Daily and Seasonal Activity Patterns of Plateau Pikas (Ochotona curzoniae) on the Qinghai–Tibet Plateau, China, and Their Relationship with Weather Condition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
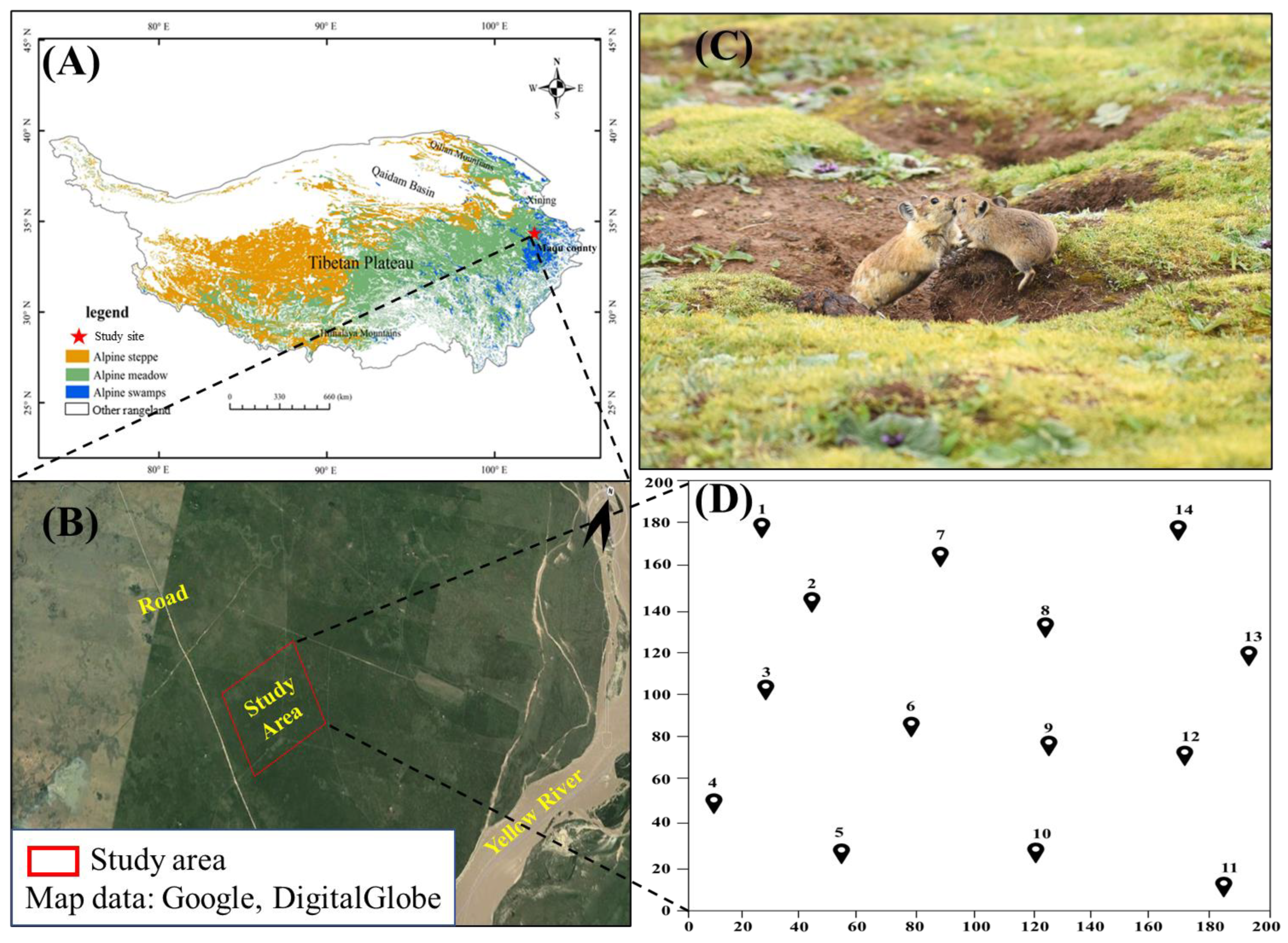
2.1. Study Area
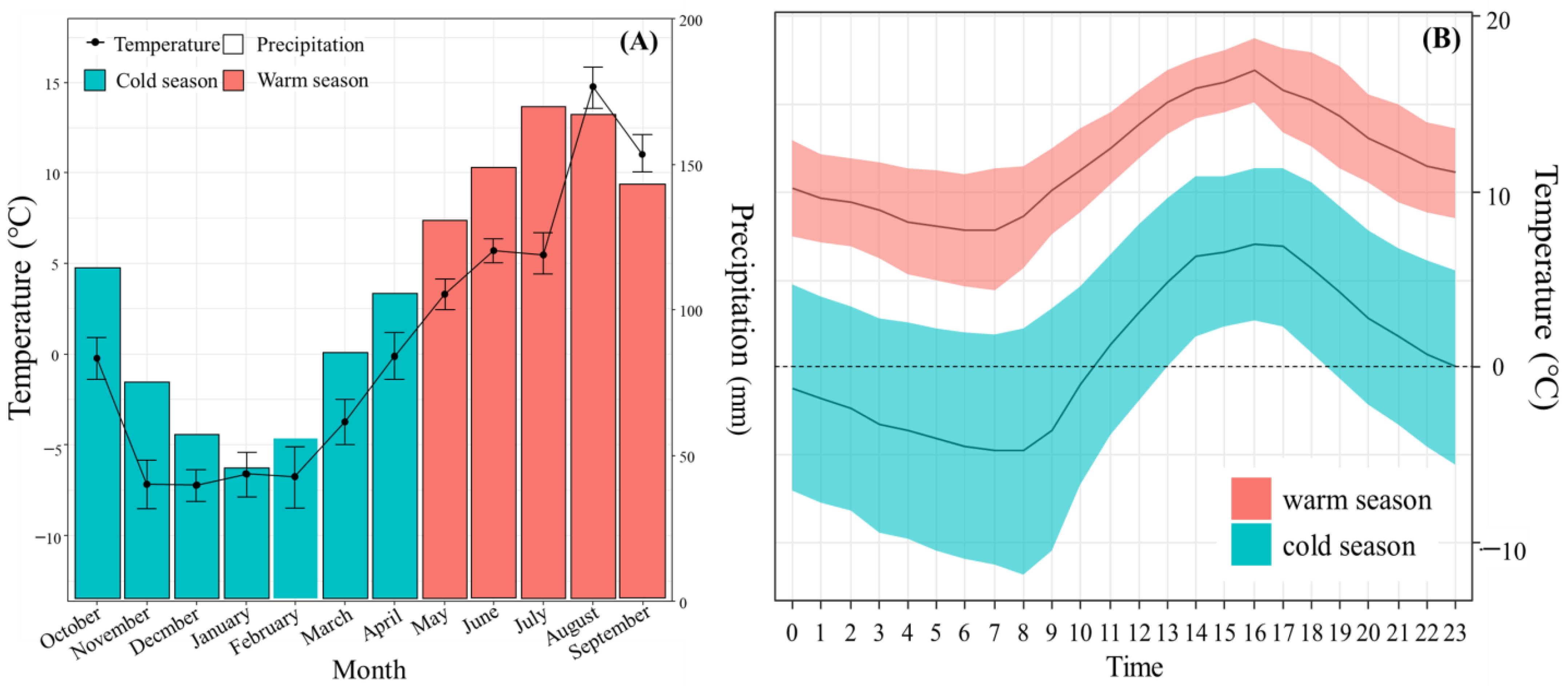
2.2. Climatic Data Record
2.3. Camera Trapping
2.4. Data Analyses
3. Results
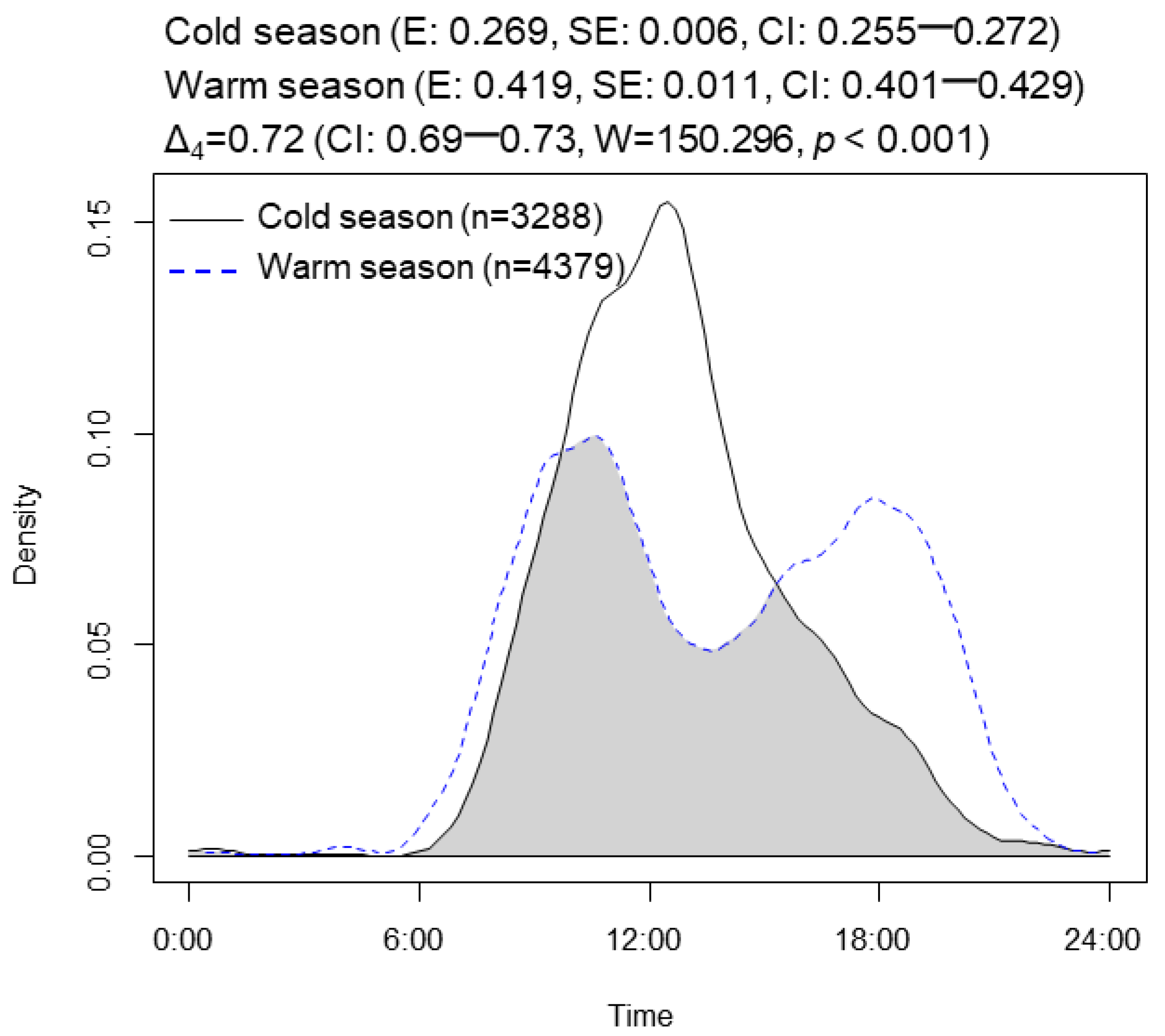
3.1. Seasonal Activity Patterns
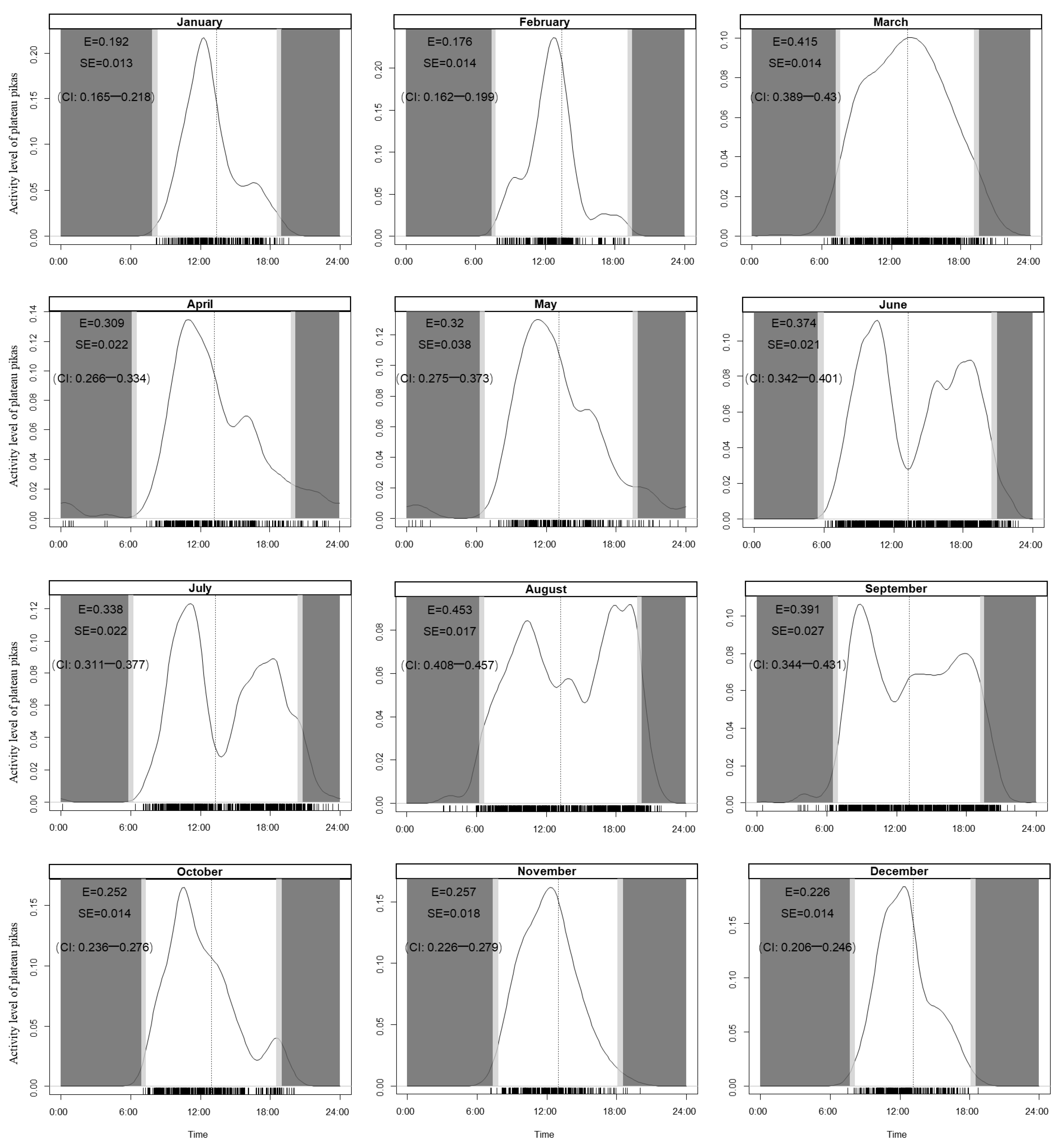
3.2. Daily Activity Patterns
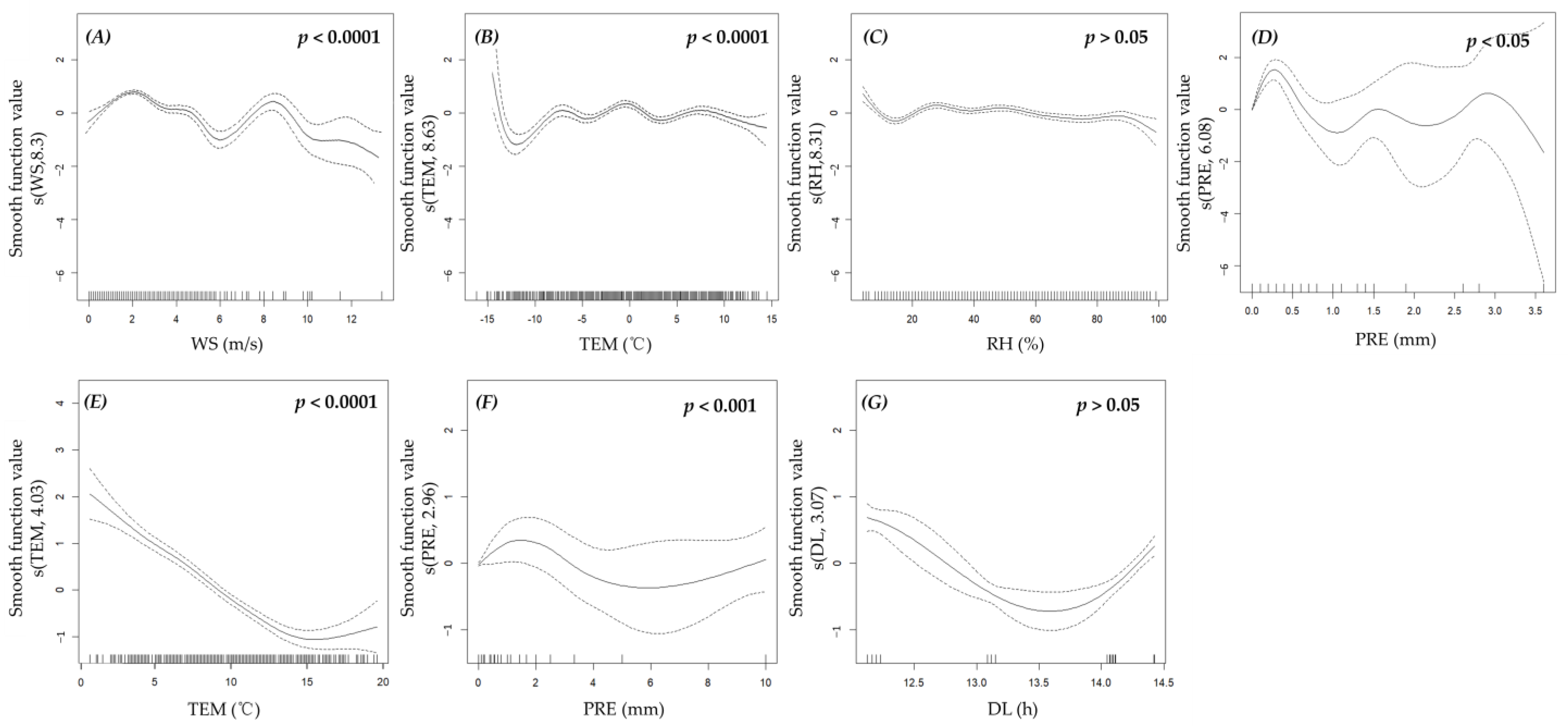
3.3. Effects of Environmental Factors
4. Discussion
4.1. Seasonal Activity Patterns
4.2. Daily Activity Patterns
4.3. Effects of Environmental Factors on the Activity of Plateau Pikas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halle, S. Ecological Relevance of Daily Activity Patterns. In Activity Patterns in Small Mammals; Springer: Berlin/Heidelberg, Germany, 2000; pp. 67–90. [Google Scholar]
- Vlasatá, T.; Šklíba, J.; Lövy, M.; Meheretu, Y.; Sillero-Zubiri, C.; Šumbera, R. Daily activity patterns in the giant root rat (Tachyoryctes macrocephalus), a fossorial rodent from the Afro-alpine zone of the Bale Mountains, Ethiopia. J. Zool. 2017, 302, 157–163. [Google Scholar] [CrossRef]
- Villafuerte, R.; Kufner, M.B.; Delibes, M.; Moreno, S. Environmental factors influencing the seasonal daily activity of the European rabbit (Oryctolagus cuniculus) in a Mediterranean area. Mammalia 1993, 57, 341–348. [Google Scholar] [CrossRef]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1649–1655. [Google Scholar] [CrossRef]
- Milling, C.R.; Rachlow, J.L.; Johnson, T.R.; Forbey, J.S.; Shipley, L.A. Seasonal variation in behavioral thermoregulation and predator avoidance in a small mammal. Behav. Ecol. 2017, 28, 1236–1247. [Google Scholar] [CrossRef]
- Monterroso, P.; Alves, P.C.; Ferreras, P. Catch Me If You Can: Diel Activity Patterns of Mammalian Prey and Predators. Ethology 2013, 119, 1044–1056. [Google Scholar] [CrossRef]
- Vignoli, L.; Luiselli, L. Better in the dark: Two Mediterranean amphibians synchronize reproduction with moonlit nights. Web Ecol. 2013, 13, 1–11. [Google Scholar] [CrossRef]
- Hut, R.A.; Kronfeld-Schor, N.; Van der Vinne, V.; De la Iglesia, H. In Search of a Temporal Niche: Environmental Factors. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 199, pp. 281–304. [Google Scholar]
- Rocha, M.; Serronha, A.; Rodrigues, M.; Alves, P.C.; Monterroso, P. Comfort over safety: Thermoregulation overshadows predation risk effects in the activity of a keystone prey. J. Zology 2021, 316, 209–222. [Google Scholar] [CrossRef]
- Hanya, G. Seasonal variations in the activity budget of Japanese macaques in the coniferous forest of Yakushima: Effects of food and temperature. Am. J. Primatol. Off. J. Am. Soc. Primatol. 2004, 63, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Melinda, B.; Francesca, P.; Norman, O.S.; Erasmus, B.F.N.; Hetem, R.S. How free-ranging ungulates with differing water dependencies cope with seasonal variation in temperature and aridity. Conserv. Physiol. 2019, 7, coz064. [Google Scholar]
- Fredericksen, T.S. Thermal Regulation and Habitat Use of the Eastern Box Turtle in Southwestern Virginia. Northeast. Nat. 2014, 21, 554–564. [Google Scholar] [CrossRef]
- Stelzner, J.K.; Hausfater, G. Posture, microclimate, and thermoregulation in yellow baboons. Primates 1986, 27, 449–463. [Google Scholar] [CrossRef]
- Maestri, R.; Marinho, J.R. Singing in the rain. Rainfall and moonlight affect daily activity patterns of rodents in a Neotropical forest. Acta Theriol. 2014, 59, 427–433. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Dayan, T. Activity patterns of rodents: The physiological ecology of biological rhythms. Biol. Rhythm Res. 2008, 39, 193–211. [Google Scholar] [CrossRef]
- Monterroso, P.; Rebelo, P.; Alves, P.C.; Ferreras, P. Niche partitioning at the edge of the range: A multidimensional analysis with sympatric martens. J. Mammal. 2016, 97, 928–939. [Google Scholar] [CrossRef]
- Veldhuis, M.P.; Hofmeester, T.R.; Balme, G.; Druce, D.J.; Pitman, R.T.; Cromsigt, J.P.G.M. Predation risk constrains herbivores’ adaptive capacity to warming. Nat. Ecol. Evol. 2020, 4, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.M.; Vander Wall, S.B. Linking summer foraging to winter survival in yellow pine chipmunks (Tamias amoenus). Oecologia 2008, 157, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, C.M. Voluntary wheel running: A review and novel interpretation. Anim. Behav. 1998, 56, 11–27. [Google Scholar] [CrossRef]
- Rowcliffe, J.M.; Carbone, C. Surveys using camera traps: Are we looking to a brighter future? Anim. Conserv. 2008, 11, 185–186. [Google Scholar] [CrossRef]
- Hoogenboom, I.; Daan, S.; Dallinga, J.; Schoenmakers, M. Seasonal change in the daily timing of behaviour of the common vole, Microtus arvalis. Oecologia 1984, 61, 18–31. [Google Scholar] [CrossRef]
- Blount, J.D.; Chynoweth, M.W.; Green, A.M.; Şekercioğlu, Ç.H. Review: COVID-19 highlights the importance of camera traps for wildlife conservation research and management. Biol. Conserv. 2021, 256, 978–984. [Google Scholar] [CrossRef]
- Burton, A.C.; Neilson, E.; Moreira, D.; Ladle, A.; Steenweg, R.; Fisher, J.T.; Bayne, E.; Boutin, S. Wildlife camera trapping: A review and recommendations for linking surveys to ecological processes. J. Appl. Ecol. 2015, 52, 675–685. [Google Scholar] [CrossRef]
- Rovero, F.; Zimmermann, F. Camera Trapping for Wildlife Research; Pelagic Publishing Ltd.: London, UK, 2016. [Google Scholar]
- Caravaggi, A.; Zaccaroni, M.; Riga, F.; Schai-Braun, S.C.; Dick, J.T.; Montgomery, W.I.; Reid, N. An invasive-native mammalian species replacement process captured by camera trap survey random encounter models. Remote Sens. Ecol. Conserv. 2016, 2, 45–58. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Smith, A.T.; Gao, W.X. Social relationships of adult black-lipped pikas (Ochotona curzoniae). J. Mammal. 1991, 72, 231–247. [Google Scholar] [CrossRef]
- Smith, A.T.; Wilson, M.C.; Hogan, B.W. Functional-trait ecology of the plateau pika Ochotona curzoniae (Hodgson, 1858) in the Qinghai-Tibetan Plateau ecosystem. Integr. Zool. 2018, 14, 87–103. [Google Scholar] [CrossRef]
- Wilson, M.C.; Smith, A.T. The pika and the watershed: The impact of small mammal poisoning on the ecohydrology of the Qinghai-Tibetan Plateau. Ambio 2015, 44, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, S.; Gao, Q.; Liu, S.; Liang, Y.; Cao, X. Responses of alpine vegetation and soils to the disturbance of plateau pika (Ochotona curzoniae) at burrow level on the Qinghai–Tibetan Plateau of China. Ecol. Eng. 2016, 88, 232–236. [Google Scholar] [CrossRef]
- Nie, H. Study on the Evolutionary Ecology of Small Herbivorous Mammals: Life History Strategy of Plateau Pika (Ochotona curzoniae); Zhejiang University: Hangzhou, China, 2005. [Google Scholar]
- Stephen, D.F.; Smith, A.T.; Xue, G.W. Social and ecological influences on dispersal and philopatry in the plateau pika (Ochotona curzoniae). Behav. Ecol. 1998, 9, 622–635. [Google Scholar]
- Liu, W.; Zhang, Y.; Wang, X.; Zhao, J.; Zhou, L. Caching selection by plateau pika and its biological significance. Acta Theriol. Sin. 2009, 29, 152–159. [Google Scholar]
- Wang, D.-h.; Sun, R.-y.; Wang, Z.-w. Effects of photoperiod and temperature on brown adipose tissue thermogenic properties in plateau pika. Zool. Res. 1999, 20, 347–351. [Google Scholar]
- Zeng, J.; Wang, Z.; Han, Y. On the daily activity rhythm of five small mammals. Acta Theriol. Sin. 1981, 1, 189–197. [Google Scholar]
- Zong, H.; Xia, W. Circadian activity rhythms of plateau pikas, Ochotona curzoniae. Acta Theriol. Sin. 1987, 7, 211–223. [Google Scholar]
- Wei, W.; Zhang, W. Architecture Characteristics of Burrow System of Plateau Pika, Ochotona curzoniae. Pak. J. Zool. 2018, 50, 311–316. [Google Scholar] [CrossRef]
- Zhou, R.; Hua, R.; Hua, X.Z.; Zhang, F.Y.; Ye, G.H.; Tang, Z.S.; Hua, L.M. Temporal and spatial dynamics of plant community structure under the disturbance of plateau pika (Ochotona curzoniae). Grassland Turf 2021, 41, 69–75. [Google Scholar]
- Linkie, M.; Ridout, M.S. Assessing tiger–prey interactions in Sumatran rainforests. J. Zool. 2011, 3, 224–229. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M. Overview of the Overlap Package. 2021. Available online: https://cran.r-project.org/web/packages/overlap/vignettes/overlap.pdf (accessed on 18 July 2019).
- Fernández-Durán, J.J. Circular Distributions Based on Nonnegative Trigonometric Sums. Biometrics 2015, 60, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Goro, H.; Yosuke, O.; Shun, H.; Takeaki, H.; Hiroki, O.; Yuma, H.; Crowther, M. Activity of wild Japanese macaques in Yakushima revealed by camera trapping: Patterns with respect to season, daily period and rainfall. PLoS ONE 2018, 13, e0190631. [Google Scholar]
- Houngbégnon, F.G.A.; Cornelis, D.; Vermeulen, C.; Sonké, B.; Doucet, J.L. Daily Activity Patterns and Co-Occurrence of Duikers Revealed by an Intensive Camera Trap Survey across Central African Rainforests. Animals 2020, 10, 2200. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B. lme4: Linear Mixed-Effects Models Using S4 Classes; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Linear Mixed-Effects Models: Basic Concepts and Examples; R Foundation for Statistical Computing: Vienna, Austria, 2000; pp. 3–56. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Wilkening, J.; Ray, C.; Varner, J. Relating sub-surface ice features to physiological stress in a climate sensitive mammal, the American pika (Ochotona princeps). PLoS ONE 2015, 10, e0119327. [Google Scholar] [CrossRef]
- Bartoń, K. Package ‘MuMIn’. In R Package Version; R Foundation for Statistical Computing: Vienna, Austria, 2014; Volume 1, Available online: http://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed on 13 June 2019).
- Bhattacharyya, S.; Adhikari, B.S.; Rawat, G.S. Influence of snow, food, and rock cover on Royle’s pika abundance in western Himalaya. Arct. Antarct. Alp. Res. 2014, 46, 558–567. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing [Computer Software Manual]; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org/ (accessed on 23 February 2023).
- Kenagy, G. Daily and seasonal limits of time and temperature to activity of degus. Rev. Chil. Hist. Nat. 2002, 75, 567–581. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Adhikari, B.S.; Rawat, G.S. Seasonal variations in the time-activity budget of Royle’s pika in the Western Himalaya, India. J. Nat. Hist. 2019, 53, 1873–1888. [Google Scholar] [CrossRef]
- Lacey, E.A.; Patton, J.L. Life Underground: The Biology of Subterranean Rodents; University of Chicago Press: Chicago, IL, USA, 2000. [Google Scholar]
- Zhou, R.; Hua, R.; Tang, Z.; Hua, L. Group-living decrease predation risk of individual: Evidence from behavior, hormones and reproduction of plateau pika. Front. Ecol. Evol. 2023, 11, 146. [Google Scholar] [CrossRef]
- Tablado, Z.; Revilla, E.; Palomares, F. Breeding like rabbits: Global patterns of variability and determinants of European wild rabbit reproduction. Ecography 2009, 32, 310–320. [Google Scholar] [CrossRef]
- Conner, D.A. Seasonal changes in activity patterns and the adaptive value of haying in pikas (Ochotona princeps). Can. J. Zool. 2011, 61, 411–416. [Google Scholar] [CrossRef]
- Hansson, L. Dynamics and trophic interactions of small rodents: Landscape or regional effects on spatial variation? Oecologia 2002, 130, 259–266. [Google Scholar] [CrossRef]
- Marchand, P.J. Life in The Cold. Bioscience 1988, 38, 2. [Google Scholar]
- Yang, H.; Qu, J.; Liu, L.; Li, S.; Zhang, Y. Effects of food supply and burrow density on mortality of the plateau pika (Ochotona curzoniae) in winter. Acta Theriol. Sin. 2011, 31, 257–264. [Google Scholar]
- Kawamichi, T. Behaviour and Daily Activities of the Japanese Pika, Ochotona hyperborea yesoensis (with 8 Text-figures and 1 Table). Summ. Dep. Sci. Hokkaido Univ. 1969, 17, 127–151. [Google Scholar]
- Kawamichi, T. Daily activities and social pattern of two himalayan pikas, Ochotona macrotis and O. roylei, observed at mt. everest (with 9 text-figures and 3 tables). Summ. Dep. Sci. Hokkaido Univ. 1971, 17, 587–609. [Google Scholar]
- Hall, E.R. Mammals of Nevada. Am. Nat. 1946, 104, 494. [Google Scholar]
- Smith, A.T. The distribution and dispersal of pikas: Influences of behavior and climate. Ecology 1974, 55, 1368–1376. [Google Scholar] [CrossRef]
- Kawamichi, T. Annual Cycle of Behaviour and Social Pattern of the Japanese Pika, Ochotona hyperborea yesoensis (with 3 Text-figures). Summ. Dep. Sci. Hokkaido Univ. 1971, 18, 173–185. [Google Scholar]
- Sogliani, D.; Cerri, J.; Turetta, R.; Crema, M.; Corsini, M.; Mori, E. Feral rabbit populations in a peri-urban area: Insights about invasion dynamics and potential management strategies. Eur. J. Wildl. Res. 2021, 6, 60–67. [Google Scholar] [CrossRef]
- Beever, E.A.; Perrine, J.D.; Rickman, T.; Flores, M.; Clark, J.P.; Waters, C.; Weber, S.S.; Yardley, B.; Thoma, D.; Chesley, P.T. Pika (Ochotona princeps) losses from two isolated regions reflect temperature and water balance, but reflect habitat area in a mainland region. J. Mammal. 2016, 97, 1495–1511. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Linderholm, H.W.; Chen, D.; Yu, Q.; Wu, D.; Haginoya, S. Observation and calculation of the solar radiation on the Tibetan Plateau. Energy Convers. Manag. 2012, 57, 23–32. [Google Scholar] [CrossRef]
- Kontsiotis, V.J.; Panteleimon, X.; Vasilios, L.; Bakaloudis, D.E. Effects of environmental and intrinsic factors on the reproduction of insular European wild rabbits (Oryctolagus cuniculus cuniculus Linnaeus 1758). Mammalia 2018, 83, 143. [Google Scholar] [CrossRef]
- Hayes, A.R.; Huntly, N.J. Effects of wind on the behavior and call transmission of pikas (Ochotona princeps). J. Mammal. 2005, 86, 974–981. [Google Scholar] [CrossRef]
- Jackson, T. The diurnal activity of brants’whistling rat (Parotomys brantsii): The effect of seasonal and physical conditions. Trans. R. Soc. S. Afr. 1998, 53, 227–236. [Google Scholar] [CrossRef]
- Larom, D.; Garstang, M.; Payne, K.; Raspet, R.; Lindeque, M. The influence of surface atmospheric conditions on the range and area reached by animal vocalizations. J. Exp. Biol. 1997, 200, 421–431. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Hua, R.; Tang, Z.; Hua, L. Daily and Seasonal Activity Patterns of Plateau Pikas (Ochotona curzoniae) on the Qinghai–Tibet Plateau, China, and Their Relationship with Weather Condition. Animals 2023, 13, 1689. https://doi.org/10.3390/ani13101689
Zhou R, Hua R, Tang Z, Hua L. Daily and Seasonal Activity Patterns of Plateau Pikas (Ochotona curzoniae) on the Qinghai–Tibet Plateau, China, and Their Relationship with Weather Condition. Animals. 2023; 13(10):1689. https://doi.org/10.3390/ani13101689
Chicago/Turabian StyleZhou, Rui, Rui Hua, Zhuangsheng Tang, and Limin Hua. 2023. "Daily and Seasonal Activity Patterns of Plateau Pikas (Ochotona curzoniae) on the Qinghai–Tibet Plateau, China, and Their Relationship with Weather Condition" Animals 13, no. 10: 1689. https://doi.org/10.3390/ani13101689




