Google Scholar] [CrossRef]
- Mentlein, R.; Gallwitz, B.; Schmidt, W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1 (7–36) amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993, 214, 829–835. [Google Scholar] [CrossRef]
- Grandt, D.; Schimiczek, M.; Beglinger, C.; Layer, P.; Goebell, H.; Eysselein, V.E.; Reeve, J.R., Jr. Two molecular forms of peptide YY (PYY) are abundant in human blood: Characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul. Pept. 1994, 51, 151–159. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Corpa, E.S.; McQuade, J.; Krasnicki, S.; Conze, D.B. Feeding after fourth ventricular administration of neuropeptide Y receptor agonists in rats. Peptides 2001, 22, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Raposinho, P.D.; Pierroz, D.D.; Broqua, P.; White, R.B.; Pedrazzini, T.; Aubert, M.L. Chronic administration of neuropeptide Y into the lateral ventricle of C57BL/6J male mice produces an obesity syndrome including hyperphagia, hyperleptinemia, insulin resistance, and hypogonadism. Mol. Cell. Endocrinol. 2001, 185, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hagan, M.M. Peptide YY: A key mediator of orexigenic behavior. Peptides 2002, 23, 377–382. [Google Scholar] [CrossRef]
- Karra, E.; Chandarana, K.; Batterham, R.L. The role of peptide YY in appetite regulation and obesity. J. Psychol. 2009, 587, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Unniappan, S.; Kieffer, T.J. Leptin extends the anorectic effects of chronic PYY (3-36) administration in ad libitum-fed rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, 51–58. [Google Scholar] [CrossRef]
- Blomqvist, A.G.; Herzog, H. Y-receptor subtypes—How many more? Trends Neurosci. 1997, 20, 294–298. [Google Scholar] [CrossRef]
- Broberger, C.; Landry, M.; Wong, H.; Walsh, J.N.; Hökfelt, T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin-and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology 1997, 66, 393–408. [Google Scholar] [CrossRef]
- Keire, D.A.; Mannon, P.; Kobayashi, M.; Walsh, J.H.; Solomon, T.E.; Reeve, J.R., Jr. Primary structures of PYY, [Pro34] PYY, and PYY-(3–36) confer different conformations and receptor selectivity. Am. J. Physiol. Gastroint. Liver. Physiol. 2000, 279, G126–G131. [Google Scholar] [CrossRef]
- Stanić, D.; Brumovsky, P.; Fetissov, S.; Shuster, S.; Herzog, H.; Hökfelt, T. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J. Comp. Neurol. 2006, 499, 357–390. [Google Scholar] [CrossRef]
- Lumb, K.J.; DeCarr, L.B.; Milardo, L.F.; Mays, M.R.; Buckholz, T.M.; Fisk, S.E.; Pellegrino, C.M.; Ortiz, A.A.; Mahle, C.D. Novel selective neuropeptide Y2 receptor PEGylated peptide agonists reduce food intake and body weight in mice. J. Med. Chem. 2007, 50, 2264–2268. [Google Scholar] [CrossRef]
- Abbott, C.R.; Small, C.J.; Kennedy, A.R.; Neary, N.M.; Sajedi, A.; Ghatei, M.A.; Bloom, S.R. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY (3–36) on food intake. Brain Res. 2005, 1043, 139–144. [Google Scholar] [CrossRef]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef]
- Conlon, J.M.; O’Harte, F. The primary structure of a PYY-related peptide from chicken intestine suggests an anomalous site of cleavage of the signal peptide in preproPYY. FEBS Lett. 1992, 313, 225–228. [Google Scholar] [CrossRef]
- Ando, R.; Kawakami, S.I.; Bungo, T.; Ohgushi, A.; Takagi, T.; Denbow, D.M.; Furuse, M. Feeding responses to several neuropeptide Y receptor agonists in the neonatal chick. Eur. J. Pharmacol. 2001, 427, 53–59. [Google Scholar] [CrossRef]
- Aoki, K.; Kondo, M.; Okuda, M.; Saneyasu, T.; Honda, K.; Kamisoyama, H. General and comparative endocrinology identification, expression analysis, and functional characterization of peptide YY in chickens (Gallus gallus domesticus). Gen. Comp. Endocrinol. 2017, 242, 1–7. [Google Scholar]
- Aoki, K.; Kondo, M.; Okuda, M.; Saneyasu, T.; Honda, K.; Kamisoyama, H. Identification, expression analysis, and functional characterization of peptide YY in chickens (Gallus gallus domesticus). Gen. Comp. Endocrinol. 2017, 242, 11–17. [Google Scholar]
- Ueno, H.; Yamaguchi, H.; Mizuta, M.; Nakazato, M. The role of PYY in feeding regulation. Regul. Pept. 2008, 145, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Martin Alonso, A. Investigating the Physiological and Pharmacological Effects of the Gut Hormone Peptide YY (PYY). Ph.D. Thesis, Imperial College London, London, UK, 2022. [Google Scholar]
- Jin, S.L.; Han, V.K.M.; Simmons, J.G.; Towle, A.C.; Lauder, J.M.; Lund, P.K. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: An immunocytochemical study. J. Comp. Neurol. 1988, 271, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Eissele, R.; Göke, R.; Willemer, S.; Harthus, H.P.; Vermeer, H.; Arnold, R.E.; Göke, B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur. J. Clin. Investig. 1992, 22, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Göke, R.; Richter, G.; Fehmann, H.C.; Arnold, R.; Göke, B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 1995, 56, 117–126. [Google Scholar] [CrossRef]
- Bell, G.I.; Santerre, R.F.; Mullenbach, G.T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 1983, 302, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.; Rotondo, A.; Mulé, F.; Tack, J. A comparison of glucagon-like peptides 1 and 2. Aliment. Pharmacol. Ther. 2013, 37, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Hiramatsu, K. Chicken intestinal L cells and glucagon-like peptide-1 secretion. J. Poult. Sci. 2020, 57, 1–6. [Google Scholar] [CrossRef]
- Drucker, D.J. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat. Clin. Pract. Endoc. 2005, 1, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Turton, M.D.; O’shea, D.; Gunn, I.; Beak, S.A.; Edwards, C.M.B.; Meeran, K.; Choi, S.J.; Taylor, G.M.; Health, M.M.; Lambert, P.D.; et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379, 69–72. [Google Scholar] [CrossRef]
- Svendsen, B.; Pedersen, J.; Albrechtsen, N.J.W.; Hartmann, B.; Toräng, S.; Rehfeld, J.F.; Poulsen, S.S.; Holst, J.J. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology 2015, 156, 847–857. [Google Scholar] [CrossRef]
- Ronveaux, C.C.; de Lartigue, G.; Raybould, H.E. Ability of GLP-1 to decrease food intake is dependent on nutritional status. Physiol. Behav. 2014, 135, 222–229. [Google Scholar] [CrossRef]
- NamKoong, C.; Kim, M.S.; Jang, B.T.; Lee, Y.H.; Cho, Y.M.; Choi, H.J. Central administration of GLP-1 and GIP decreases feeding in mice. Biochem. Biophys. Res. Commun. 2017, 490, 247–252. [Google Scholar] [CrossRef]
- Van Dijk, G.; Thiele, T.E.; Seeley, R.J.; Woods, S.C.; Bernstein, I.L. Glucagon-like peptide-1 and satiety. Nature 1997, 385, 214. [Google Scholar] [CrossRef]
- Meeran, K.; O’Shea, D.; Edwards, C.M.B.; Turton, M.D.; Heath, M.M.; Gunn, I.; Abusnana, S.; Rossi, M.; Small, C.J.; Goldstone, A.P.; et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 1999, 140, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Verdich, C.; Flint, A.; Gutzwiller, J.P.; Naslund, E.; Beglinger, C.; Hellstrom, P.M.; Long, S.J.; Morgan, L.M.; Holst, J.J.; Astrup, A. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J. Clin. Endocr. 2001, 86, 4382–4389. [Google Scholar]
- Näslund, E.; Barkeling, B.; King, N.; Gutniak, M.; Blundell, J.E.; Holst, J.J.; Rössner, S.; Hellström, P.M. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int. J. Obes. 1999, 23, 304–311. [Google Scholar] [CrossRef]
- Verdich, C.; Toubro, S.; Buemann, B.; Lysgård Madsen, J.; Juul Holst, J.; Astrup, A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—Effect of obesity and weight reduction. Int. J. Obes. 2001, 25, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Moghadam, A.A.; Cordner, Z.A.; Liang, N.C.; Moran, T.H. Long term exendin-4 treatment reduces food intake and body weight and alters expression of brain homeostatic and reward markers. Endocrinology 2014, 155, 3473–3483. [Google Scholar] [CrossRef] [PubMed]
- Ruska, Y.; Szilvásy-Szabó, A.; Kővári, D.; Kádár, A.; Mácsai, L.; Sinkó, R.; Hrabovszky, E.; Gereben, B.; Fekete, C. Expression of glucagon-like peptide 1 receptor in neuropeptide Y neurons of the arcuate nucleus in mice. Brain. Struct. Funct. 2022, 227, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.J.; Tang-Christensen, M.; Jessop, D.S. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology 1997, 138, 4445–4455. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Secher, A.; Hecksher-Sørensen, J.; Pyke, C. Long-acting glucagon-like peptide-1 receptor agonists have direct access to and effects on pro-opiomelanocortin/cocaine-and amphetamine-stimulated transcript neurons in the mouse hypothalamus. J. Diabetes Investig. 2016, 7, 56–63. [Google Scholar] [CrossRef]
- Péterfi, Z.; Szilvásy-Szabó, A.; Farkas, E.; Ruska, Y.; Pyke, C.; Knudsen, L.B.; Fekete, C. GLP-1 regulates the POMC neurons of the arcuate nucleus both directly and indirectly via presynaptic action. Neuroendocrinology 2021, 111, 986–997. [Google Scholar] [CrossRef]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef]
- Park, S. Neuroendocrine control of food intake. J. Korean Endocr. Soc. 2007, 22, 391–396. [Google Scholar] [CrossRef][Green Version]
- Hiramatsu, K.; Yamasaki, A.; Karasawa, Y. Comparative study on the distribution of glucagon-like peptide-1 (GLP-1)-immunoreactive cells in the intestine of chicken and ostrich. J. Poult. Sci. 2003, 40, 39–44. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Yamasaki, A.; Shioji, T. Immunohistochemical and morphometrical studies on the distribution of glucagon-like peptide-1 (GLP-1)-immunoreactive cells in the chicken intestine. J. Poult. Sci. 2005, 42, 223–229. [Google Scholar] [CrossRef]
- Nishimura, K.; Hiramatsu, K.; Watanabe, T.; Makino, R.; Sasaki, N.; Kita, K. Amino acid supplementation to diet influences the activity of the L cells in chicken small intestine. J. Poult. Sci. 2015, 52, 221–226. [Google Scholar] [CrossRef]
- Burcelin, R. The incretins: A link between nutrients and well-being. Br. J. Nutr. 2005, 93, 147–156. [Google Scholar] [CrossRef]
- Tachibana, T.; Matsumoto, M.; Furuse, M.; Hasegawa, S.; Yoshizawa, F.; Sugahara, K. Central, but not peripheral, glucagon-like peptide-1 inhibits crop emptying in chicks. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 134, 777–781. [Google Scholar] [CrossRef]
- Honda, K.; Saneyasu, T.; Yamaguchi, T.; Shimatani, T.; Aoki, K.; Nakanishi, K.; Kamisoyama, H. Intracerebroventricular administration of novel glucagon-like peptide suppresses food intake in chicks. Peptides 2014, 52, 98–103. [Google Scholar] [CrossRef]
- Shousha, S.; Nakahara, K.; Nasu, T.; Sakamoto, T.; Murakami, N. Effect of glucagon-like peptide-1 and-2 on regulation of food intake, body temperature and locomotor activity in the Japanese quail. Neurosci. Lett. 2007, 415, 102–107. [Google Scholar] [CrossRef]
- Tachibana, T.; Oikawa, D.; Adachi, N.; Boswell, T.; Furuse, M. Intracerebroventricular injection of glucagon-like peptide-1 changes lipid metabolism in chicks. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 1104–1108. [Google Scholar] [CrossRef]
- Richards, M.P.; McMurtry, J.P. Expression of proglucagon and proglucagon-derived peptide hormone receptor genes in the chicken. Gen. Comp. Endocrinol. 2008, 156, 323–338. [Google Scholar] [CrossRef]
- Huang, G.; Li, J.; Fu, H.; Yan, Z.; Bu, G.; He, X.; Wang, Y. Characterization of glucagon-like peptide 1 receptor (GLP1R) gene in chickens: Functional analysis, tissue distribution, and identification of its transcript variants. Domest. Anim. Endocrinol. 2012, 43, 1–15. [Google Scholar] [CrossRef]
- Nishimura, K.; Hiramatsu, K.; Monir, M.M.; Takemoto, C.; Watanabe, T. Ultrastructural study on colocalization of glucagon-like peptide (GLP)-1 with GLP-2 in chicken intestinal L-cells. J. Vet. Med. Sci. 2013, 75, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Sam, A.H.; Troke, R.C.; Tan, T.M.; Bewick, G.A. The role of the gut/brain axis in modulating food intake. Neuropharmacology 2012, 63, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Nagell, C.F.; Wettergren, A.; Pedersen, J.F.; Mortensen, D.; Holst, J.J. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. Scand. J. Gastroenterol. 2004, 39, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Yusta, B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu. Rev. Physiol. 2014, 76, 561–583. [Google Scholar] [CrossRef]
- Drucker, D.J.; Habener, J.F.; Holst, J.J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Investig. 2017, 127, 4217–4227. [Google Scholar] [CrossRef]
- Wismann, P.; Pedersen, S.L.; Hansen, G.; Mannerstedt, K.; Pedersen, P.J.; Jeppesen, P.B.; Vrang, N.; Fosgerau, K.; Jelsing, J. Novel GLP-1/GLP-2 co-agonists display marked effects on gut volume and improves glycemic control in mice. Physiol. Behav. 2018, 192, 72–81. [Google Scholar] [CrossRef]
- Munroe, D.G.; Gupta, A.K.; Kooshesh, F.; Vyas, T.B.; Rizkalla, G.; Wang, H.; Demchshyn, L.; Yang, Z.J.; Kamboj, R.K.; Chen, H.; et al. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc. Natl. Acad. Sci. USA 1999, 96, 1569–1573. [Google Scholar] [CrossRef]
- Tang-Christensen, M.; Larsen, P.J.; Thulesen, J.; Rømer, J.; Vrang, N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat. Med. 2000, 6, 802–807. [Google Scholar] [CrossRef]
- Lovshin, J.; Estall, J.; Yusta, B.; Brown, T.J.; Drucker, D.J. Glucagon-like peptide (GLP)-2 action in the murine central nervous system is enhanced by elimination of GLP-1 receptor signaling. J. Biol. Chem. 2001, 276, 21489–21499. [Google Scholar] [CrossRef]
- Baldassano, S.; Bellanca, A.L.; Serio, R.; Mule, F. Food intake in lean and obese mice after peripheral administration of glucagon-like peptide 2. J. Endocrinol. 2012, 213, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. The CNS glucagon-like peptide-2 receptor in the control of energy balance and glucose homeostasis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Saneyasu, T.; Shimatani, T.; Aoki, K.; Yamaguchi, T.; Nakanishi, K.; Kamisoyama, H. Intracerebroventricular administration of chicken glucagon-like peptide-2 potently suppresses food intake in chicks. Anim. Sci. J. 2015, 86, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Shimatani, T.; Aoki, K.; Yamaguchi, T.; Kondo, M.; Saneyasu, T.; Kamisoyama, H. Glucagon-like Peptide-2 Functions as an Anorexigenic Peptide not only in the Cebntral Nervous System but also in the Peripheral Circulation in Broiler Chicks. J. Poult. Sci. 2015, 52, 183–187. [Google Scholar] [CrossRef][Green Version]
- Kewan, A.; Shimatani, T.; Saneyasu, T.; Kamisoyama, H.; Honda, K. Comparison of the effects of intracerebroventricular administration of glucagon-like peptides 1 and 2 on hypothalamic appetite regulating factors and sleep-like behavior in chicks. Neurosci. Lett. 2022, 768, 136362. [Google Scholar] [CrossRef]
- Ghatei, M.A.; Uttenthal, L.O.; Christofides, N.D.; Bryant, M.G.; Bloom, S.R. Molecular forms of human enteroglucagon in tissue and plasma: Plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J. Clin. Endocrinol. Metab. 1983, 57, 488–495. [Google Scholar] [CrossRef]
- Dakin, C.L.; Gunn, I.; Small, C.J.; Edwards, C.M.B.; Hay, D.L.; Smith, D.M.; Ghatei, M.A.; Bloom, S.R. Oxyntomodulin inhibits food intake in the rat. Endocrinology 2001, 142, 4244–4250. [Google Scholar] [CrossRef]
- Dakin, C.L.; Small, C.J.; Batterham, R.L.; Neary, N.M.; Cohen, M.A.; Patterson, M.; Ghatei, M.A.; Bloom, S.R. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology 2004, 145, 2687–2695. [Google Scholar] [CrossRef]
- Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Batterham, R.L.; Park, A.; Patterson, M.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Oxyntomodulin suppresses appetite and reduces food intake in humans. J. Clin. Endocr. 2003, 88, 4696–4701. [Google Scholar] [CrossRef]
- Wynne, K.; Park, A.J.; Small, C.J.; Patterson, M.; Ellis, S.M.; Murphy, K.G.; Wren, A.M.; Frost, G.S.; Meeran, K.; Ghatei, M.A.; et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: A double-blind, randomized, controlled trial. Diabetes 2005, 54, 2390–2395. [Google Scholar] [CrossRef]
- Baggio, L.L.; Huang, Q.; Brown, T.J.; Drucker, D.J. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 2004, 127, 546–558. [Google Scholar] [CrossRef]
- Baldissera, F.G.; Holst, J.J.; Knuhtsen, S.; Hilsted, L.; Nielsen, O.V. Oxyntomodulin (glicentin-(33–69)): Pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul. Pept. 1988, 21, 151–166. [Google Scholar] [CrossRef]
- Schjoldager, B.T.G.; Baldissera, F.G.A.; Mortensen, P.E.; Holst, J.J.; Christiansen, J. Oxyntomodulin: A potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. Eur. J. Clin. Investig. 1988, 18, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.A.; Bowden, C.N.; Nandar, W.; Rogers, J.O. Central oxyntomodulin causes anorexigenic effects associated with the hypothalamus and alimentary canal in chicks (Gallus gallus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Saneyasu, T.; Yamaguchi, T.; Shimatani, T.; Aoki, K.; Nakanishi, K.; Kamisoyama, H. Intracerebroventricular administration of chicken oxyntomodulin suppresses food intake and increases plasma glucose and corticosterone concentrations in chicks. Neurosci. Lett. 2014, 564, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Halter, B.; Chowdhury, V.S.; Gilbert, E.R.; Cline, M.A. Oxyntomodulin induces satiety and activates the arcuate nucleus of the hypothalamus in Japanese quail. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 247, 110721. [Google Scholar] [CrossRef] [PubMed]
- Shurlock, T.G.H.; Forbes, J.M. Evidence for hepatic glucostatic regulation of food intake in the domestic chicken and its interaction with gastro-intestinal control. Br. Poult. Sci. 1981, 22, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Lacy, M.P.; van Krey, H.P.; Skewes, P.A.; Denbow, D.M. Effect of intrahepatic glucose infusions on feeding in heavy and light breed chicks. Poult. Sci. 1985, 64, 751–756. [Google Scholar] [CrossRef]
- Honda, K.; Kamisoyama, H.; Saito, N.; Kurose, Y.; Sugahara, K.; Hasegawa, S. Central administration of glucagon suppresses food intake in chicks. Neurosci. Lett. 2007, 416, 198–201. [Google Scholar] [CrossRef]
- Gibbs, J.; Young, R.C.; Smith, G.P. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature 1973, 245, 323–325. [Google Scholar] [CrossRef]
- Buffa, R.; Solcia, E.; Go, V.L.W. Immunohistochemical identification of the cholecystokinin cell in the intestinal mucosa. Gastroenterology 1976, 70, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A.; Goldfine, I.D.; Rosen, M.S.; Taplitz, R.A.; Williams, J.A. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J. Clin. Investig. 1985, 75, 1144–1152. [Google Scholar] [CrossRef]
- Moran, T.H.; McHugh, P.R. Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1982, 242, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Kissileff, H.R.; Pi-Sunyer, F.X.; Thornton, J.; Smith, G.P. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am. J. Clin. Nutr. 1981, 34, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.H.; Robinson, P.H.; Goldrich, M.S.; McHugh, P.R. Two brain cholecystokinin receptors: Implications for behavioral actions. Brain Res. 1986, 362, 175–179. [Google Scholar] [CrossRef]
- Smith, G.P.; Jerome, C.; Cushin, B.J.; Eterno, R.; Simansky, K.J. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 1981, 213, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Melville, L.D.; Smith, G.P.; Gibbs, J. Devazepide antagonizes the inhibitory effect of cholecystokinin on intake in sham-feeding rats. Pharmacol. Biochem. Behav. 1992, 43, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Gutzwiller, J.P.; Drewe, J.; Ketterer, S.; Hildebrand, P.; Krautheim, A.; Beglinger, C. Interaction between CCK and a preload on reduction of food intake is mediated by CCK-A receptors in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, 189–195. [Google Scholar] [CrossRef]
- Tachibana, T.; Matsuda, K.; Kawamura, M.; Ueda, H.; Khan, M.S.I.; Cline, M.A. Feeding-suppressive mechanism of sulfated cholecystokinin (26–33) in chicks. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 161, 372–378. [Google Scholar] [CrossRef]
- Dunn, I.C.; Meddle, S.L.; Wilson, P.W.; Wardle, C.A.; Law, A.S.; Bishop, V.R.; Hindar, C.; Robertson, G.W.; Burt, D.W.; Ellison, S.J.H.; et al. Decreased expression of the satiety signal receptor CCKAR is responsible for increased growth and body weight during the domestication of chickens. Am. J. Physiol. Endocrinol. Metab. 2013, 304, 909–921. [Google Scholar] [CrossRef]
- Jønson, L.; Schoeman, N.; Saayman, H.; Naudé, R.; Jensen, H.; Johnsen, A.H. Identification of ostrich and chicken cholecystokinin cDNA and intestinal peptides. Peptides 2000, 21, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Lucini, C. An immunohistochemical study on the endocrine cells in the gastrointestinal tract of domestic duck. Eur. J. Basic. Appl. Histochem. 1991, 35, 131–143. [Google Scholar] [PubMed]
- Castaldo, L.; Lucini, C. Ontogenesis of some endocrine cells in the duck gastrointestinal tract. Eur. J. Histochem. 1994, 38, 319–326. [Google Scholar] [PubMed]
- Côté, C.D.; Zadeh-Tahmasebi, M.; Rasmussen, B.A.; Duca, F.A.; Lam, T.K. Hormonal signaling in the gut. J. Biol. Chem. 2014, 289, 11642–11649. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, I.B.; Svensson, S.P.; Monstein, H.J. Molecular cloning of an unusual bicistronic cholecystokinin receptor mRNA expressed in chicken brain: A structural and functional expression study. Regul. Pept. 2003, 114, 37–43. [Google Scholar] [CrossRef]
- Ohkubo, T.; Shamoto, K.; Ogino, T. Structure and tissue distribution of cholecystokinin-1 receptor in chicken. J. Poult. Sci. 2007, 44, 98–104. [Google Scholar] [CrossRef][Green Version]
- Dupre, J.; Ross, S.A.; Watson, D.; Brown, J.C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J. Clin. Endocrinol. Metab. 1973, 37, 826–828. [Google Scholar] [CrossRef]
- Andersen, D.K.; Elahi, D.; Brown, J.C.; Tobin, J.D.; Andres, R. Oral glucose augmentation of insulin secretion: Interactions of gastric inhibitory polypeptide with ambient glucose and insulin levels. J. Clin. Investig. 1978, 62, 152–161. [Google Scholar] [CrossRef]
- Usdin, T.B.; Mezey, E.; Button, D.C.; Brownstein, M.J.; Bonner, T.I. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993, 133, 2861–2870. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes. Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef]
- Szalowska, E.; Meijer, K.; Kloosterhuis, N.; Razaee, F.; Priebe, M.; Vonk, R.J. Sub-chronic administration of stable GIP analog in mice decreases serum LPL activity and body weight. Peptides 2011, 32, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging roles for serotonin in regulating metabolism: New implications for an ancient molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Sohn, J.W.; Xu, Y.; Jones, J.E.; Wickman, K.; Williams, K.W.; Elmquist, J.K. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 2011, 71, 488–497. [Google Scholar] [CrossRef]
- Vlaev, I.; Crockett, M.J.; Clark, L.; Müller, U.; Robbins, T.W. Serotonin enhances the impact of health information on food choice. Cogn. Affect. Behav. Neurosci. 2017, 17, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Tecott, L.H.; Sun, L.M.; Akana, S.F.; Strack, A.M.; Lowenstein, D.H.; Dallman, M.F.; Julius, D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 1995, 374, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.D.; Liu, C.; Sohn, J.W.; Liu, T.; Kim, M.H.; Lee, C.E.; Vianna, C.R.; Williams, K.W.; Xu, Y.; Elmquist, J.K. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Investig. 2013, 123, 5061–5070. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.E.; Oh, J.H.; Seo, K.W.; Song, K.H. Leptin, adiponectin and serotonin levels in lean and obese dogs. BMC Vet. Res. 2014, 10, 113. [Google Scholar] [CrossRef]
- Shen, Y.B.; Voilqué, G.; Kim, J.D.; Odle, J.; Kim, S.W. Effects of increasing tryptophan intake on growth and physiological changes in nursery pigs. J. Anim. Sci. 2012, 90, 2264–2275. [Google Scholar] [CrossRef]
- Kwon, W.B.; Soto, J.A.; Stein, H.H. Effects of dietary leucine and tryptophan on serotonin metabolism and growth performance of growing pigs. J. Anim. Sci. 2022, 100, skab356. [Google Scholar] [CrossRef]
- Linh, N.T.; Guntoro, B.; Qui, N.H. Immunomodulatory, behavioral, and nutritional response of tryptophan application on poultry. Vet. World. 2021, 14, 2244. [Google Scholar] [CrossRef]
- Monteiro, M.P.; Batterham, R.L. The importance of the gastrointestinal tract in controlling food intake and regulating energy balance. Gastroenterology 2017, 152, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Grunddal, K.V.; Ratner, C.F.; Svendsen, B.; Sommer, F.; Engelstoft, M.S.; Madsen, A.N.; Pedersen, J.; Nøhr, M.K.; Egerod, K.L.; Nawrocki, A.R.; et al. Neurotensin is coexpressed, coreleased, and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology 2016, 157, 176–194. [Google Scholar] [CrossRef]
- Ratner, C.; Skov, L.J.; Raida, Z.; Bächler, T.; Bellmann-Sickert, K.; Le Foll, C.; Sivertsen, B.; Dalbøge, L.S.; Hartmann, B.; Beck-Sickinger, A.G.; et al. Effects of peripheral neurotensin on appetite regulation and its role in gastric bypass surgery. Endocrinology 2016, 157, 3482–3492. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.B.; Gribble, F.M.; Reimann, F. Nutrient-induced cellular mechanisms of gut hormone secretion. Nutrients 2021, 13, 883. [Google Scholar] [CrossRef]
- Augustin, R.; Mayoux, E. Mammalian sugar transporters. In Glucose Homeostasis; IntechOpen: London, UK, 2014. [Google Scholar]
- Wright, E.M.; Ghezzi, C.; Loo, D.D. Novel and unexpected functions of SGLTs. Physiology 2017, 32, 435–443. [Google Scholar] [CrossRef]
- Wright, E.M.; Hirsch, J.R.; Loo, D.D.; Zampighi, G.A. Regulation of Na+/glucose cotransporters. J. Exp. Biol. 1997, 200, 287–293. [Google Scholar] [CrossRef]
- Singh, S.K.; Bartoo, A.C.; Krishnan, S.; Boylan, M.O.; Schwartz, J.H.; Wolfe, M.M. Glucose-dependent insulinotropic polypeptide (GIP) stimulates transepithelial glucose transport. Obesity 2008, 16, 2412–2416. [Google Scholar] [CrossRef]
- Cheeseman, C.I.; Tsang, R. The effect of GIP and glucagon-like peptides on intestinal basolateral membrane hexose transport. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 271, 477–482. [Google Scholar] [CrossRef]
- Cheeseman, C.I.; O’neill, D. Basolateral D-glucose transport activity along the crypt-villus axis in rat jejunum and upregulation induced by gastric inhibitory peptide and glucagon-like peptide-2. Exp. Physiol. 1998, 83, 605–616. [Google Scholar] [CrossRef]
- Ogawa, E.; Hosokawa, M.; Harada, N.; Yamane, S.; Hamasaki, A.; Toyoda, K.; Fujimoto, S.; Fujita, Y.; Fukuda, K.; Tsukiyama, K.; et al. The effect of gastric inhibitory polypeptide on intestinal glucose absorption and vintestinal motility in mice. Biochem. Biophys. Res. Commun. 2011, 404, 115–120. [Google Scholar] [CrossRef]
- Smith, K.; Azari, E.K.; LaMoia, T.E.; Hussain, T.; Vargova, V.; Karolyi, K.; Veldhuis, P.P.; Arnoletti, J.P.; de la Fuente, S.G.; Pratley, R.E.; et al. T1R2 receptor-mediated glucose sensing in the upper intestine potentiates glucose absorption through activation of local regulatory pathways. Mol. Metab. 2018, 17, 98–111. [Google Scholar] [CrossRef]
- Powell, D.R.; Smith, M.; Greer, J.; Harris, A.; Zhao, S.; DaCosta, C.; Mseeh, F.; Shadoan, M.K.; Sands, A.; Zambrowicz, B.; et al. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)–mediated absorption of intestinal glucose. J. Pharmacol. Exp. Ther. 2013, 345, 250–259. [Google Scholar] [CrossRef]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.T.; Murillo, M.D.; Rodriguez-Yoldi, M.C.; Alcalde, A.I.; Mesonero, J.E.; Rodriguez-Yoldi, M.J. Effects of serotonin on the physiology of the rabbit small intestine. Can. J. Physiol. Pharm. 2000, 78, 359–366. [Google Scholar] [CrossRef]
- Hirsh, A.J.; Cheeseman, C.I. Cholecystokinin decreases intestinal hexose absorption by a parallel reduction in SGLT1 abundance in the brush-border membrane. J. Biol. Chem. 1998, 273, 14545–14549. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; King, A.; Samuelson, L.C.; Kindel, T.L.; Rider, T.; Jandacek, R.J.; Raybould, H.E.; Woods, S.C.; Tso, P. Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity. Gastroenterology 2010, 138, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Harada, N.; Ikeguchi-Ogura, E.; Sankoda, A.; Hatoko, T.; Lu, X.; Yasuda, T.; Yamane, S.; Inagaki, N. Gene expression of nutrient-sensing molecules in I cells of CCK reporter male mice. J. Mol. Endocrinol. 2021, 66, 11–22. [Google Scholar] [CrossRef]
- Sandoval, D.A.; D’Alessio, D.A. Physiology of proglucagon peptides: Role of glucagon and GLP-1 in health and disease. Physiol. Rev. 2015, 95, 513–548. [Google Scholar] [CrossRef]
- Campbell, J.E. Targeting the GIPR for obesity: To agonize or antagonize? Potential mechanisms. Mol. Metab. 2021, 46, 101139. [Google Scholar] [CrossRef]
- Sundaresan, S.; Shahid, R.; Riehl, T.E.; Chandra, R.; Nassir, F.; Stenson, W.F.; Liddle, R.A.; Abumrad, N.A. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 2013, 27, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Little, T.J.; Isaacs, N.J.; Young, R.L.; Ott, R.; Nguyen, N.Q.; Rayner, C.K.; Horowitz, M.; Feinle-Bisset, C. Characterization of duodenal expression and localization of fatty acid-sensing receptors in humans: Relationships with body mass index. Am. J. Physiol. Gastroint. Liver. Physiol. 2014, 307, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Demenis, C.; McLaughlin, J.; Smith, C.P. Sulfated cholecystokinin-8 promotes CD36—Mediated fatty acid uptake into primary mouse duodenal enterocytes. Front. Physiol. 2017, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Dai, D.; Wang, X.; Ding, Z.; Li, C.; Mehta, J.L. GLP-1 agonists inhibit ox-LDL uptake in macrophages by activating protein kinase A. J. Cardiovasc. Pharmacol. 2014, 64, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, K.; Wang, W.; Wen, Z.; Wang, P.; Liu, L.; Wang, D.W. Glucagon-like peptide-1 ameliorates cardiac lipotoxicity in diabetic cardiomyopathy via the PPAR α pathway. Aging Cell. 2018, 17, e12763. [Google Scholar] [CrossRef]
- Markovic, M.A.; Srikrishnaraj, A.; Tsang, D.; Brubaker, P.L. Requirement for the intestinal epithelial insulin-like growth factor-1 receptor in the intestinal responses to glucagon-like peptide-2 and dietary fat. FASEB J. 2020, 34, 6628–6640. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Parker, M.C.; Ferris, C.F.; Leeman, S.E. Neurotensin stimulates [3H] oleic acid translocation across rat small intestine. Am. J. Physiol. Gastrointest. Liver. Physiol. 1986, 251, 823–829. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Zaytseva, Y.Y.; Liu, Y.; Rychahou, P.; Jiang, K.; Starr, M.E.; Kim, J.T.; Harris, J.W.; Yiannikouris, F.B.; et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature 2016, 533, 411–415. [Google Scholar] [CrossRef]
- Grenier, E.; Garofalo, C.; Delvin, E.; Levy, E. Modulatory role of PYY in transport and metabolism of cholesterol in intestinal epithelial cells. PLoS ONE 2012, 7, e40992. [Google Scholar] [CrossRef]
- Johnson, L.R. Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Ganapathy, V.; Gupta, N.; Martindale, R.G. Protein digestion and absorption. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1667–1692. [Google Scholar]
- Coon, S.D.; Rajendran, V.M.; Schwartz, J.H.; Singh, S.K. Glucose-dependent insulinotropic polypeptide-mediated signaling pathways enhance apical PepT1 expression in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver. Physiol. 2015, 308, 56–62. [Google Scholar] [CrossRef]
- Cheeseman, C.I. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997, 273, 1965–1971. [Google Scholar] [CrossRef]
- Shin, E.D.; Estall, J.L.; Izzo, A.; Drucker, D.J.; Brubaker, P.L. Mucosal adaptation to enteral nutrients is dependent on the physiologic actions of glucagon-like peptide-2 in mice. Gastroenterology 2005, 128, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.L. Glucagon-like peptide-2 and the regulation of intestinal growth and function. Compr. Physiol. 2011, 8, 1185–1210. [Google Scholar]
- Lee, J.; Koehler, J.; Yusta, B.; Bahrami, J.; Matthews, D.; Rafii, M.; Pencharz, P.B.; Drucker, D.J. Enteroendocrine-derived glucagon-like peptide-2 controls intestinal amino acid transport. Mol. Metab. 2017, 6, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Coon, S.D.; Schwartz, J.H.; Rajendran, V.M.; Jepeal, L.; Singh, S.K. Glucose-dependent insulinotropic polypeptide regulates dipeptide absorption in mouse jejunum. Am. J. Physiol. Gastrointest. Liver. Physiol. 2013, 305, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.P.; McKenzie, M.E.; Dayton, A.D. Relationship of coccidial lesion scores and weight gain in infections of Eimeria acervulina, E. maxima and E. tenella in broilers. Avian. Pathol. 1990, 19, 489–496. [Google Scholar] [CrossRef]
- Chapman, H.D. Milestones in avian coccidiosis research: A review. Poult. Sci. 2014, 93, 501–511. [Google Scholar] [CrossRef]
- Lillehoj, H.S.; Jang, S.I.; Lee, S.H.; Lillehoj, E.P. Chapter 4: Avian coccidiosis as a prototype intestinal disease—Host protective immunity and novel disease control strategies. In Intestinal Health: Key to Maximise Growth Performance in Livestock; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; p. 79. [Google Scholar]
- Teng, P.Y.; Choi, J.; Tompkins, Y.; Lillehoj, H.; Kim, W. Impacts of increasing challenge with Eimeria maxima on the growth performance and gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet. Res. 2021, 52, 81. [Google Scholar] [CrossRef]
- Kipper, M.; Andretta, I.; Lehnen, C.R.; Lovatto, P.A.; Monteiro, S.G. Meta-analysis of the performance variation in broilers experimentally challenged by Eimeria spp. Vet. Parasitol. 2013, 196, 77–84. [Google Scholar] [CrossRef]
- Bozkurt, M.; Aysul, N.; Küçükyilmaz, K.; Aypak, S.; Ege, G.; Catli, A.U.; Aksit, H.; Çöven, F.; Seyrek, K.; Çınar, M. Efficacy of in-feed preparations of an anticoccidial, multienzyme, prebiotic, probiotic, and herbal essential oil mixture in healthy and Eimeria spp.-infected broilers. Poult. Sci. 2014, 93, 389–399. [Google Scholar] [CrossRef]
- Miska, K.B.; Fetterer, R.H. The effect of Eimeria maxima infection on the expression of amino acid and sugar transporters aminopeptidase, as well as the di-and tri-peptide transporter PepT1, is not solely due to decreased feed intake. Poult. Sci. 2018, 97, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Abdelhady, A.Y.; El-Safty, S.A.; Hashim, M.; Ibrahim, M.A.; Mohammed, F.F.; Elbaz, A.M.; Abdel-Moneim, A.M.E. Comparative evaluation of single or combined anticoccidials on performance, antioxidant status, immune response, and intestinal architecture of broiler chickens challenged with mixed Eimeria species. Poult. Sci. 2021, 100, 101162. [Google Scholar] [CrossRef]
- Teng, P.Y.; Liu, G.; Choi, J.; Yadav, S.; Wei, F.; Kim, W.K. Effects of levels of methionine supplementations in forms of L-or DL-methionine on the performance, intestinal development, immune response, and antioxidant system in broilers challenged with Eimeria spp. Poult. Sci. 2023, 102, 102586. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, Y.H.; Choi, J.; Teng, P.Y.; Yamada, M.; Sugiyama, T.; Kim, W.K. Reduced bone formation and increased bone resorption drive bone loss in Eimeria infected broilers. Sci. Rep. 2023, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, W. Interactions of microbiota and mucosal immunity in the ceca of broiler chickens infected with Eimeria tenella. Vaccines 2022, 10, 1941. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Teng, P.Y.; Singh, A.K.; Choi, J.; Kim, W.K. Influence of Brassica spp. rapeseed and canola meal, and supplementation of bioactive compound (AITC) on growth performance, intestinal-permeability, oocyst shedding, lesion score, histomorphology, and gene expression of broilers challenged with E. maxima. Poult. Sci. 2022, 101, 101583. [Google Scholar] [CrossRef]
- Yadav, S.; Teng, P.Y.; Dos Santos, T.S.; Gould, R.L.; Craig, S.W.; Fuller, A.L.; Pazdro, R.; Kim, W.K. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020, 99, 5936–5945. [Google Scholar] [CrossRef]
- Morris, B.C.; Danforth, H.D.; Caldwell, D.J.; Pierson, F.W.; McElroy, A.P. Intestinal mucosal mast cell immune response and pathogenesis of two Eimeria acervulina isolates in broiler chickens. Poult. Sci. 2004, 83, 1667–1674. [Google Scholar] [CrossRef]
- Kim, E.; Leung, H.; Akhtar, N.; Li, J.; Barta, J.R.; Wang, Y.; Yang, C.; Kiarie, E. Growth performance and gastrointestinal responses of broiler chickens fed corn-soybean meal diet without or with exogenous epidermal growth factor upon challenge with Eimeria. Poult. Sci. 2017, 96, 3676–3686. [Google Scholar] [CrossRef]
- Lal, K.; Bromley, E.; Oakes, R.; Prieto, J.H.; Sanderson, S.J.; Kurian, D.; Hunt, L.; Yates III, J.R.; Wastling, J.M.; Sinden, R.E.; et al. Proteomic comparison of four Eimeria tenella life-cycle stages: Unsporulated oocyst, sporulated oocyst, sporozoite and second-generation merozoite. Proteomics 2009, 9, 4566–4576. [Google Scholar] [CrossRef]
- Cowper, B.; Matthews, S.; Tomley, F. The molecular basis for the distinct host and tissue tropisms of coccidian parasites. Mol. Biochem. Parasitol. 2012, 186, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rochell, S.J.; Parsons, C.M.; Dilger, R.N. Effects of Eimeria acervulina infection severity on growth performance, apparent ileal amino acid digestibility, and plasma concentrations of amino acids, carotenoids, and α1-acid glycoprotein in broilers. Poult. Sci. 2016, 95, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.Y.; Yadav, S.; de Souza Castro, F.L.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, S.A.; Ajuwon, K.M.; Romero, L.F.; Adeola, O. Ileal endogenous amino acid losses: Response of broiler chickens to fiber and mild coccidial vaccine challenge. Poult. Sci. 2012, 91, 899–907. [Google Scholar] [CrossRef]
- Amerah, A.M.; Ravindran, V. Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. Poult. Sci. 2015, 94, 673–680. [Google Scholar] [CrossRef]
- Kiarie, E.G.; Leung, H.; Akbari Moghaddam Kakhki, R.; Patterson, R.; Barta, J.R. Utility of feed enzymes and yeast derivatives in ameliorating deleterious effects of coccidiosis on intestinal health and function in broiler chickens. Front. Vet. Sci. 2019, 6, 473. [Google Scholar] [CrossRef]
- Teng, P.Y.; Yadav, S.; Shi, H.; Kim, W.K. Evaluating endogenous loss and standard ileal digestibility of amino acids in response to the graded severity levels of E. maxima infection. Poult. Sci. 2021, 100, 101426. [Google Scholar] [CrossRef]
- Teng, P.Y.; Choi, J.; Yadav, S.; Marshall, B.; Castro, F.L.S.; Ferrel, J.; Kim, W.K. Evaluation of a dacitic (rhyolitic) tuff breccia use on performance, inflammatory, and antioxidant responses in broilers mildly challenged with Eimeria spp. Poult. Sci. 2023, 102, 102697. [Google Scholar] [CrossRef]
- Fetterer, R.H.; Miska, K.B.; Jenkins, M.C.; Wong, E.A. Expression of nutrient transporters in duodenum, jejunum, and ileum of Eimeria maxima-infected broiler chickens. Parasitol. Res. 2014, 113, 3891–3894. [Google Scholar] [CrossRef]
- Miska, K.B.; Fetterer, R.H. The mRNA expression of amino acid and sugar transporters, aminopeptidase, as well as the di-and tri-peptide transporter PepT1 in the intestines of Eimeria infected broiler chickens. Poult. Sci. 2017, 96, 465–473. [Google Scholar] [CrossRef]
- Su, S.; Miska, K.B.; Fetterer, R.H.; Jenkins, M.C.; Wong, E.A. Expression of digestive enzymes and nutrient transporters in Eimeria-challenged broilers. Exp. Parasitol. 2015, 150, 13–21. [Google Scholar] [CrossRef]
- Castro, F.L.; Teng, P.Y.; Yadav, S.; Gould, R.L.; Craig, S.; Pazdro, R.; Kim, W.K. The effects of L-Arginine supplementation on growth performance and intestinal health of broiler chickens challenged with Eimeria spp. Poult. Sci. 2020, 99, 5844–5857. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.L.S.; Tompkins, Y.H.; Pazdro, R.; Kim, W.K. The effects of total sulfur amino acids on the intestinal health status of broilers challenged with Eimeria spp. Poult. Sci. 2020, 99, 5027–5036. [Google Scholar] [CrossRef]
- Xie, H.; Rath, N.C.; Huff, G.R.; Huff, W.E.; Balog, J.M. Effects of Salmonella typhimurium lipopolysaccharide on broiler chickens. Poult. Sci. 2000, 79, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Vandeplas, S.; Dauphin, R.D.; Thiry, C.; Beckers, Y.; Welling, G.W.; Thonart, P.; Thewis, A. Efficiency of a Lactobacillus plantarum-xylanase combination on growth performances, microflora populations, and nutrient digestibilities of broilers infected with Salmonella typhimurium. Poult. Sci. 2009, 88, 1643–1654. [Google Scholar] [CrossRef]
- Marcq, C.; Cox, E.; Szalo, I.M.; Thewis, A.; Beckers, Y. Salmonella typhimurium oral challenge model in mature broilers: Bacteriological, immunological, and growth performance aspects. Poult. Sci. 2011, 90, 59–67. [Google Scholar] [CrossRef]
- Adhikari, P.; Yadav, S.; Cosby, D.E.; Cox, N.A.; Jendza, J.A.; Kim, W.K. Research Note: Effect of organic acid mixture on growth performance and Salmonella typhimurium colonization in broiler chickens. Poult. Sci. 2020, 99, 2645–2649. [Google Scholar] [CrossRef]
- Choi, J.; Marshall, B.; Ko, H.; Shi, H.; Singh, A.K.; Thippareddi, H.; Holladay, S.; Gogal, R.M., Jr.; Kim, W.K. Antimicrobial and immunomodulatory effects of tannic acid supplementation in broilers infected with Salmonella typhimurium. Poult. Sci. 2022, 101, 102111. [Google Scholar] [CrossRef]
- Moharreri, M.; Vakili, R.; Oskoueian, E.; Rajabzadeh, G. Effects of microencapsulated essential oils on growth performance and biomarkers of inflammation in broiler chickens challenged with Salmonella enteritidis. J. Saudi Soc. Agric. Sci. 2022, 21, 349–357. [Google Scholar] [CrossRef]
- Jazi, V.; Foroozandeh, A.D.; Toghyani, M.; Dastar, B.; Koochaksaraie, R.R. Effects of Pediococcus acidilactici, mannan-oligosaccharide, butyric acid and their combination on growth performance and intestinal health in young broiler chickens challenged with Salmonella typhimurium. Poult. Sci. 2018, 97, 2034–2043. [Google Scholar] [CrossRef]
- Rajani, J.; Dastar, B.; Samadi, F.; Karimi Torshizi, M.A.; Abdulkhani, A.; Esfandyarpour, S. Effect of extracted galactoglucomannan oligosaccharides from pine wood (Pinus brutia) on Salmonella typhimurium colonisation, growth performance and intestinal morphology in broiler chicks. Br. Poult. Sci. 2016, 57, 682–692. [Google Scholar] [PubMed]
- Gan, L.; Fan, H.; Mahmood, T.; Guo, Y. Dietary supplementation with vitamin C. ameliorates the adverse effects of Salmonella enteritidis—Challenge in broilers by shaping intestinal microbiota. Poult. Sci. 2020, 99, 3663–3674. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.; Shao, Y.; Gong, X.; Wu, Y.; Geng, Y.; Wang, Z.; Guo, Y. Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella enteritidis. Poult. Sci. 2018, 97, 2654–2666. [Google Scholar] [CrossRef]
- Omrani, A.H.; Mousavi, S.N.; Foroudi, F.; Jafarabadi, G.A.; Hosseini, S.A.; Alahyaribeik, S. The effects of probiotic and threonine application on the carcass yield, internal organ development, intestinal morphology and cecal microbiota of broilers challenged with Clostridium perfringens. Res. Vet. Sci. 2023, 160, 1–10. [Google Scholar] [CrossRef]
- Shini, S.; Zhang, D.; Aland, R.C.; Li, X.; Dart, P.J.; Callaghan, M.J.; Speight, R.E.; Bryden, W.L. Probiotic Bacillus amyloliquefaciens H57 ameliorates subclinical necrotic enteritis in broiler chicks by maintaining intestinal mucosal integrity and improving feed efficiency. Poult. Sci. 2020, 99, 4278–4293. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Tahir, M.; Naz, S.; Khan, N.A.; Ullah Khan, R. In vitro efficacy and ameliorating effect of Moringa oleifera on growth, carcass, stress and digestibility of nutrients in Escherichia coli-infected broilers. J. Appl. Anim. Res. 2020, 50, 118–124. [Google Scholar] [CrossRef]
- Hite, J.L.; Cressler, C.E. Parasite-mediated anorexia and nutrition modulate virulence evolution. Integr. Comp. Biol. 2019, 59, 1264–1274. [Google Scholar] [CrossRef]
- Taylor, J.; Sakkas, P.; Kyriazakis, I. Starving for nutrients: Anorexia during infection with parasites in broilers is affected by diet composition. Poult. Sci. 2022, 101, 101535. [Google Scholar] [CrossRef]
- Bottje, W.G.; Harrison, P.C. Effect of carbonated water on growth performance of cockerels subjected to constant and cyclic heat stress temperatures. Poult. Sci. 1985, 64, 1285–1292. [Google Scholar] [CrossRef]
- Al-Fataftah, A.R.; Abdelqader, A. Effects of dietary Bacillus subtilis on heat-stressed broilers performance, intestinal morphology and microflora composition. Anim. Feed Sci. Technol. 2014, 198, 279–285. [Google Scholar] [CrossRef]
- Yi, D.; Hou, Y.; Tan, L.; Liao, M.; Xie, J.; Wang, L.; Ding, B.; Yang, Y.; Gong, J. N-acetylcysteine improves the growth performance and intestinal function in the heat-stressed broilers. Anim. Feed Sci. Technol. 2016, 220, 83–92. [Google Scholar] [CrossRef]
- Castro, F.L.S.; Kim, Y.; Xu, H.; Kim, W.K. The effect of total sulfur amino acid levels on growth performance and bone metabolism in pullets under heat stress. Poult. Sci. 2020, 99, 5783–5791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ou, J.; Luo, Z.; Kim, I.H. Effect of dietary β-1, 3-glucan supplementation and heat stress on growth performance, nutrient digestibility, meat quality, organ weight, ileum microbiota, and immunity in broilers. Poult. Sci. 2020, 99, 4969–4977. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef]
- Habashy, W.S.; Milfort, M.C.; Fuller, A.L.; Attia, Y.A.; Rekaya, R.; Aggrey, S.E. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int. J. Biometeorol. 2017, 61, 2111–2118. [Google Scholar] [CrossRef]
- Bonnet, S.; Geraert, P.A.; Lessire, M.; Carre, B.; Guillaumin, S. Effect of high ambient temperature on feed digestibility in broilers. Poult. Sci. 1997, 76, 857–863. [Google Scholar] [CrossRef]
- Turcotte, L.P.; Srivastava, A.K.; Chiasson, J.L. Fasting increases plasma membrane fatty acid-binding protein (FABPPM) in red skeletal muscle. Mol. Cell. Biochem. 1997, 166, 153–158. [Google Scholar] [CrossRef]
- Orhan, C.; Tuzcu, M.; Deeh, P.B.D.; Sahin, N.; Komorowski, J.R.; Sahin, K. Organic chromium form alleviates the detrimental effects of heat stress on nutrient digestibility and nutrient transporters in laying hens. Biol. Trace Elem. Res. 2019, 189, 529–537. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Sheikhahmadi, A.; Wang, Y.; Jiao, H.; Lin, H.; Song, Z. Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus gallus domesticus). Int. J. Biometeorol. 2015, 59, 127–135. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.D.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- Song, X.; Luo, J.; Fu, D.; Zhao, X.; Bunlue, K.; Xu, Z.; Qu, M. Traditional Chinese medicine prescriptions enhance growth performance of heat stressed beef cattle by relieving heat stress responses and increasing apparent nutrient digestibility. Asian-Austral. J. Anim. Sci. 2014, 27, 1513. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS ONE 2015, 10, e0138975. [Google Scholar] [CrossRef]
- Abdelqader, A.; Al-Fataftah, A.R. Effect of dietary butyric acid on performance, intestinal morphology, microflora composition and intestinal recovery of heat-stressed broilers. Livest. Sci. 2016, 183, 78–83. [Google Scholar] [CrossRef]
- Liu, L.; Fu, C.; Yan, M.; Xie, H.; Li, S.; Yu, Q.; He, S.; He, J. Resveratrol modulates intestinal morphology and HSP70/90, NF-κB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016, 7, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017, 96, 4325–4332. [Google Scholar] [CrossRef]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic heat stress alters hypothalamus integrity, the serum indexes and attenuates expressions of hypothalamic appetite genes in broilers. J. Therm. Biol. 2019, 81, 110–117. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zhou, Y.; Feng, J.; Zhang, M. Effects of heat stress on gut-microbial metabolites, gastrointestinal peptides, glycolipid metabolism, and performance of broilers. Animals 2021, 11, 1286. [Google Scholar] [CrossRef]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 2018, 98, 4471–4478. [Google Scholar] [CrossRef]
- Mtileni, B.J.; Nephawe, K.A.; Nesamvuni, A.E.; Benyi, K. The influence of stocking density on body weight, egg weight, and feed intake of adult broiler breeder hens. Poult. Sci. 2007, 86, 1615–1619. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Kalogeraki, E.; Goliomytis, M.; Charismiadou, M.A.; Triantaphyllopoulos, K.; Ayoutanti, A.; Niforou, K.; Hager-Theodorides, A.L.; Deligeorgis, S.G. Impact of stocking density on broiler growth performance, meat characteristics, behavioural components and indicators of physiological and oxidative stress. Bri. Poult. Sci. 2012, 53, 721–730. [Google Scholar] [CrossRef]
- Ligaraba, T.J.; Benyi, K.; Baloyi, J.J. Effects of genotype and stocking density on broiler performance under three feeding regimes. Trop. Anim. Health Prod. 2016, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Astaneh, I.Y.; Chamani, M.; Mousavi, S.N.; Sadeghi, A.A.; Afshar, M.A. Effects of stocking density on performance and immunity in Ross 308 broiler chickens. Kafkas Üniversitesi Vet. Fakültesi Derg. 2018, 24, 483–489. [Google Scholar]
- Feddes, J.J.; Emmanuel, E.J.; Zuidhoft, M.J. Broiler performance, body weight variance, feed and water intake, and carcass quality at different stocking densities. Poult. Sci. 2002, 81, 774–779. [Google Scholar] [CrossRef]
- Uzum, M.H.; Toplu, H.O. Effects of stocking density and feed restriction on performance, carcass, meat quality characteristics and some stress parameters in broilers under heat stress. Rev. Med.Vet. 2013, 164, 546–554. [Google Scholar]
- Shakeri, M.; Zulkifli, I.; Soleimani, A.F.; O’Reilly, E.L.; Eckersall, P.D.; Anna, A.A.; Kumari, S.; Eckersall, P.D.; Abdullah, F.F.J. Response to dietary supplementation of L-glutamine and L-glutamate in broiler chickens reared at different stocking densities under hot, humid tropical conditions. Poult. Sci. 2014, 93, 2700–2708. [Google Scholar] [CrossRef]
- Kridtayopas, C.; Rakangtong, C.; Bunchasak, C.; Loongyai, W. Effect of prebiotic and synbiotic supplementation in diet on growth performance, small intestinal morphology, stress, and bacterial population under high stocking density condition of broiler chickens. Poult. Sci. 2019, 98, 4595–4605. [Google Scholar] [CrossRef] [PubMed]
- Kamel, N.F.; Hady, M.M.; Ragaa, N.M.; Mohamed, F.F. Effect of nucleotides on growth performance, gut health, and some immunological parameters of broiler chicken exposed to high stocking density. Livest. Sci. 2021, 253, 104703. [Google Scholar] [CrossRef]
- Worthington, J.J.; Samuelson, L.C.; Grencis, R.K.; McLaughlin, J.T. Adaptive immunity alters distinct host feeding pathways during nematode induced inflammation, a novel mechanism in parasite expulsion. PLoS Pathog. 2013, 9, e1003122. [Google Scholar] [CrossRef]
- O’Hara, J.R.; Skinn, A.C.; MacNaughton, W.K.; Sherman, P.M.; Sharkey, K.A. Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cell. Microbiol. 2006, 8, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, R.; Vigliano, F.; Quiroga, M.I.; Nieto, J.M.; Bosi, G.; Domeneghini, C. Immunohistochemical study on the neuroendocrine system of the digestive tract of turbot, Scophthalmus maximus (L.), infected by Enteromyxum scophthalmi (Myxozoa). Fish Shellfish. Immunol. 2007, 22, 252–263. [Google Scholar] [CrossRef]
- Keller, J.; Binnewies, U.; Rösch, M.; Juul Holst, J.; Beglinger, C.; Andresen, V.; Layer, P. Gastric emptying and disease activity in inflammatory bowel disease. Eur. J. Clin. Investig. 2015, 45, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).