Genome-Wide Association Study of Lactation Traits in Chinese Holstein Cows in Southern China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Phenotypic Data
2.2. Genotyping and Quality Control
2.3. Phenotypic Data Processing
2.4. Fixed-Effects ANOVA
2.5. Estimation of Individual Breeding Values
2.6. Population Structure and Kinship Identification
2.7. Genome-Wide Association Study
2.8. Identification of Candidate Genes and Functional Enrichment Analysis
3. Results
3.1. Descriptive Statistics and Estimated Breeding Values of Lactation Ttraits
3.2. Population Stratification
3.3. Genome-Wide Association Results
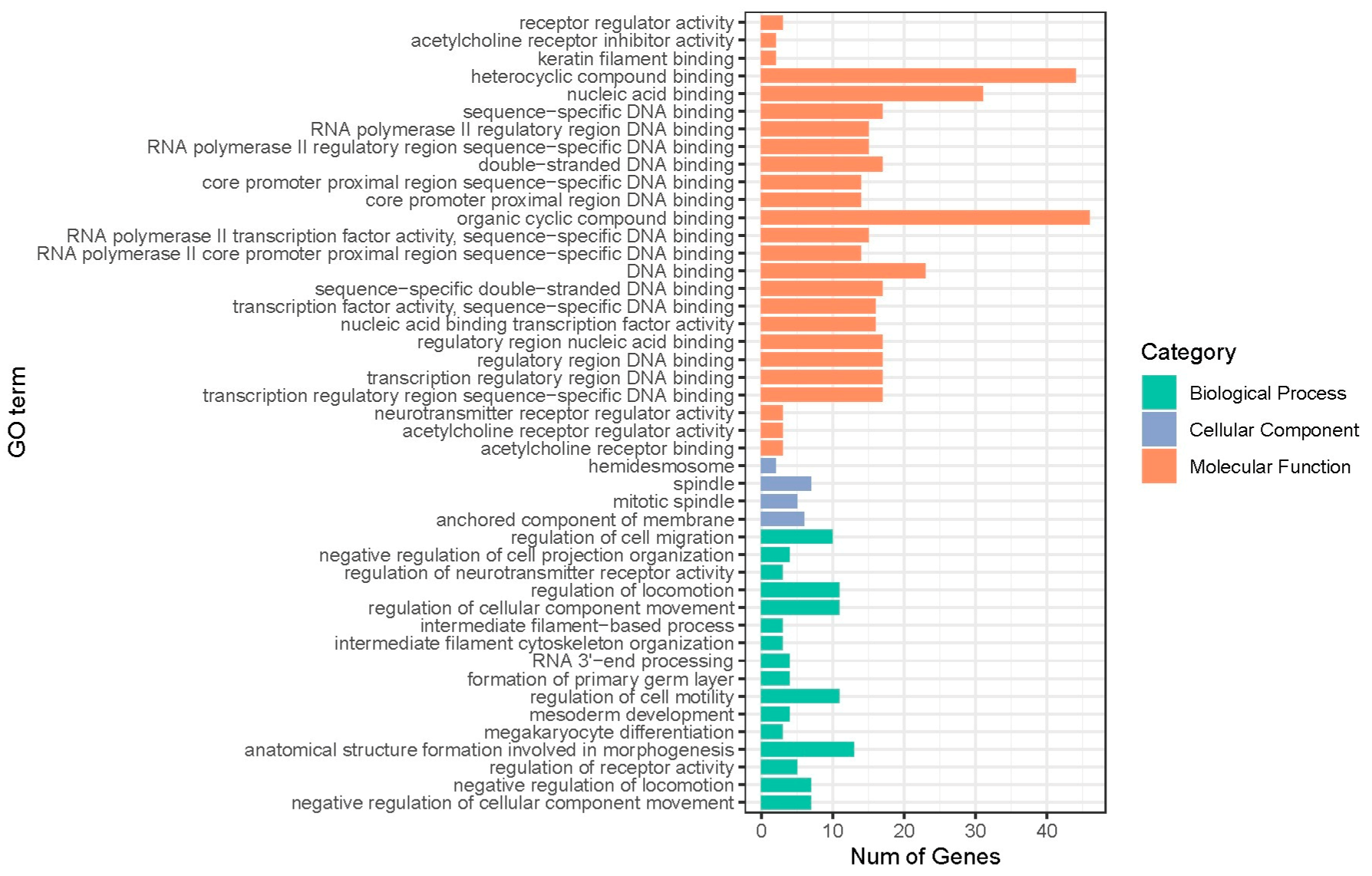
3.4. GO and KEGG Analyses for Lactation Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahi, A.K.; Nitter, G. Developing breeding schemes for pasture based dairy production systems in Kenya: I. Derivation of economic values using profit functions. Livest. Prod. Sci. 2004, 88, 161–177. [Google Scholar] [CrossRef]
- Qian, L.; Zhao, A.; Zhang, Y.; Chen, T.; Zeisel, S.H.; Jia, W.; Cai, W. Metabolomic Approaches to Explore Chemical Diversity of Human Breast-Milk, Formula Milk and Bovine Milk. Int. J. Mol. Sci. 2016, 17, 2128. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Chai, M.; Tian, C.; Li, Y.; Deng, T.; Wu, H.; Liu, X. Genetic variants of fatty acid elongase 6 in Chinese Holstein cow. Gene 2018, 670, 123–129. [Google Scholar] [CrossRef]
- West, J.W. Effects of Heat-Stress on Production in Dairy Cattle. J. Dairy. Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Cowley, F.C.; Barber, D.G.; Houlihan, A.V.; Poppi, D.P. Immediate and residual effects of heat stress and restricted intake on milk protein and casein composition and energy metabolism. J. Dairy. Sci. 2015, 98, 2356–2368. [Google Scholar] [CrossRef] [Green Version]
- Gorniak, T.; Meyer, U.; Südekum, K.H.; Dänicke, S. Impact of mild heat stress on dry matter intake, milk yield and milk composition in mid-lactation Holstein dairy cows in a temperate climate. Arch. Anim. Nutr. 2014, 68, 358–369. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Q.; Yi, M.; Pang, Z.H.; Xiong, B.H. Effects of seasonal change and parity on raw milk composition and related indices in Chinese Holstein cows in northern China. J. Dairy. Sci. 2013, 96, 6863–6869. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Z.; Zhuang, X.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Identification of circRNA-Associated-ceRNA Networks Involved in Milk Fat Metabolism under Heat Stress. Int. J. Mol. Sci. 2020, 21, 4162. [Google Scholar] [CrossRef]
- Sigdel, A.; Abdollahi-Arpanahi, R.; Aguilar, I.; Peñagaricano, F. Whole Genome Mapping Reveals Novel Genes and Pathways Involved in Milk Production Under Heat Stress in US Holstein Cows. Front. Genet. 2019, 10, 928. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, J.; Sun, D.; Ma, P.; Ding, X.; Yu, Y.; Zhang, Q. Genome wide association studies for milk production traits in Chinese Holstein population. PLoS ONE 2010, 5, e13661. [Google Scholar] [CrossRef] [Green Version]
- Iung, L.H.S.; Petrini, J.; Ramírez-Díaz, J.; Salvian, M.; Rovadoscki, G.A.; Pilonetto, F.; Dauria, B.D.; Machado, P.F.; Coutinho, L.L.; Wiggans, G.R.; et al. Genome-wide association study for milk production traits in a Brazilian Holstein population. J. Dairy. Sci. 2019, 102, 5305–5314. [Google Scholar] [CrossRef] [Green Version]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Ranjitkar, S.; Bu, D.; Van Wijk, M.; Ma, Y.; Ma, L.; Zhao, L.; Shi, J.; Liu, C.; Xu, J. Will heat stress take its toll on milk production in China? Clim. Chang. 2020, 161, 637–652. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Gao, X.; Song, W.; Chen, C.; Yao, D.; Ma, J.; Xu, L.; Ma, Y. Genome-wide association study of milk components in Chinese Holstein cows using single nucleotide polymorphism. Livest. Sci. 2020, 233, 103951. [Google Scholar] [CrossRef]
- Nayeri, S.; Sargolzaei, M.; Abo-Ismail, M.K.; May, N.; Miller, S.P.; Schenkel, F.; Moore, S.S.; Stothard, P. Genome-wide association for milk production and female fertility traits in Canadian dairy Holstein cattle. BMC Genet. 2016, 17, 75. [Google Scholar] [CrossRef] [Green Version]
- Nayeri, S.; Sargolzaei, M.; Abo-Ismail, M.K.; Miller, S.; Schenkel, F.; Moore, S.S.; Stothard, P. Genome-wide association study for lactation persistency, female fertility, longevity, and lifetime profit index traits in Holstein dairy cattle. J. Dairy. Sci. 2017, 100, 1246–1258. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Ning, C.; Liu, J.F.; Zhang, Q.; Jiang, L. Short communication: Replication of genome-wide association studies for milk production traits in Chinese Holstein by an efficient rotated linear mixed model. J. Dairy. Sci. 2019, 102, 2378–2383. [Google Scholar] [CrossRef] [Green Version]
- Prakapenka, D.; Liang, Z.; Jiang, J.; Ma, L.; Da, Y. A Large-Scale Genome-Wide Association Study of Epistasis Effects of Production Traits and Daughter Pregnancy Rate in U.S. Holstein Cattle. Genes 2021, 12, 1089. [Google Scholar] [CrossRef]
- Pausch, H.; Wurmser, C.; Reinhardt, F.; Emmerling, R.; Fries, R. Short communication: Validation of 4 candidate causative trait variants in 2 cattle breeds using targeted sequence imputation. J. Dairy. Sci. 2015, 98, 4162–4167. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Liu, X.; Yang, J.; Wang, H.; Jiang, J.; Liu, L.; He, S.; Ding, X.; Liu, J.; Zhang, Q. Targeted resequencing of GWAS loci reveals novel genetic variants for milk production traits. BMC Genom. 2014, 15, 1105. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Ma, L.; Prakapenka, D.; VanRaden, P.M.; Cole, J.B.; Da, Y. A Large-Scale Genome-Wide Association Study in U.S. Holstein Cattle. Front. Genet. 2019, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lian, Z.; Liu, G.; Deng, M.; Sun, B.; Guo, Y.; Liu, D.; Li, Y. Identification of genetic markers associated with milk production traits in Chinese Holstein cattle based on post genome-wide association studies. Anim. Biotechnol. 2021, 32, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wan, Z.; Zhang, J.; Xu, L.; Han, B.; Sun, D. Genome-Wide Association Studies for the Concentration of Albumin in Colostrum and Serum in Chinese Holstein. Animals 2020, 10, 2211. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Michal, J.J.; Wu, X.L.; Pan, Z.; MacNeil, M.D. The heparan and heparin metabolism pathway is involved in regulation of fatty acid composition. Int. J. Biol. Sci. 2011, 7, 659–663. [Google Scholar] [CrossRef] [Green Version]
- Mehla, K.; Magotra, A.; Choudhary, J.; Singh, A.K.; Mohanty, A.K.; Upadhyay, R.C.; Srinivasan, S.; Gupta, P.; Choudhary, N.; Antony, B.; et al. Genome-wide analysis of the heat stress response in Zebu (Sahiwal) cattle. Gene 2014, 533, 500–507. [Google Scholar] [CrossRef]
- Silanikove, N.; Maltz, E.; Halevi, A.; Shinder, D. Metabolism of Water, Sodium, Potassium, and Chlorine by High Yielding Dairy Cows at the Onset of Lactation. J. Dairy. Sci. 1997, 80, 949–956. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Osorio, J.S.; Lohakare, J.; Bionaz, M. Biosynthesis of milk fat, protein, and lactose: Roles of transcriptional and posttranscriptional regulation. Physiol. Genom. 2016, 48, 231–256. [Google Scholar] [CrossRef] [Green Version]
- Thaller, G.; Krämer, W.; Winter, A.; Kaupe, B.; Erhardt, G.; Fries, R. Effects of DGAT1 variants on milk production traits in German cattle breeds. J. Anim. Sci. 2003, 81, 1911–1918. [Google Scholar] [CrossRef]
- Schennink, A.; Stoop, W.M.; Visker, M.H.; Heck, J.M.; Bovenhuis, H.; van der Poel, J.J.; van Valenberg, H.J.; van Arendonk, J.A. DGAT1 underlies large genetic variation in milk-fat composition of dairy cows. Anim. Genet. 2007, 38, 467–473. [Google Scholar] [CrossRef]
- Cases, S.; Smith, S.J.; Zheng, Y.W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collins, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.; Dad, R.; Worku, T.; Xu, S.; Ullah, F.; Zhang, M.; Liang, X.; Den, T.; Fan, M.; Zhang, S. The functions and mechanisms of sequence differences of DGAT1 gene on milk fat synthesis between dairy cow and buffalo. J. Dairy. Res. 2020, 87, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Aragão, D.P.; da Silva Souza, B.; de Brito, T.V.; de Araújo Bastos Santana, L.; de Paiva Silva, R.M.; de Oliveira, A.P.; da Cunha Pereira, A.C.T.; Ferreira, G.P.; Dos Reis Barbosa, A.L.; de Oliveira, J.S. The anti-inflammatory and antinociceptive activity of albumins from Crotalaria retusa seeds. Biomed. Pharmacother. 2017, 93, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Metallo, C.M.; Gameiro, P.A.; Bell, E.L.; Mattaini, K.R.; Yang, J.; Hiller, K.; Jewell, C.M.; Johnson, Z.R.; Irvine, D.J.; Guarente, L.; et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2011, 481, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Martins, R.; Machado, P.C.; Pinto, L.F.B.; Silva, M.R.; Schenkel, F.S.; Brito, L.F.; Pedrosa, V.B. Genome-wide association study and pathway analysis for fat deposition traits in nellore cattle raised in pasture-based systems. J. Anim. Breed. Genet. 2021, 138, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Marai, I.F.; Habeeb, A.A.; Farghaly, H.M. Productive, physiological and biochemical changes in imported and locally born Friesian and Holstein lactating cows under hot summer conditions of Egypt. Trop. Anim. Health Prod. 1999, 31, 233–243. [Google Scholar] [CrossRef]
- Kuo, M.L.; Lee, M.B.; Tang, M.; den Besten, W.; Hu, S.; Sweredoski, M.J.; Hess, S.; Chou, C.M.; Changou, C.A.; Su, M.; et al. PYCR1 and PYCR2 Interact and Collaborate with RRM2B to Protect Cells from Overt Oxidative Stress. Sci. Rep. 2016, 6, 18846. [Google Scholar] [CrossRef] [Green Version]
- Phang, J.M.; Pandhare, J.; Liu, Y. The metabolism of proline as microenvironmental stress substrate. J. Nutr. 2008, 138, 2008s–2015s. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hyde, A.S.; Simpson, M.A.; Barycki, J.J. Emerging regulatory paradigms in glutathione metabolism. Adv. Cancer Res. 2014, 122, 69–101. [Google Scholar] [CrossRef] [Green Version]
- Pederzolli, C.D.; Sgaravatti, A.M.; Braum, C.A.; Prestes, C.C.; Zorzi, G.K.; Sgarbi, M.B.; Wyse, A.T.; Wannmacher, C.M.; Wajner, M.; Dutra-Filho, C.S. 5-Oxoproline reduces non-enzymatic antioxidant defenses in vitro in rat brain. Metab. Brain Dis. 2007, 22, 51–65. [Google Scholar] [CrossRef]
- Lu, X.; Arbab, A.A.I.; Abdalla, I.M.; Liu, D.; Zhang, Z.; Xu, T.; Su, G.; Yang, Z. Genetic Parameter Estimation and Genome-Wide Association Study-Based Loci Identification of Milk-Related Traits in Chinese Holstein. Front. Genet. 2021, 12, 799664. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Chang, W.C.; Shibuya, A.; Yoshida, A. Human stomach aldehyde dehydrogenase cDNA and genomic cloning, primary structure, and expression in Escherichia coli. J. Biol. Chem. 1992, 267, 3030–3037. [Google Scholar] [CrossRef] [PubMed]
- Audrito, V.; Messana, V.G.; Deaglio, S. NAMPT and NAPRT: Two Metabolic Enzymes With Key Roles in Inflammation. Front. Oncol. 2020, 10, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wibowo, T.A.; Gaskins, C.T.; Newberry, R.C.; Thorgaard, G.H.; Michal, J.J.; Jiang, Z. Genome assembly anchored QTL map of bovine chromosome 14. Int. J. Biol. Sci. 2008, 4, 406–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Huang, J.; Jiang, M.; Lin, H. Tissue-specific transplantation antigen P35B (TSTA3) immune response-mediated metabolism coupling cell cycle to postreplication repair network in no-tumor hepatitis/cirrhotic tissues (HBV or HCV infection) by biocomputation. Immunol. Res. 2012, 52, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Buaban, S.; Lengnudum, K.; Boonkum, W.; Phakdeedindan, P. Genome-wide association study on milk production and somatic cell score for Thai dairy cattle using weighted single-step approach with random regression test-day model. J. Dairy. Sci. 2022, 105, 468–494. [Google Scholar] [CrossRef]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]


| Traits | n | Mean | Min | Max | SD | CV (%) |
|---|---|---|---|---|---|---|
| MY | 392 | 9807.07 | 4870.42 | 14205.58 | 1538.60 | 15.64 |
| FP | 392 | 3.54 | 1.97 | 5.07 | 0.48 | 13.56 |
| PP | 392 | 3.19 | 2.34 | 3.76 | 0.23 | 7.21 |
| Item | MY | FP | PP |
|---|---|---|---|
| Calving age | <0.001 *** | <0.001 *** | 0.797 |
| Parity | 0.101 | 0.002 ** | 0.296 |
| Calving season | <0.001 *** | 0.007 ** | 0.054 |
| Traits | n | Mean | Min | Max | SD | Heritability |
|---|---|---|---|---|---|---|
| MY | 392 | 70.25 | −1013.32 | 1118.44 | 408.94 | 0.07 |
| FP | 392 | −0.03 | −0.45 | 0.41 | 0.16 | 0.12 |
| PP | 392 | −0.01 | −0.34 | 0.26 | 0.10 | 0.20 |
| Traits | SNP Name | BTA | Position (bp) | p-Value | Nearest Gene Name | Distance (bp) |
|---|---|---|---|---|---|---|
| FP | ARS-BFGL-NGS-4939 | 14 | 609,870 | 2.85 × 10−9 | DGAT1 | Within |
| Chr14_1765835 | 14 | 580,019 | 2.96 × 10−9 | SLC52A2 | Within | |
| BovineHD1400000216 | 14 | 550,784 | 3.12 × 10−9 | CPSF1 | Within | |
| Chr14_1757935 | 14 | 572,120 | 2.74 × 10−8 | ADCK5 | 1622 | |
| Chr14_2022745 | 14 | 831,004 | 6.44 × 10−8 | GRINA | 938 | |
| Chr14_1699016 | 14 | 513,203 | 8.36 × 10−8 | VPS28 | 384 | |
| BovineHD1400000282 | 14 | 859,251 | 9.51 × 10−8 | PLEC | Within | |
| BovineHD1400000287 | 14 | 883,732 | 9.51 × 10−8 | PLEC | Within | |
| BovineHD1400000275 | 14 | 2,019,390 | 1.00 × 10−7 | TSNARE1 | Within | |
| ARS-BFGL-NGS-57820 | 14 | 465,742 | 1.46 × 10−7 | FOXH1 | 3390 | |
| BovineHD1400000206 | 14 | 494,621 | 2.25 × 10−7 | TONSL | 1650 | |
| Chr14_1653693 | 14 | 468,124 | 2.52 × 10−7 | FOXH1 | 1008 | |
| BovineHD1400000204 | 14 | 487,527 | 3.05 × 10−7 | CYHR1 | Within | |
| DB-813-seq-rs110906821 | 14 | 758,854 | 8.04 × 10−7 | EXOSC4 | 1844 | |
| BovineHD1200022127 | 12 | 73,935,024 | 2.02 × 10−6 | HS6ST3 | Within | |
| BovineHD1400000271 | 14 | 810,116 | 5.73 × 10−6 | SPATC1 | 3725 | |
| PP | Hapmap32084-BTA-147824 | 21 | 6,095,419 | 1.40 × 10−6 | CERS3 | Within |
| Hapmap25132-BTA-96391 | 13 | 27,232,535 | 2.37 × 10−6 | FZD8 | 445,190 | |
| ARS-BFGL-NGS-4300 | 21 | 21,278,489 | 3.94 × 10−6 | AP3S2 | Within | |
| ARS-BFGL-NGS-40264 | 11 | 2,382,261 | 6.04 × 10−6 | SNRNP200 | Within | |
| BovineHD2100000735 | 21 | 4,285,575 | 8.19 × 10−6 | GABRA5 | Within | |
| BovineHD1100000821 | 11 | 2,385,285 | 9.53 × 10−6 | SNRNP200 | Within | |
| BovineHD1100000817 | 11 | 2,381,644 | 1.10 × 10−5 | SNRNP200 | Within |
| Term | Description | Gene Count | % | p-Value | Gene |
|---|---|---|---|---|---|
| bta01100 | Metabolic pathways | 16 | 11.1 | 0.03333 | CERS3, DGAT1, GPAA1, IDH2, GPT, PYCR3, OPLAH, HDDC3, ALDH1A3, MAN2A2, NAPRT, CYP11B1, ANPEP, CYC1, GPAT2, GFUS |
| bta00480 | Glutathione metabolism | 3 | 2.1 | 0.04989 | ANPEP, IDH2, OPLAH |
| bta01230 | Biosynthesis of amino acids | 3 | 2.1 | 0.06663 | IDH2, GPT, PYCR3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.; Lin, X.; Xiao, Z.; She, Y.; Deng, M.; Liu, G.; Sun, B.; Guo, Y.; Liu, D.; Li, Y. Genome-Wide Association Study of Lactation Traits in Chinese Holstein Cows in Southern China. Animals 2023, 13, 2545. https://doi.org/10.3390/ani13152545
Su M, Lin X, Xiao Z, She Y, Deng M, Liu G, Sun B, Guo Y, Liu D, Li Y. Genome-Wide Association Study of Lactation Traits in Chinese Holstein Cows in Southern China. Animals. 2023; 13(15):2545. https://doi.org/10.3390/ani13152545
Chicago/Turabian StyleSu, Minqiang, Xiaojue Lin, Zupeng Xiao, Yuanhang She, Ming Deng, Guangbin Liu, Baoli Sun, Yongqing Guo, Dewu Liu, and Yaokun Li. 2023. "Genome-Wide Association Study of Lactation Traits in Chinese Holstein Cows in Southern China" Animals 13, no. 15: 2545. https://doi.org/10.3390/ani13152545





