Strategies and Mechanisms of Thermal Compensation in Newborn Water Buffaloes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Hypothermia in Newborn Ruminants
3. Intrinsic Factors Affecting the Temperature in the Newborn Buffalo
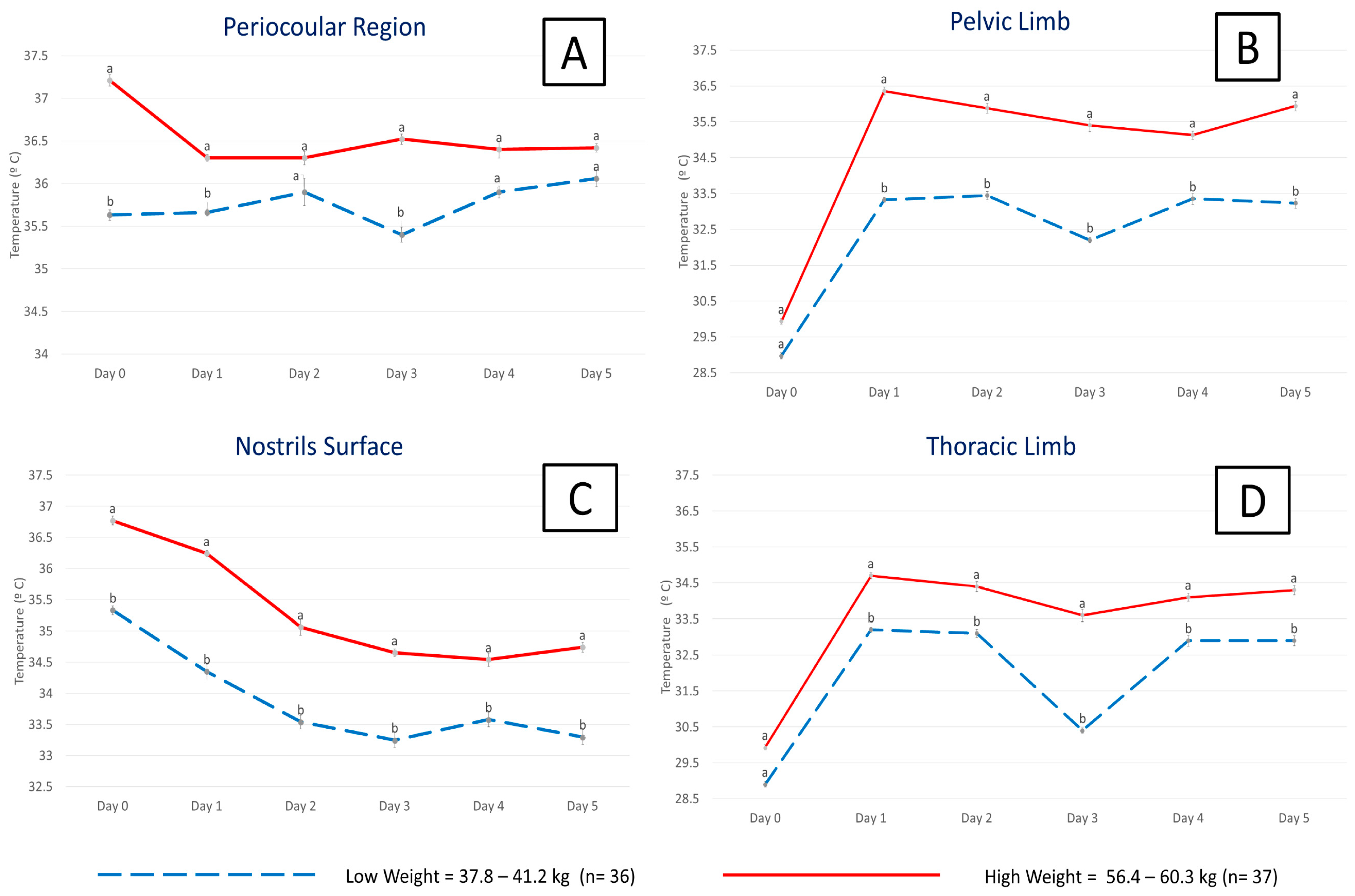
3.1. Birth Weight
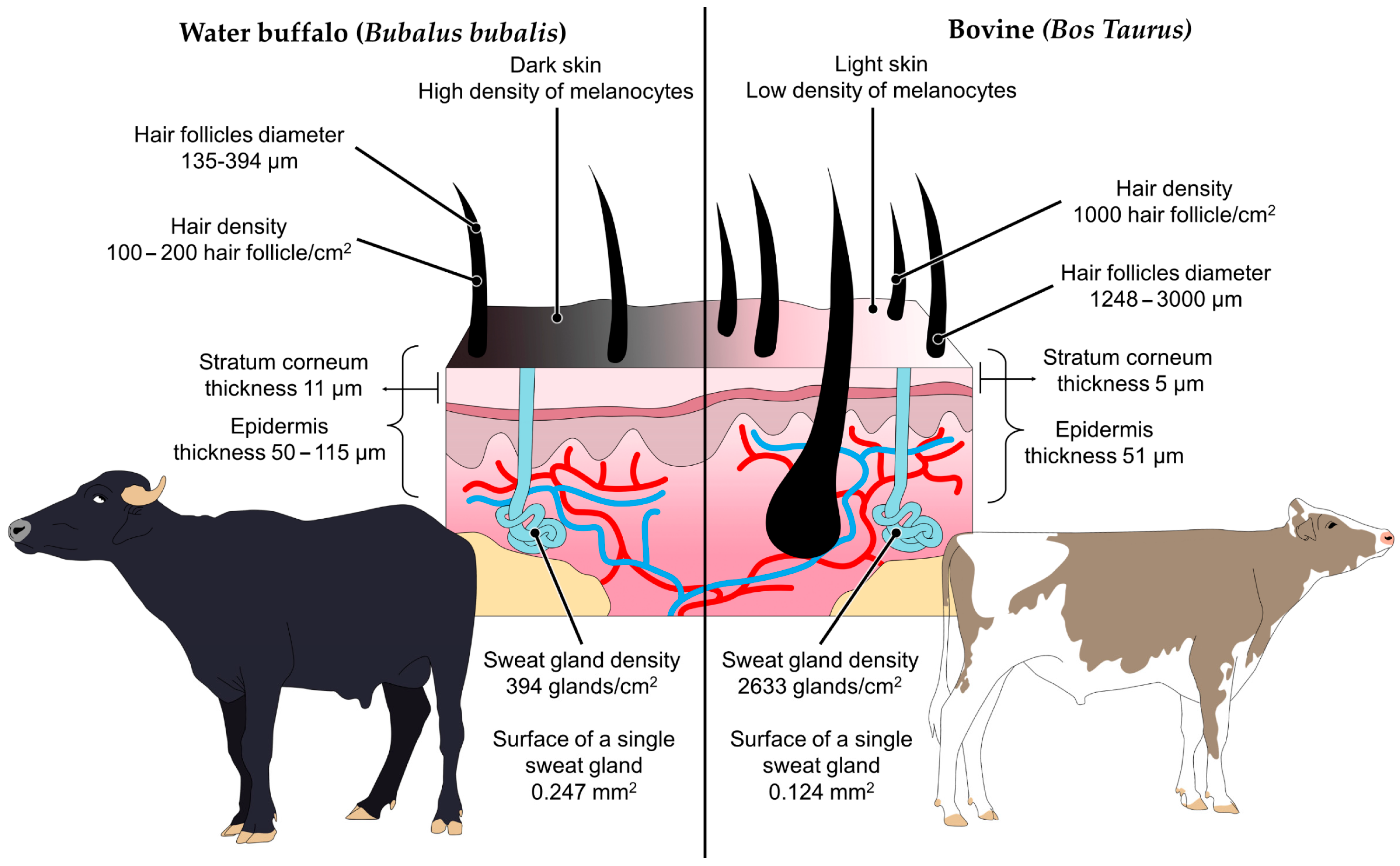
3.2. Skin Features
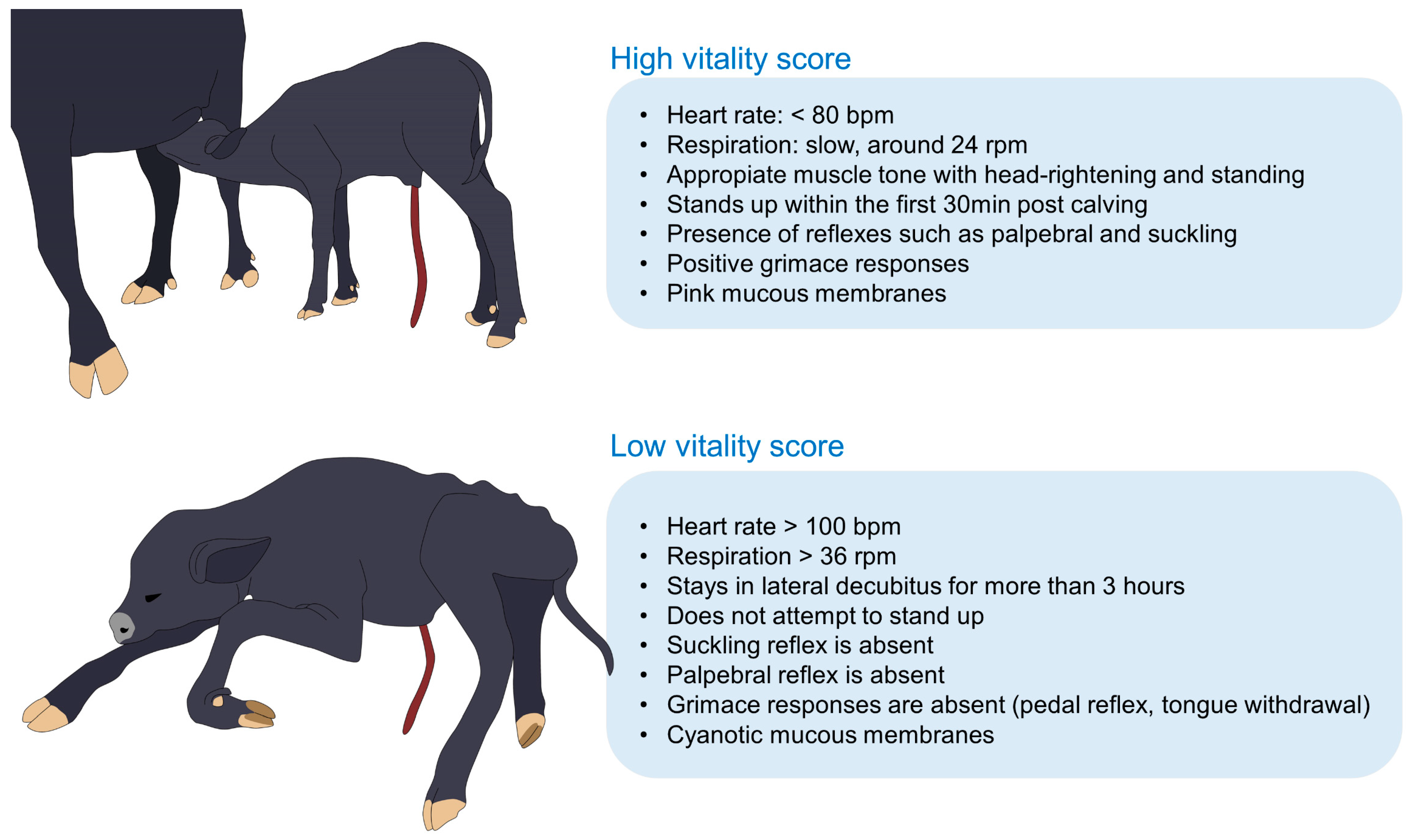
3.3. Neonatal Vitality
4. Effect of Environmental Factors
5. Thermoregulatory Mechanisms Triggered to Face Neonatal Hypothermia
5.1. Vasomotor Control
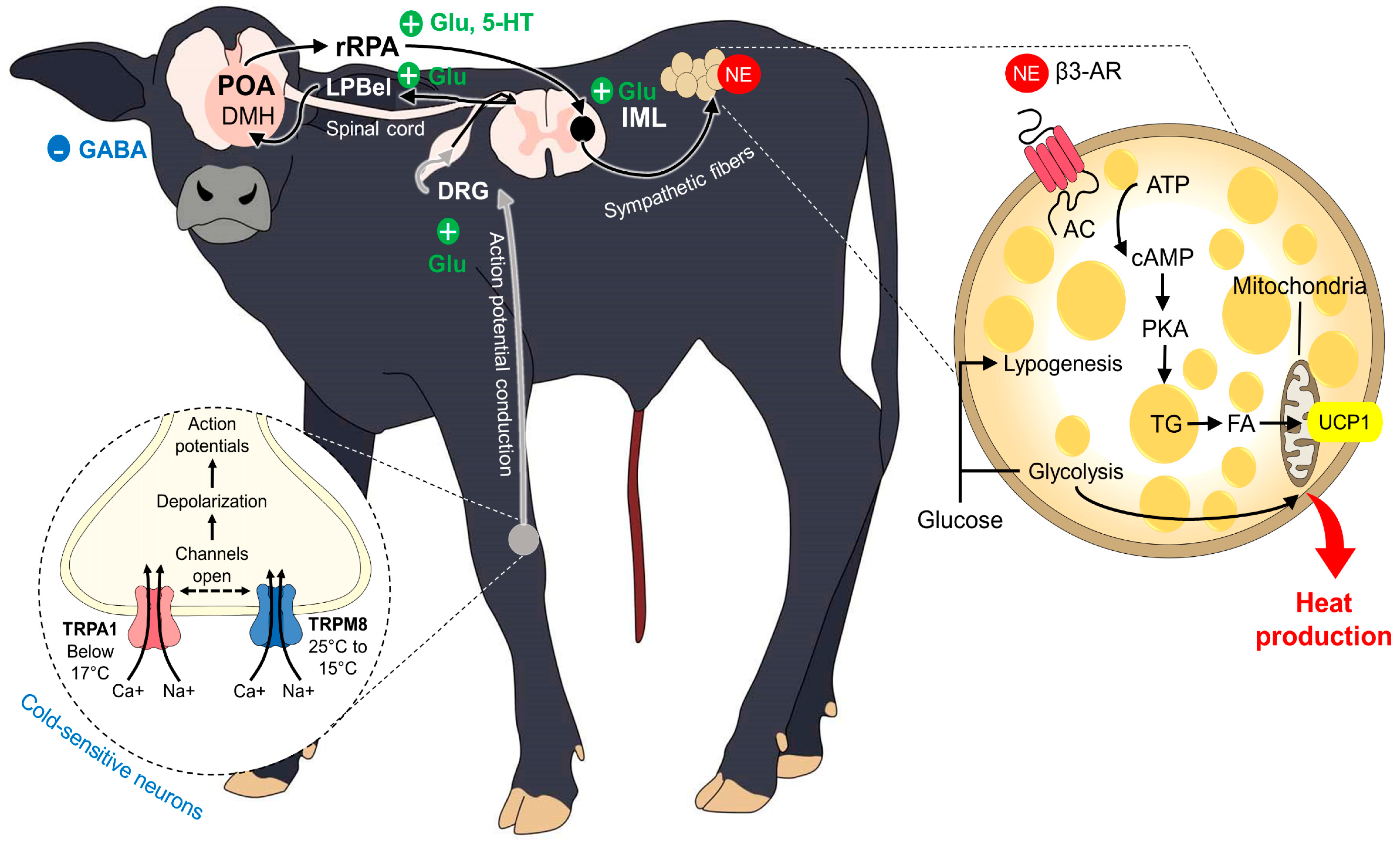
5.2. BAT Thermogenesis
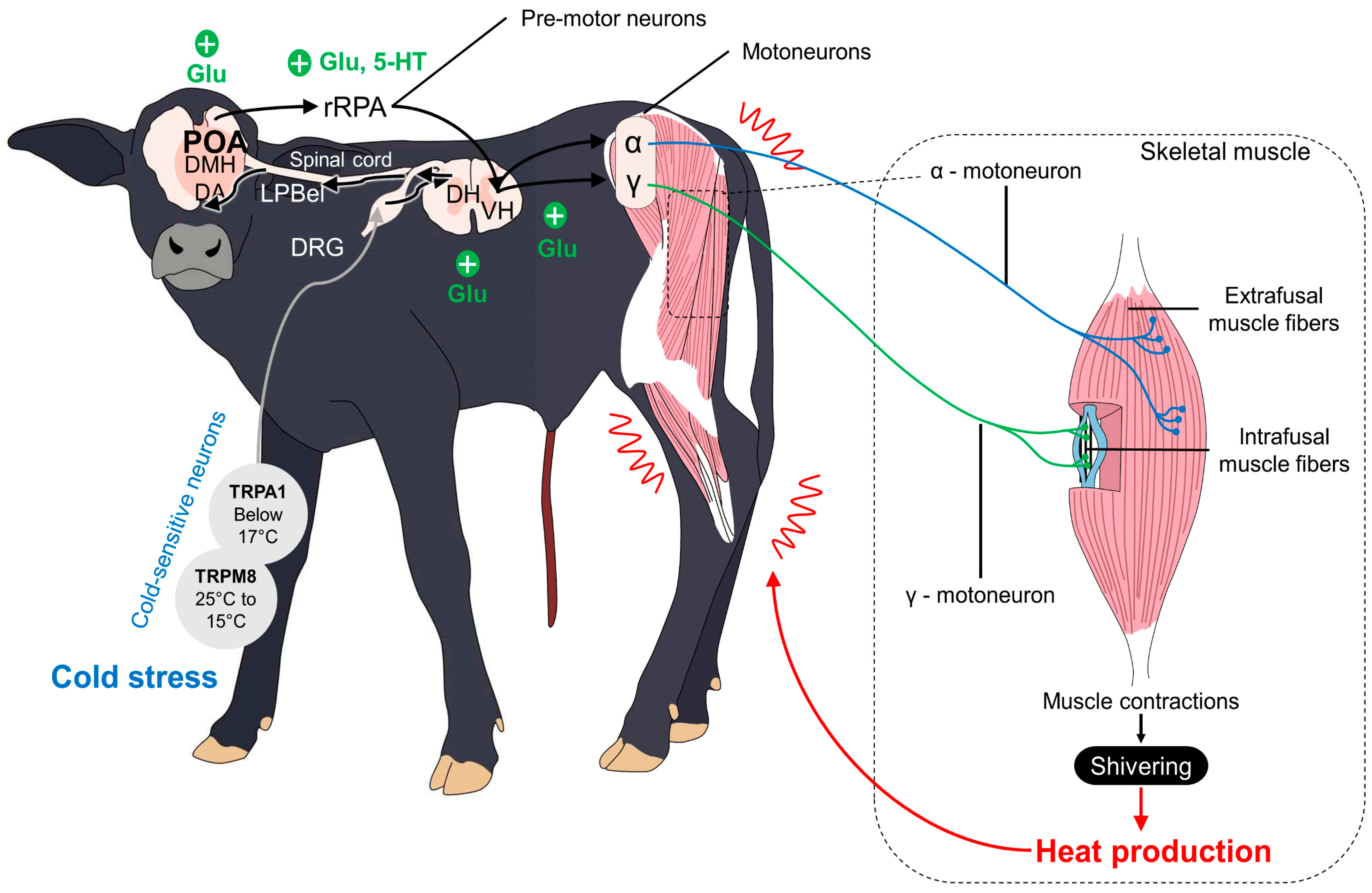
5.3. Shivering Thermogenesis
5.4. Changes in Behavior and Posture in the Face of Cold Stress
6. Colostrum Intake: Nutritional, Immunological, and Physicochemical Aspects
7. Evaluation of the Thermal Response through Infrared Thermography (IRT)
7.1. Central Thermal Windows
7.2. Peripheral Thermal Windows
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kozat, S. Hypothermia in newborn calves. J. Istanbul Vet. Sci. 2018, 2, 30–37. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mota-Rojas, D.; Martínez-Burnes, J.; Villanueva-García, D.; Domínguez-Oliva, A.; Gómez-Prado, J.; Mora-Medina, P.; Casas-Alvarado, A.; Olmos-Hernández, A.; Soto, P.; et al. Strategies for hypothermia compensation in altricial and precocial newborn mammals and their monitoring by infrared thermography. Vet. Sci. 2022, 9, 246. [Google Scholar] [CrossRef]
- Napolitano, F.; Bragaglio, A.; Braghieri, A.; El-Aziz, A.H.A.; Titto, C.G.; Villanueva-García, D.; Mora-Medina, P.; Pereira, A.M.F.; Hernández-Avalos, I.; José-Pérez, N.; et al. The effect of birth weight and time of day on the thermal response of newborn water buffalo calves. Front. Vet. Sci. 2023, 10, 84092. [Google Scholar] [CrossRef]
- Rodríguez-González, D.; Minervino, A.H.H.; Orihuela, A.; Bertoni, A.; Morales-Canela, D.A.D.A.; Álvarez-Macías, A.; José-Pérez, N.; Domínguez-Oliva, A.; Mota-Rojas, D.; Hamad, A.M.; et al. Handling and physiological aspects of the dual-purpose water buffalo production system in the mexican humid tropics. Animals 2022, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Yaqoob, M.; Hashmi, N.; Zaman, M.A.; Amjad, M.S. Farmers’ attitude towards interventions regarding buffalo calf health care and management practices under field conditions. Pak. Vet. J. 2009, 29, 125–128. [Google Scholar]
- Khan, Z.Z.U.; Khan, S.; Ahmad, N.; Raziq, A. Investigation of mortality incidence and managemental practices in buffalo calves at commercial dairy farms in Peshawar City. J. Agric. Biol. Sci. 2007, 2, 16–22. [Google Scholar]
- Hancock, R.D.; Coe, A.J.; Conde de Albite Silva, F. Perinatal mortality in lambs in Southern Brazil. Trop. Anim. Health Prod. 1996, 28, 266–272. [Google Scholar] [CrossRef]
- Hassan, N.; Shaheen, M.; Bashir, S. Hypothermia in a Lamb: A case report. J. Entomol. Zool. Stud. 2020, 8, 1777–1778. [Google Scholar]
- Powers, D.R.; Langland, K.M.; Wethington, S.M.; Powers, S.D.; Graham, C.H.; Tobalske, B.W. Hovering in the heat: Effects of environmental temperature on heat regulation in foraging hummingbirds. R. Soc. Open Sci. 2017, 4, 171056. [Google Scholar] [CrossRef] [Green Version]
- Lotito, D.; Pacifico, E.; Matuozzo, S.; Musco, N.; Iommelli, P.; Tudisco, R.; Lombardi, P. Colostrum management for buffalo calves: A review. Vet. Sci. 2023, 10, 358. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D.; la Fuente, V.C.; et al. Physiological and behavioral mechanisms of thermoregulation in mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Napolitano, F.; Braghieri, A.; Guerrero-Legarreta, I.; Bertoni, A.; Martínez-Burnes, J.; Cruz-Monterrosa, R.; Gómez, J.; Ramírez-Bribiesca, E.; Barrios-García, H.; et al. Thermal biology in river buffalo in the humid tropics: Neurophysiological and behavioral responses assessed by infrared thermography. J. Anim. Behav. Biometeorol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; de Mira Geraldo, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals 2021, 11, 3472. [Google Scholar] [CrossRef]
- Bertoni, A.; Napolitano, F.; Mota-Rojas, D.; Sabia, E.; Álvarez-Macías, A.; Mora-Medina, P.; Morales-Canela, A.; Berdugo-Gutiérrez, J.; Guerrero- Legarreta, I.; Mendoza, A.B.; et al. Similarities and differences between river buffaloes and cattle: Health, physiological, behavioral and productivity aspects. J. Buffalo Sci. 2020, 9, 92–109. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Martínez-Burnes, J.; Casas-Alvarado, A.; Gómez-Prado, J.; Hernández-Ávalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Jacome-Romero, J.; Rodríguez-González, D.; Pereira, A.M.F. Clinical usefulness of infrared thermography to detect sick animals: Frequent and current cases. CABI Rev. 2022, 2022, 1–27. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Cook, N.; Tessaro, S.V.; Deregt, D.; Desroches, G.; Dubeski, P.L.; Tong, A.K.W.; Godson, D.L. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Verduzco-Mendoza, A.; Bueno-Nava, A.; Wang, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Casas, A.; Domínguez, A.; Mota-Rojas, D. Experimental applications and factors involved in validating thermal windows using infrared thermography to assess the health and thermostability of laboratory animals. Animals 2021, 11, 3448. [Google Scholar] [CrossRef]
- Gómez-Prado, J.; Pereira, A.M.F.; Wang, D.; Villanueva-García, D.; Domínguez-Oliva, A.; Mora-Medina, P.; Hernández-Avalos, I.; Martínez-Burnes, J.; Casas-Alvarado, A.; Olmos-Hernández, A.; et al. Thermoregulation mechanisms and perspectives for validating thermal windows in pigs with hypothermia and hyperthermia: An overview. Front. Vet. Sci. 2022, 9, 1023294. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Wang, D.; Titto, C.G.; Gómez-Prado, J.; Carvajal-de la Fuente, V.; Ghezzi, M.; Boscato-Funes, L.; Barrios-García, H.; Torres-Bernal, F.; Casas-Alvarado, A.; et al. Pathophysiology of Fever and Application of Infrared Thermography (IRT) in the Detection of Sick Domestic Animals: Recent Advances. Animals 2021, 11, 2316. [Google Scholar] [CrossRef]
- Cuttance, E.; Laven, R. Perinatal mortality risk factors in dairy calves. Vet. J. 2019, 253, 105394. [Google Scholar] [CrossRef]
- Ratanapob, N.; VanLeeuwen, J.; McKenna, S.; Wichtel, M.; Stryhn, H.; Rodriguez-Lecompte, J.C.; Menzies, P.; Wichtel, J. Management factors associated with perinatal lamb mortality in Prince Edward Island flocks. Prev. Vet. Med. 2020, 180, 105035. [Google Scholar] [CrossRef] [PubMed]
- Slee, J.; Griffiths, R.G.; Samson, D.E. Hypothermia in newborn lambs induced by experimental immersion in a water bath and by natural exposure outdoors. Res. Vet. Sci. 1980, 1, 275–280. [Google Scholar] [CrossRef]
- Refshauge, G.; Brien, F.D.; Hinch, G.N.; van de Ven, R. Neonatal lamb mortality: Factors associated with the death of Australian lambs. Anim. Prod. Sci. 2016, 56, 726. [Google Scholar] [CrossRef] [Green Version]
- Khounsy, S.; Nampanya, S.; Inthavong, P.; Yang, M.; Khamboungheung, B.; Avery, M.; Bush, R.; Rast, L.; Windsor, P.A. Significant mortality of large ruminants due to hypothermia in northern and central Lao PDR. Trop. Anim. Health Prod. 2012, 44, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Wang, D.D.-H.; Titto, C.G.; Martínez-Burnes, J.; Villanueva-García, D.; Lezama, K.; Domínguez, A.; Hernández-Avalos, I.; Mora-Medina, P.; Verduzco, A.; et al. Neonatal infrared thermography images in the hypothermic ruminant model: Anatomical-morphological-physiological aspects and mechanisms for thermoregulation. Front. Vet. Sci. 2022, 9, 963205. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Bragaglio, A.; Braghieri, A.; Napolitano, F.; Domínguez-Oliva, A.; Mora-Medina, P.; Álvarez-Macías, A.; De Rosa, G.; Pacelli, C.; José, N.; et al. Dairy buffalo behavior: Calving, imprinting and allosuckling. Animals 2022, 12, 2899. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Haeeb, A.A.M. Buffalo’s biological functions as affected by heat stress—A review. Livest. Sci. 2010, 127, 89–109. [Google Scholar] [CrossRef]
- Londoño, C.R.; Sánchez, E.N.; Prada Sanmiguel, G.A.A.; Londoño, R.C.; Sánchez, M.E.N.; Prada Sanmiguel, G.A.A. Parámetros fisiológicos y valores hematológicos normales en búfalos (Bubalus bubalis) del Magdalena Medio colombiano. Rev. Med. Vet. 2012, 1, 51. [Google Scholar] [CrossRef]
- Asakura, H. Fetal and Neonatal Thermoregulation. J. Nippon Med. Sch. 2004, 71, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Rook, J.S.; Scholman, G.; Wing-Proctor, S.; Shea, M. Diagnosis and control of neonatal losses in sheep. Vet. Clin. North Am. Food Anim. Pract. 1990, 6, 531–562. [Google Scholar] [CrossRef]
- Menant, O.; Ungerfeld, R.; Pérez-Clariget, R.; Freitas-de-Melo, A. Is body surface temperature measured on the single lambs’ back a reliable indicator of the ewe-lamb bond around birth? J. Therm. Biol. 2020, 93, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-García, D.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Mora-Medina, P.; Salmerón, C.; Gómez, J.; Boscato, L.; Gutiérrez-Pérez, O.; Cruz, V.; et al. Hypothermia in newly born piglets: Mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometeorol. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Olson, D.; Ritter, R.; Papasian, C.; Gutenberger, S. Sympathoadrenal and adrenal hormonal responses of newborn calves to hypothermia. Can. J. Comp. Med. 1981, 45, 321–326. [Google Scholar] [PubMed]
- Jain, A.K.; Tripathi, R.K.; Sharma, I.J.; Quadri, M.A.; Agrawal, R.G. Relationship of serum lipids with development of hypothermia in neonatal bovines. Buffalo Bull. 2007, 26, 67–71. [Google Scholar]
- Mellor, D.J.; Stafford, K.J. Animal welfare implications of neonatal mortality and morbidity in farm animals. Vet. J. 2004, 168, 118–133. [Google Scholar] [CrossRef]
- Tyler, H.; Ramsey, H. Hypoxia in neonatal calves: Effect on selected metabolic parameters. J. Dairy Sci. 1991, 74, 1957–1962. [Google Scholar] [CrossRef]
- Nuñez, A.; Benavente, I.; Blanco, D.; Boix, H.; Cabañas, F.; Chaffanel, M.; Fernández-Colomer, B.; Fernández-Lorenzo, J.R.; Loureiro, B.; Moral, M.T.; et al. Estrés oxidativo en la asfixia perinatal y la encefalopatía hipóxico-isquémica. An. Pediatría 2018, 88, 228.e1–228.e9. [Google Scholar] [CrossRef]
- Marcato, F.; van den Brand, H.; Kemp, B.; van Reenen, K. Evaluating potential biomarkers of health and performance in veal calves. Front. Vet. Sci. 2018, 5, 133. [Google Scholar] [CrossRef] [Green Version]
- Dubey, P.; Singh, R.R.; Choudhary, S.S.; Verma, K.K.; Kumar, A.; Gamit, P.M.; Dubey, S.; Prajapati, K. Post parturient neonatal behaviour and their relationship with maternal behaviour score, parity and sex in surti buffaloes. J. Appl. Anim. Res. 2018, 46, 360–364. [Google Scholar] [CrossRef]
- Metz, J.; Metz, J.H.M. Maternal influence on defecation and urination in the newborn calf. Appl. Anim. Behav. Sci. 1986, 16, 325–333. [Google Scholar] [CrossRef]
- Jainudeen, M.; Hafez, E. Cattle and buffalo. In Reproduction in Farm Animals; Hafez, E., Hafez, B., Eds.; Blackwell Publishing: Philadelphia, PA, USA, 2000; pp. 159–171. [Google Scholar]
- Lammoglia, M.A.; Bellows, R.A.; Grings, E.E.; Bergman, J.W. Effects of prepartum supplementary fat and muscle hypertrophy genotype on cold tolerance in newborn calves. J. Anim. Sci. 1999, 77, 2227. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, L.; Gimeno, A.; Parra-Llorca, A.; Vento, M. Early neonatal death: A challenge worldwide. Semin. Fetal Neonatal Med. 2017, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, A.; Mota-Rojas, D.; Strappini, A.; Serrapica, F.; Braghieri, A.; Mora-Medina, P.; Napolitano, F. Neurophysiological mechanisms of cow–calf bonding in buffalo and other farm animals. Animals 2021, 11, 1968. [Google Scholar] [CrossRef] [PubMed]
- Mora-Medina, P.; Napolitano, F.; Mota-Rojas, D.; Berdugo-Gutiérrez, J.; Ruiz-Buitrago, J.; Guerrero-Legarreta, I. Imprinting, sucking and allosucking behaviors in buffalo calves. J. Buffalo Sci. 2018, 7, 49–57. [Google Scholar] [CrossRef]
- Whalin, L.; Weary, D.M.; von Keyserlingk, M.A.G. Understanding behavioural development of calves in natural settings to inform calf management. Animals 2021, 11, 2446. [Google Scholar] [CrossRef]
- Barrier, A.C.; Ruelle, E.; Haskell, M.J.; Dwyer, C.M. Effect of a difficult calving on the vigour of the calf, the onset of maternal behaviour, and some behavioural indicators of pain in the dam. Prev. Vet. Med. 2012, 103, 248–256. [Google Scholar] [CrossRef]
- Fitzgerald, K.T.; Newquist, K.L. Husbandry of the neonate. Small Anim. Pediatr. 2010, 2011, 44–52. [Google Scholar] [CrossRef]
- Aoki, M.; Nomura, F.; Kawata, H.; Mayer, J.E. Effect of calcium and preischemic hypothermia on recovery of myocardial function after cardioplegic ischemia in neonatal lambs. J. Thorac. Cardiovasc. Surg. 1993, 105, 207–213. [Google Scholar] [CrossRef]
- Wood, T.; Thoresen, M. Physiological responses to hypothermia. Semin. Fetal Neonatal Med. 2015, 20, 87–96. [Google Scholar] [CrossRef]
- Thoresen, M.; Simmonds, M.; Satas, S.; Tooley, J.; Silver, I.A. Effective selective head cooling during posthypoxic hypothermia in newborn piglets. Pediatr. Res. 2001, 49, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Burnes, J.; Muns, R.; Barrios-García, H.; Villanueva-García, D.; Domínguez-Oliva, A.; Mota-Rojas, D. Parturition in Mammals: Animal Models, Pain and Distress. Animals 2021, 11, 2960. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.A.F. The effect of stillbirth on reproductive and productive performance of pure Egyptian buffaloes and their crosses with Italian buffaloes. Theriogenology 2017, 103, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Ávalos, I.; José, N.; Casas-Alvarado, A.; Gómez, J.; Mora-Medina, P. Thermal homeostasis in the newborn puppy: Behavioral and physiological responses. J. Anim. Behav. Biometeorol. 2021, 9, 1–25. [Google Scholar] [CrossRef]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Mora-Medina, P.; Ogi, A.; Mariti, C.; Olmos-Hernández, A.; Martínez-Burnes, J.; Hernández-Ávalos, I.; Sánchez-Millán, J.; Gazzano, A. Blood biomarker profile alterations in newborn canines: Effect of the mother′s weight. Animals 2021, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- Lezama-García, K.; Martínez-Burnes, J.; Marcet-Rius, M.; Gazzano, A.; Olmos-Hernández, A.; Mora-Medina, P.; Domínguez-Oliva, A.; Pereira, A.M.F.; Hernández-Ávalos, I.; Baqueiro-Espinosa, U.; et al. Is the weight of the newborn puppy related to its thermal balance? Animals 2022, 12, 3536. [Google Scholar] [CrossRef]
- Bellows, R.A. Factors affecting calving difficulty. In Proceedings of the Range Beef Cow Symposium XIII, Cheyenne, WY, USA, 6–8 December 1993; Volume 43, pp. 2–14. [Google Scholar]
- Mota-Rojas, D.; Villanueva-García, D.; Mota-Reyes, A.; Orihuela, A.; Hernández-Ávalos, I.; Domínguez-Oliva, A.; Casas-Alvarado, A.; Flores-Padilla, K.; Jacome-Romero, J.; Martínez-Burnes, J. Meconium aspiration syndrome in animal models: Inflammatory process, apoptosis, and surfactant inactivation. Animals 2022, 12, 3310. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; López, A.; Martínez-Burnes, J.; Muns, R.; Villanueva-García, D.; Mora-Medina, P.; González-Lozano, M.; Olmos-Hernández, A.; Ramírez-Necoechea, R. Is vitality assessment important in neonatal animals? CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Lezama-García, K.; Martínez-Burnes, J.; Pérez-Jiménez, J.C.; Domínguez-Oliva, A.; Mora-Medina, P.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Mota-Rojas, D. Relation between the dam’s weight on superficial temperature of her puppies at different stages of the post-partum. Vet. Sci. 2022, 9, 673. [Google Scholar] [CrossRef]
- Napolitano, F.; Mota-Rojas, D.; Braghieri, A.; Guerrero-Legarreta, I.; Cruz-Monterrosa, R.G.; José-Pérez, N.; Álvarez-Macías, A.; Domínguez-Oliva, A.; Rodríguez-González, D.; Lezama-García, K.; et al. Colostrum in the water buffalo: Immunological, nutritional and physicochemical aspects. Soc. Rural. Prod. Medio Ambient. 2022, 42, 125–142. [Google Scholar]
- Plush, K.; Brien, F.D.; Hebart, M.L.; Hynd, P.I. Thermogenesis and physiological maturity in neonatal lambs: A unifying concept in lamb survival. Anim. Prod. Sci. 2016, 56, 736–745. [Google Scholar] [CrossRef]
- Rowan, T.G. Thermoregulation in neonatal ruminants. BSAP Occas. Publ. 1992, 15, 13–24. [Google Scholar] [CrossRef]
- Bienboire-Frosini, C.; Wang, D.; Marcet-Rius, M.; Villanueva-García, D.; Gazzano, A.; Domínguez-Oliva, A.; Olmos-Hernández, A.; Hernández-Avalos, I.; Lezama-García, K.; Verduzco-Mendoza, A.; et al. The role of brown adipose tissue and energy metabolism in mammalian thermoregulation during the perinatal period. Animals 2023, 13, 1542. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Mandujano, E.; Reis-de-Souza, T.C.; Ramírez-Rodríguez, E.; Mariscal-Landín, G. Impacto del peso al nacimiento del lechón sobre los balances de nitrógeno y energía en la fase de crecimiento. Rev. Mex. Ciencias Pecu. 2019, 10, 903–916. [Google Scholar] [CrossRef]
- Barreto, J.V.P.; Pertile, S.F.N.; de Almeida Rego, F.C.; Patelli, T.H.C.; Nascimento, S.T.; Lorenzetti, E.; da Cunha Filho, L.F.C. Prediction of vitality and survival of newborn lambs using a modified Apgar score. Appl. Anim. Behav. Sci. 2021, 238, 105281. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Morgan, C.A. Maintenance of body temperature in the neonatal lamb: Effects of breed, birth weight, and litter size1. J. Anim. Sci. 2006, 84, 1093–1101. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Pérez, R.; Avendaño-Reyes, L.; Correa-Calderón, A.; Mellado, M.; Meza-Herrera, C.A.; Montañez-Valdez, O.D.; Macías-Cruz, U. Relationships of body surface thermography with core temperature, birth weight and climatic variables in neonatal lambs born during early spring in an arid region. J. Therm. Biol. 2019, 82, 142–149. [Google Scholar] [CrossRef]
- Johanson, J.M.; Berger, P.J. Birth Weight as a predictor of calving ease and perinatal mortality in holstein cattle. J. Dairy Sci. 2003, 86, 3745–3755. [Google Scholar] [CrossRef] [Green Version]
- Nowak, R.; Poindron, P. From birth to colostrum: Early steps leading to lamb survival. Reprod. Nutr. Dev. 2006, 46, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Lezama-García, K.; Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Ghezzi, M.; Domínguez, A.; Gómez, J.; de Mira Geraldo, A.; Lendez, P.; Hernández-Ávalos, I.; et al. Transient Receptor Potential (TRP) and thermoregulation in animals: Structural biology and neurophysiological aspects. Animals 2022, 12, 106. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.-E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Sheikh, I.U.; Rajkhowa, J. Epidermal thickness in the skin of Yak (Poephagus poephagus). Indian J. Vet. Anat. 2003, 15, 73–76. [Google Scholar]
- Bazzaz, A.A. Poor nociceptive innervation of neck skin in three domestic ruminants. Eur. J. Mol. Clin. Med. 2020, 7, 111–117. [Google Scholar]
- Hafez, E.S.E.; Badreldin, A.L.; Shafei, M.M. Skin structure of Egyptian buffaloes and cattle with particular reference to sweat glands. J. Agric. Sci. 1955, 46, 19–30. [Google Scholar] [CrossRef]
- Ibrahim, R.S.; Hussin, A.M.H. Comparative Histological study of the integument in buffallo and cow. Diyala J. Agric. Sci. 2018, 10, 24–34. [Google Scholar]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Pereira, M.F.A.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Ávalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical applications and factors involved in validating thermal windows in large rumiants to assess health and productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef]
- Muralidharan, M.R.; Ramesh, V. Histological and biochemical studies of the skin of cattle and buffalo. Indian J. Anim. Res. 2005, 39, 41–44. [Google Scholar]
- Debbarma, D.; Uppal, V.; Bansal, N.; Gupta, A. Histomorphometrical study on regional variation in distribution of sweat glands in buffalo skin. Dermatol. Res. Pract. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Lendez, P.A.; Martínez Cuesta, L.; Nieto Farías, M.V.; Vater, A.A.; Ghezzi, M.D.; Mota-Rojas, D.; Dolcini, G.L.; Ceriani, M.C. Effect of heat stress on TNF-α, TNFRI and TNFRII expression in BLV infected dairy cattle. J. Therm. Biol. 2023, 114, 103568. [Google Scholar] [CrossRef]
- Shafies, M.M.; El-Khair, M.M.A. Activity of the sebaceous glands of bovines in hot climates. United Arab Repub. J. Anim. Prod. 1970, 10, 81–98. [Google Scholar]
- Mota-Rojas, D.; Habeeb, A.; Ghezzi, M.D.; Kanth, P.; Napolitano, F.; Lendez, P.A.; Cuibus, A.; Ceriani, M.C.; Sarubbi, J.; Braghieri, A.; et al. Termorregulación del búfalo de agua: Mecanismos neurobiológicos, cambios microcirculatorios y aplicaciones prácticas de la termografía infrarroja. In El Búfalo de Agua en Latinoamérica, Hallazgos Recientes; Napolitano, F., Mota-Rojas, D., Guerrero-Legarreta, I., Orihuela, A., Eds.; BM Editores: Ciudad de México, Mexico, 2020; pp. 923–958. Available online: https://www.lifescienceglobal.com/journals/journal-of-buffalo-science/97-abstract/jbs/4550-el-bufalo-de-agua-enlatinoamerica-hallazgos-recientes (accessed on 5 June 2020).
- Flinn, T.; Kleemann, D.O.; Swinbourne, A.M.; Kelly, J.M.; Weaver, A.C.; Walker, S.K.; Gatford, K.L.; Kind, K.L.; van Wettere, W.H.E.J. Neonatal lamb mortality: Major risk factors and the potential ameliorative role of melatonin. J. Anim. Sci. Biotechnol. 2020, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Bienboire-Frosini, C.; Muns, R.; Marcet-Rius, M.; Gazzano, A.; Villanueva-García, D.; Martínez-Burnes, J.; Domínguez-Oliva, A.; Lezama-García, K.; Casas-Alvarado, A.; Mota-Rojas, D. Vitality in Newborn Farm Animals: Adverse Factors, Physiological Responses, Pharmacological Therapies, and Physical Methods to Increase Neonate Vigor. Animals 2023, 13, 1542. [Google Scholar] [CrossRef] [PubMed]
- Lezama-García, K.; Martínez-Burnes, J.; Baquerio-Espinosa, U.; Olmos-Hernández, A.; Hernández-Avalos, I.; Domínguez-Oliva, A.; Mota-Rojas, D. Assessment of vitality, blood profile, and degree of meconium staining on the skin in neonate dogs according to its birth weight. Vet. Sci. 2023; In revision. [Google Scholar]
- Mülling, M. Asphyxie des neugeborenen Kalbes. Prakt. Tierarzt Coll. 1976, 58, 78–80. [Google Scholar]
- Szenci, O. Importance of monitoring fetal and neonatal vitality in bovine practices. Animals 2023, 13, 1081. [Google Scholar] [CrossRef]
- Mee, J.F. Newborn dairy calf management. Vet. Clin. North Am. Food Anim. Pract. 2008, 24, 1–17. [Google Scholar] [CrossRef]
- Nel, C.L.; Cloete, S.W.P.; Kruger, A.C.M.; Dzama, K. Long term genetic selection for reproductive success affects neonatal lamb vitality across cold stress conditions. J. Therm. Biol. 2021, 98, 102908. [Google Scholar] [CrossRef]
- Probo, M.; Veronesi, M.C. Clinical scoring systems in the newborn calf: An overview. Animals 2022, 12, 3013. [Google Scholar] [CrossRef]
- Afzal, M.M.H.; Anjum, A. Calf mortality: Season pattern, age distribution and causes of calf mortality. Pak. Vet. J. 1983, 3, 30–33. [Google Scholar]
- Vannucchi, C.I.; Rodrigues, J.A.; Silva, L.C.G.; Lúcio, C.F.; Veiga, G.A.L. Effect of dystocia and treatment with oxytocin on neonatal calf vitality and acid-base, electrolyte and haematological status. Vet. J. 2015, 203, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, C.I.C.; Rodrigues, J.J.A.; Silva, L.L.C.G.; Lúcio, C.C.F.; Veiga, G.A.L.G. A clinical and hemogasometric survey of neonatal lambs. Small Rumin. Res. 2012, 108, 107–112. [Google Scholar] [CrossRef]
- Murray, C.F.; Leslie, K.E. Newborn calf vitality: Risk factors, characteristics, assessment, resulting outcomes and strategies for improvement. Vet. J. 2013, 198, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Villanueva-García, D.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Domínguez-Oliva, A.; Lezama-García, K.; Miranda-Cortés, A.; Martínez-Burnes, J. Cardiorespiratory and Neuroprotective Effects of Caffeine in Neonate Animal Models. Animals 2023, 13, 1769. [Google Scholar] [CrossRef]
- Lanzoni, L.; Chincarini, M.; Giammarco, M.; Fusaro, I.; Gloria, A.; Contri, A.; Ferri, N.; Vignola, G. Maternal and neonatal behaviour in italian mediterranean buffaloes. Animals 2021, 11, 1584. [Google Scholar] [CrossRef]
- Singh, A.; Prachakar, S.; Brar, P.; Uppal, S.; Singh, P.; Gandotra, V. Blood biochemical profiles and physical-activity parameters in neonatal buffalo calves under normal and forced calving. Indian J. Anim. Sci. 2011, 81, 570–574. [Google Scholar]
- Diesch, T.; Mellor, D.; Stafford, K.; Ward, R. The physiological and physical status of single calves at birth in a dairy herd in New Zealand. N. Z. Vet. J. 2004, 52, 250–255. [Google Scholar] [CrossRef]
- Haughey, K.G. Cold injury in newborn lambs. Aust. Vet. J. 1973, 49, 554–563. [Google Scholar] [CrossRef]
- Graña-Baumgartner, A.; Dukkipati, V.S.R.; Kenyon, P.R.; Blair, H.T.; López-Villalobos, N.; Gedye, K.; Biggs, P.J. RNAseq Analysis of brown adipose tissue and thyroid of newborn lambs subjected to short-term cold exposure reveals signs of early whitening of adipose tissue. Metabolites 2022, 12, 996. [Google Scholar] [CrossRef]
- Sreedhar, S.; Ranganadham, M.; Mohan, E.M. Calf mortality in indigenous buffaloes. Indian Vet. J. 2010, 87, 197–198. [Google Scholar]
- Torell, R.; Kvasnicke, B.; Bruce, B. Care of Hypothermic (Cold Stressed) Newborn Beef Cows. Available online: https://www.thecattlesite.com/articles/1317/caring-for-hypothermic-cold-stressed-newborn-beef-calves (accessed on 11 May 2023).
- Yáñez-Pizaña, A.; de la Cruz-Cruz, L.A.; Tarazona-Morales, A.; Roldan-Santiago, P.; Ballesteros-Rodea, G.; Pineda-Reyes, R.; Orozco-Gregorio, H. Physiological and behavioral changes of water buffalo in hot and cold systems: Review. J. Buffalo Sci. 2020, 9, 110–120. [Google Scholar] [CrossRef]
- Da Silva, J.A.R.; de Andrade Pantoja, M.H.; da Silva, W.C.; de Almeida, J.C.F.; de Paula PachechoNoronha, R.; Barbosa, A.V.C.; de Brito Lourenço Júnior, J. Thermoregulatory reactions of female buffaloes raised in the sun and in the shade, in the climatic conditions of the rainy season of the Island of Marajó, Pará, Brazil. Front. Vet. Sci. 2022, 9, 998544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Choi, C.; Li, D.; Yan, G.; Li, H.; Shi, Z. Effects of airspeed on the respiratory rate, rectal temperature, and immunity parameters of dairy calves housed individually in an axial-fan-ventilated barn. Animals 2021, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Drillich, M.; Klein-Jöbstl, D.; Iwersen, M. Invited review: Influence of climatic conditions on the development, performance, and health of calves. J. Dairy Sci. 2016, 99, 2438–2452. [Google Scholar] [CrossRef] [Green Version]
- Hill, T.M.; Bateman, H.G.; Suarez-Mena, F.X.; Dennis, T.S.; Schlotterbeck, R.L. Short communication: Changes in body temperature of calves up to 2 months of age as affected by time of day, age, and ambient temperature. J. Dairy Sci. 2016, 99, 8867–8870. [Google Scholar] [CrossRef] [Green Version]
- Merlet, C.; Leandri, J.; Rey, P.; Tchobroutsky, C. Effect of localized cooling on starting respiration in lambs at birth. J. Physiol. 1967, 59, 457–458. [Google Scholar]
- Harned, H.S.; Herrington, R.T.; Ferreiro, J.I. The effects of immersion and temperature on respiration in newborn lambs. Pediatrics 1970, 45, 598–605. [Google Scholar] [CrossRef]
- Farrag, B. Effect of seasonal variations during dry and wet seasons on reproductive performance and biological and economic criteria of hair sheep under Halaieb rangeland conditions. Arch. Anim. Breed. 2022, 65, 319–327. [Google Scholar] [CrossRef]
- Hulbert, L.E.; Moisá, S.J. Stress, immunity, and the management of calves. J. Dairy Sci. 2016, 99, 3199–3216. [Google Scholar] [CrossRef] [Green Version]
- Buijs, T.J.; McNaughton, P.A. The role of cold-sensitive ion channels in peripheral thermosensation. Front. Cell. Neurosci. 2020, 14, 262. [Google Scholar] [CrossRef]
- McKemy, D.D. How Cold is It? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol. Pain 2005, 1, 1733. [Google Scholar] [CrossRef]
- Zhao, Z.-D.; Yang, W.Z.; Gao, C.; Fu, X.; Zhang, W.; Zhou, Q.; Chen, W.; Ni, X.; Lin, J.-K.; Yang, J.; et al. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 2042–2047. [Google Scholar] [CrossRef] [Green Version]
- Rothhaas, R.; Chung, S. Role of the preoptic area in sleep and thermoregulation. Front. Neurosci. 2021, 15, 664781. [Google Scholar] [CrossRef] [PubMed]
- Guseynov, N.A.; Hammouri, M.H.; Muraev, A.A.; Ivanov, S.Y.; Lukianova, E.A.; Klimenko, A.S.; Noeerazlighi, M.A. Local hardware hypothermia influence on the physiological processes. Rudn. J. Med. 2022, 26, 243–258. [Google Scholar] [CrossRef]
- Polli, V.A.; Vaz, R.Z.; Carvalho, S.; Costa, P.T.; de Oliveira Mello, R.; Restle, J.; Nigeliskii, A.F.; Silveira, I.D.B.; Pissinin, D. Thermal comfort and performance of feedlot lambs finished in two climatic conditions. Small Rumin. Res. 2019, 174, 163–169. [Google Scholar] [CrossRef]
- Van Someren, E.J.W. Age-related changes in thermoreception and thermoregulation. In Handbook of the Biology of Aging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 463–478. [Google Scholar]
- Vilela, R.A.; de Brito Lourenço Junior, J.; Jacintho, M.A.C.; Barbosa, A.V.C.; Pantoja, M.H.d.A.; Oliveira, C.M.C.; Garcia, A.R. Dynamics of thermolysis and skin microstructure in water buffaloes reared in humid tropical climate—A microscopic and thermographic study. Front. Vet. Sci. 2022, 9, 871206. [Google Scholar] [CrossRef]
- Pressicce, G.A. The Buffalo (Bubalus bubalis): Production and Research; Bentham Science Publishers: Sharjah, United Arab Emirates, 2017. [Google Scholar]
- Nazari, S.; Kourosh-Arami, M.; Komaki, A.; Hajizadeh, S. Relative contribution of central and peripheral factors in superficial blood flow regulation following cold exposure. Physiol. Pharmacol. 2020, 24, 89–100. [Google Scholar] [CrossRef]
- Slee, J. Body temperature and vasomotor responses in Scottish Blackface and Tasmanian Merino sheep subjected to slow cooling. Anim. Sci. 1968, 10, 265–282. [Google Scholar] [CrossRef]
- Turnpenny, J.; Wathes, C.; Clark, J.; McArthur, A. Thermal balance of livestock. Agric. For. Meteorol. 2000, 101, 29–52. [Google Scholar] [CrossRef]
- Bianco, A.C.; Silva, J.E. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J. Clin. Invest. 1987, 79, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Alexander, G. Development of brown fat in the sheep fetus: Preparation for neonatal thermogenesis. Proc. Aust. Physiol. Pharmacol. Soc. 1981, 12, 31–35. [Google Scholar]
- Gunn, T.R.; Gluckman, P.D. Perinatal thermogenesis. Early Hum. Dev. 1995, 42, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Romert, L.; Sundin, U.; Barnard, T. Morphology and biochemical properties of perirenal adipose tissue from lamb (Ovis aries). A comparison with brown adipose tissue. Comp. Biochem. Physiol. Part B Comp. Biochem. 1977, 56, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.; Symonds, M.E. Thermoregulation in newborn lambs: Influence of feeding and ambient temperature on brown adipose tissue. Exp. Physiol. 1998, 83, 651–657. [Google Scholar] [CrossRef]
- Nowack, J.; Vetter, S.G.; Stalder, G.; Painer, J.; Kral, M.; Smith, S.; Le, M.H.; Jurcevic, P.; Bieber, C.; Arnold, W.; et al. Muscle nonshivering thermogenesis in a feral mammal. Sci. Rep. 2019, 9, 6378. [Google Scholar] [CrossRef] [Green Version]
- Louveau, I.; Perruchot, M.-H.; Bonnet, M.; Gondret, F. Invited review: Pre- and postnatal adipose tissue development in farm animals: From stem cells to adipocyte physiology. Animal 2016, 10, 1839–1847. [Google Scholar] [CrossRef]
- Soppela, P.; Sormunen, R.; Saarela, S.; Huttunen, P.; Nieminen, M. Localization, cellular morphology and respiratory capacity of “brown” adipose tissue in newborn reindeer. Comp. Biochem. Physiol. Part A Physiol. 1992, 101, 281–293. [Google Scholar] [CrossRef]
- Carstens, G.E. Cold thermoregulation in the newborn calf. Vet. Clin. North Am. Food Anim. Pract. 1994, 10, 69–106. [Google Scholar] [CrossRef]
- Tansey, E.A.; Johnson, C.D. Recent advances in thermoregulation. Adv. Physiol. Educ. 2015, 39, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Legendre, L.J.; Davesne, D. The evolution of mechanisms involved in vertebrate endothermy. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190136. [Google Scholar] [CrossRef] [Green Version]
- Hohtola, E. Shivering Thermogenesis in Birds and Mammals. In Life in the Cold: Evolution, Mechanisms, Adaptation, and Application; Barnes, M., Carey, H.V., Eds.; ARCUS: Fairbanks, AK, USA, 2004; pp. 241–252. [Google Scholar]
- Haman, F. Shivering in the cold: From mechanisms of fuel selection to survival. J. Appl. Physiol. 2006, 100, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Rowland, L.A.; Bal, N.C.; Periasamy, M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol. Rev. 2015, 90, 1279–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mila, H.; Feugier, A.; Grellet, A.; Anne, J.; Gonnier, M.; Martin, M.; Rossig, L.; Chastant-Maillard, S. Immunoglobulin G concentration in canine colostrum: Evaluation and variability. J. Reprod. Immunol. 2015, 112, 24–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kienzle, E.; Zentek, J.; Meyer, H. Body composition of puppies and young dogs. J. Nutr. 1998, 128, 2680S–2683S. [Google Scholar] [CrossRef] [Green Version]
- Berthon, D.; Herpin, P.; Bertin, R.; De Marco, F.; le Dividich, J. Metabolic changes associated with sustained 48-Hr shivering thermogenesis in the newborn pig. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1996, 114, 327–335. [Google Scholar] [CrossRef]
- Curtis, S. Environmental—Thermoregulatory interactions and neonatal piglet survival. J. Anim. Sci. 1970, 31, 576–587. [Google Scholar] [CrossRef]
- Herpin, P.; Vincent, A.; Damon, M. Effect of breed and body weight on thermoregulatory abilities of European (Piétrain×(Landrace×Large White)) and Chinese (Meishan) piglets at birth. Livest. Prod. Sci. 2004, 88, 17–26. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Gregorio, O.; Villanueva, D.; Bonilla, H.; Suárez, X.; Hernandez, R.; Roldan, P.; Trujillo, M. Foetal and neonatal energy metabolism in pigs and humans: A review. Vet. Med. 2011, 56, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Alexander, G.; Williams, D. Shivering and non-shivering thermogenesis during summit metabolism in young lambs. J. Physiol. 1968, 198, 251–276. [Google Scholar] [CrossRef]
- Smith, S.; Carstens, G. Ontogeny and metabolism of brown adipose tissue in livestock species. In Biology of Growing Animals; Burrin, D., Mersmann, H., Eds.; Elsevier: Philadephia, PA, USA, 2005; pp. 303–322. [Google Scholar]
- Lourenço, M.L.G.; Machado, L.H.A. Particularidades do período de transição fetal-neonatal em neonatos caninos. Rev. Bras. Reprod. Anim 2013, 37, 303–308. [Google Scholar]
- Rödel, H.G.; Bautista, A.; García-Torres, E.; Martínez-Gómez, M.; Hudson, R. Why do heavy littermates grow better than lighter ones? A study in wild and domestic European rabbits. Physiol. Behav. 2008, 95, 441–448. [Google Scholar] [CrossRef] [PubMed]
- García-Torres, E.; Hudson, R.; Castelán, F.; Martínez-Gómez, M.; Bautista, A. Differential metabolism of brown adipose tissue in newborn rabbits in relation to position in the litter huddle. J. Therm. Biol. 2015, 51, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Carlton, P.; Marks, R. Cold exposure and heat reinforced operant behavior. Science 1958, 128, 1344. [Google Scholar] [CrossRef] [PubMed]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferner, K.; Schultz, J.A.; Zeller, U. Comparative anatomy of neonates of the three major mammalian groups (monotremes, marsupials, placentals) and implications for the ancestral mammalian neonate morphotype. J. Anat. 2017, 231, 798–822. [Google Scholar] [CrossRef] [Green Version]
- Hrupka, B.J.; Leibbrandt, V.D.; Crenshaw, T.D.; Benevenga, N.J.; Hrupka, B.J.; Leibbrandt, V.D.; Crenshaw, T.D.; Benevenga, N.J. Effect of sensory stimuli on huddling behavior of pigs. J. Anim. Sci. 2000, 78, 592–596. [Google Scholar] [CrossRef] [Green Version]
- Lezama-García, K.; Mariti, C.; Mota-Rojas, D.; Martínez-Burnes, J.; Barrios-García, H.; Gazzano, A. Maternal behaviour in domestic dogs. Int. J. Vet. Sci. Med. 2019, 7, 20–30. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Conington, J.; Corbiere, F.; Holmøy, I.H.; Muri, K.; Nowak, R.; Rooke, J.; Vipond, J.; Gautier, J.-M. Invited review: Improving neonatal survival in small ruminants: Science into practice. Animal 2016, 10, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Harri, M.; Mononen, J.; Haapanen, K.; Korhonen, H. Postnatal changes in hypothermic response in farmborn blue foxes and raccoon dogs. J. Therm. Biol. 1991, 16, 71–76. [Google Scholar] [CrossRef]
- Coroian, A.; Erler, S.; Matea, C.T.; Mireșan, V.; Răducu, C.; Bele, C.; Coroian, C.O. Seasonal changes of buffalo colostrum: Physicochemical parameters, fatty acids andcholesterol variation. Chem. Cent. J. 2013, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Scheuer, B.H.; Zbinden, Y.; Schneiter, P.; Tappy, L.; Blum, J.W.; Hammon, H.M. Effects of colostrum feeding and glucocorticoid administration on insulin-dependent glucose metabolism in neonatal calves. Domest. Anim. Endocrinol. 2006, 31, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.C.; Silva, D.G.; Rocha, T.G.; Monteiro, B.M.; Pereira, G.T.; Fiori, L.C.; Viana, R.B.; Fagliari, J.J. Serum biochemical profile of neonatal buffalo calves. Arq. Bras. Med. Vet. Zootec. 2019, 71, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Quigley, J.D.; Drewry, J.J. Nutrient and immunity transfer from cow to calf pre- and postcalving. J. Dairy Sci. 1998, 81, 2779–2790. [Google Scholar] [CrossRef] [PubMed]
- Ojha, B.K.; Dutta, N.; Pattanaik, A.K.; Singh, S.K.; Narang, A. Effect of pre-partum strategic supplementation of concentrates on colostrum quality and performance of buffalo calves. Anim. Nutr. Feed Technol. 2015, 15, 41. [Google Scholar] [CrossRef]
- Dang, A.K.; Kapila, S.; Purohit, M.; Singh, C. Changes in colostrum of Murrah buffaloes after calving. Trop. Anim. Health Prod. 2009, 41, 1213–1217. [Google Scholar] [CrossRef]
- Farmer, C.; Quesnel, H. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 2009, 87, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Guy, M.A.; McFadden, T.B.; Cockrell, D.C.; Besser, T.E. Regulation of colostrum formation in beef and dairy cows. J. Dairy Sci. 1994, 77, 3002–3007. [Google Scholar] [CrossRef]
- Castro, N.; Capote, J.; Bruckmaier, R.M.; Argüello, A. Management effects on colostrogenesis in small ruminants: A review. J. Appl. Anim. Res. 2011, 39, 85–93. [Google Scholar] [CrossRef]
- Erdem, H.; Okuyucu, I.C. Non-genetic factors affecting some colostrum quality traits in Holstein cattle. Pak. J. Zool. 2020, 52, 557–564. [Google Scholar] [CrossRef]
- Mellor, D.; Murray, L. Making the most of colostrum at lambing. Vet. Rec. 1986, 118, 351–353. [Google Scholar] [CrossRef]
- McGee, M.; Drennan, M.J.; Caffrey, P.J. Effect of age and nutrient restriction pre partum on beef suckler cow serum immunoglobulin concentrations, colostrum yield, composition and immunoglobulin concentration and immune status of their progeny. Irish J. Agric. Food Res. 2006, 45, 157–171. [Google Scholar]
- Abdel-Hamid, M.; Yang, P.; Mostafa, I.; Osman, A.; Romeih, E.; Yang, Y.; Huang, Z.; Awad, A.A.; Li, L. Changes in whey proteome between Mediterranean and Murrah buffalo colostrum and mature milk reflect their pharmaceutical and medicinal value. Molecules 2022, 27, 1575. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, N. Chemical composition, hormonal levels and immunoglobin G concentration in colostrums, milk and blood plasma of Egyptian buffaloes following calving. Int. J. Adv. Res. 2015, 3, 471–478. [Google Scholar]
- Qureshi, T.M.; Yaseen, M.; Nadeem, M.; Murtaza, M.A.; Munir, M. Physico–chemical composition and antioxidant potential of buffalo colostrum, transition milk, and mature milk. J. Food Process. Preserv. 2020, 44, e14763. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.M.; Abd Rabo, F.H.; EL-Dieb, S.M.; El-Kashef, H.A. Changes in composition of colostrum of Egyptian buffaloes and Holstein cows. BMC Vet. Res. 2012, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Luo, G.; Gao, S.; Zhang, X.; Chen, C.; Yao, Z.; Zhao, J.; Lv, H.; Niu, K.; Nie, P.; et al. Evaluation of parity effect on characteristics and minerals in buffalo (Bubalus Bubalis) colostrum and mature milk. Foods 2023, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Barmaiya, S.; Dixit, A.; Mishra, A.; Jain, A.K.; Gupta, A.; Paul, A.; Quadri, M.A.; Madan, A.K.; Sharma, I.J. Quantitation of serum immunoglobulins of neonatal buffalo calves and cow calves through elisa and page: Status of immune-competence. Buffalo Bull. 2009, 28, 85–94. [Google Scholar]
- Erdem, H.; Okuyucu, I.C.; Demirci, H. Components and specific gravity of colostrum from Anatolian buffalo cows and effects on growth of buffalo calves. S. Afr. J. Anim. Sci. 2022, 52, 316–325. [Google Scholar] [CrossRef]
- Silva, F.L.M.; Miqueo, E.; da Silva, M.D.; Torrezan, T.M.; Rocha, N.B.; Salles, M.S.V.; Bittar, C.M.M. Thermoregulatory responses and performance of dairy calves fed different amounts of colostrum. Animals 2021, 11, 703. [Google Scholar] [CrossRef]
- Wang, F.-K.; Shih, J.-Y.; Juan, P.-H.; Su, Y.-C.; Wang, Y.-C. Non-invasive cattle body temperature measurement using infrared thermography and auxiliary sensors. Sensors 2021, 21, 2425. [Google Scholar] [CrossRef]
- Tattersall, G.J.; Cadena, V. Insights into animal temperature adaptations revealed through thermal imaging. Imaging Sci. J. 2010, 58, 261–268. [Google Scholar] [CrossRef]
- Giro, A.; de Campos Bernardi, A.C.; Barioni Junior, W.; Lemes, A.P.; Botta, D.; Romanello, N.; Barreto, A.d.N.; Garcia, A.R. Application of microchip and infrared thermography for monitoring body temperature of beef cattle kept on pasture. J. Therm. Biol. 2019, 84, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Casas-Alvarado, A.; Martínez-Burnes, J.; Mora-Medina, P.; Hernández-Avalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Gómez-Prado, J.; Mota-Rojas, D. Thermal and circulatory changes in diverse body regions in dogs and cats evaluated by infrared thermography. Animals 2022, 12, 789. [Google Scholar] [CrossRef]
- Dela Ricci, G.; Silva-Miranda, K.O.; Titto, C.G. Infrared thermography as a non-invasive method for the evaluation of heat stress in pigs kept in pens free of cages in the maternity. Comput. Electron. Agric. 2019, 157, 403–409. [Google Scholar] [CrossRef]
- Stewart, M.; Wilson, M.T.; Schaefer, A.L.; Huddart, F.; Sutherland, M.A. The use of infrared thermography and accelerometers for remote monitoring of dairy cow health and welfare. J. Dairy Sci. 2017, 100, 3893–3901. [Google Scholar] [CrossRef]
- Lowe, G.; Sutherland, M.; Waas, J.; Schaefer, A.; Cox, N.; Stewart, M. Infrared thermography—A non-invasive method of measuring respiration rate in calves. Animals 2019, 9, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, H.; Li, Y.; Fang, T.; Xing, M.; Sun, F.; Chen, X.; Bindelle, J.; Wang, W.; Guo, L. Evaluation of the best region for measuring eye temperature in dairy cows exposed to heat stress. Front. Vet. Sci. 2022, 9, 857777. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Worth, G.M.; Dowling, S.K.; Lowe, G.L.; Cave, V.M.; Stewart, M. Evaluation of infrared thermography as a non-invasive method of measuring the autonomic nervous response in sheep. PLoS ONE 2020, 15, e0233558. [Google Scholar] [CrossRef]
- Labeur, L.; Villiers, G.; Small, A.H.; Hinch, G.N.; Schmoelzl, S. Infrared thermal imaging as a method to evaluate heat loss in newborn lambs. Res. Vet. Sci. 2017, 115, 517–522. [Google Scholar] [CrossRef]
- Bertoni, A.; Mota-Rojas, D.; Álvarez-Macias, A.; Mora-Medina, P.; Guerrero-Legarreta, I.; Morales-Canela, A.; Gómez-Prado, J.; José-Pérez, N.; Martínez-Burnes, J. Scientific findings related to changes in vascular microcirculation using infrared thermography in the river buffalo. J. Anim. Behav. Biometeorol. 2020, 8, 288–297. [Google Scholar] [CrossRef]
- Manani, M.; Jegatheesan, P.; DeSandre, G.; Song, D.; Showalter, L.; Govindaswami, B. Elimination of admission hypothermia in preterm very low-birth-weight infants by standardization of delivery room management. Perm. J. 2013, 17, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islas, P.; Mota-Rojas, D.; Martínez-Burnes, J.; Mora-Medina, P.; González-Lozano, M.; Santiago, R.; Greenwell-Beare, V.; González-Hernández, M.; Vega-Manríquez, X.; Gregorio, O. Physiological and metabolic responses in newborn piglets associated with the birth order. Anim. Reprod. Sci. 2018, 197, 247–256. [Google Scholar] [CrossRef]
- Gloria, A.; Chincarini, M.; Vignola, G.; Ferri, N.; Contri, A. Venous blood gas parameters in healthy Mediterranean buffalo calves in the first 72 hours of life. Theriogenology 2020, 157, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, D.; Guerrero-Legarreta, I.; Cruz Monterrosa, R.G.; Napolitano, F.; Gonçalves-Titto, C.; El-Aziz, A.H.A.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Oliva- Domínguez, A.; Mota-Rojas, D. Assessment of thermal changes in water buffalo mobilized from the paddock and transported by short journeys. Front. Vet. Sci. 2023, 10, 1184577. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Rojas, D.; Braghieri, A.; Ghezzi, M.; Ceriani, M.C.; Martínez-Burnes, J.; Lendez, P.A.; Pereira, A.M.F.; Lezama-García, K.; Domínguez-Oliva, A.; Casas-Alvarado, A.; et al. Strategies and Mechanisms of Thermal Compensation in Newborn Water Buffaloes. Animals 2023, 13, 2161. https://doi.org/10.3390/ani13132161
Mota-Rojas D, Braghieri A, Ghezzi M, Ceriani MC, Martínez-Burnes J, Lendez PA, Pereira AMF, Lezama-García K, Domínguez-Oliva A, Casas-Alvarado A, et al. Strategies and Mechanisms of Thermal Compensation in Newborn Water Buffaloes. Animals. 2023; 13(13):2161. https://doi.org/10.3390/ani13132161
Chicago/Turabian StyleMota-Rojas, Daniel, Ada Braghieri, Marcelo Ghezzi, María Carolina Ceriani, Julio Martínez-Burnes, Pamela Anahí Lendez, Alfredo M. F. Pereira, Karina Lezama-García, Adriana Domínguez-Oliva, Alejandro Casas-Alvarado, and et al. 2023. "Strategies and Mechanisms of Thermal Compensation in Newborn Water Buffaloes" Animals 13, no. 13: 2161. https://doi.org/10.3390/ani13132161










