Characterization of Fat Quality in Cow Milk from Alpine Farms as Influenced by Seasonal Variations of Diets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farming Conditions and Diets
2.2. Feedstuffs and Milk Sampling
| Summer | Winter | |||||
|---|---|---|---|---|---|---|
| AS | BS | AW | BW | |||
| Only pasture 100% SDM | Fresh Forages (31.7% SDM) | |||||
| Daily-cut fresh grass | 35 kg | |||||
| TMR (58% SDM) | TMR (100% SDM) | TMR (89.8% SDM) | ||||
| -Alfalfa hay | 6 kg | -Alfalfa hay | 10 kg | -Meadow hay | 12.5 kg | |
| -Concentrate A * | 5 kg | -Mixed grass hay | 4.5 kg | -Concentrate A * | 5.5 kg | |
| -Commercial concentrate ** | 3 kg | -Concentrate (CP 18%) | 4.5 kg | -Commercial concentrate ** | 3.5 kg | |
| -Molasses | 0.5 kg | -Flaked corn | 2 kg | -Molasses | 1 kg | |
| Total TMR | 14.5 kg | -Beet pulps | 1 kg | Total TMR | 22.5 kg | |
| -Straw | 1 kg | |||||
| -Whey | 12 kg | |||||
| Total TMR | 35 kg | |||||
| Concentrate at Milking (10.3% SDM) | Concentrate at Milking (10.2% SDM) | |||||
| Concentrate B *** | 2.5 kg | Concentrate B *** | 2.5 kg | |||
2.3. Composition and Fatty Acid Content of Dietary Feedstuffs
2.4. Fatty Acid Profile of Milk
2.5. Data Analysis
3. Results and Discussion
3.1. Diets Composition
3.2. Milk Composition
3.2.1. Saturated Fatty Acids (SFAs)
3.2.2. Odd- and Branched-Chain Fatty Acids (OBCFAs)
- -
- They have been recognized as biomarkers of the rumen functionality, with their proportions strictly related to the ruminal microflora system (mainly, differences between cellulosolytic and amylolitic bacteria);
- -
3.2.3. Unsaturated Fatty Acids (UFAs)
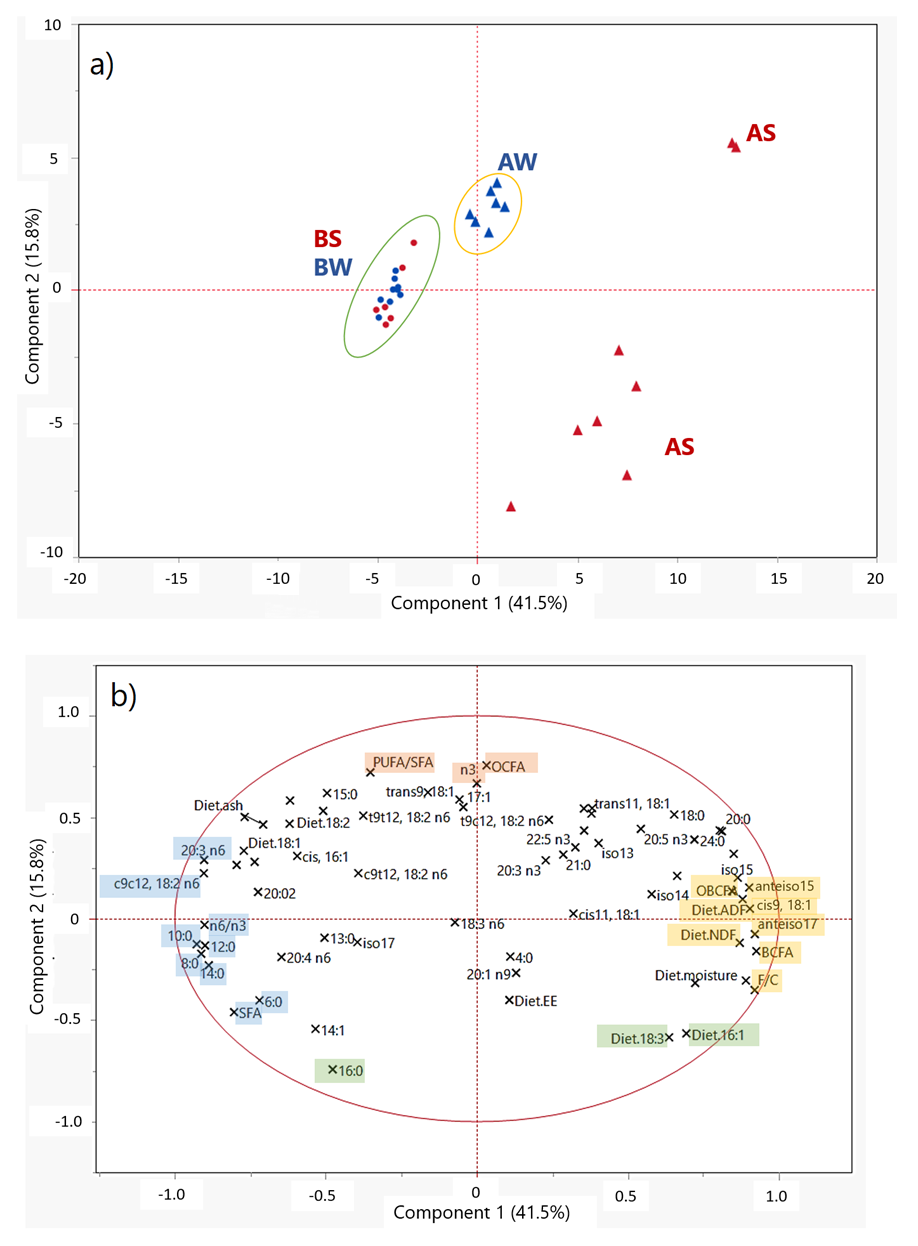
3.3. Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacDonald, D.; Crabtree, J.R.; Wiesinger, G.; Dax, T.; Stamou, N.; Fleury, P.; Gutierrez Lazpita, J.; Gibon, A. Agricultural abandonment in mountain areas of Europe: Environmental consequences and policy response. J. Environ. Manag. 2000, 59, 47–69. [Google Scholar] [CrossRef]
- Montrasio, R.; Mattiello, S.; Zucaro, M.; Genovese, D.; Battaglini, L. The perception of ecosystem services of mountain farming and of a local cheese: An analysis for the touristic valorization of an inner alpine area. Sustainability 2020, 12, 8017. [Google Scholar] [CrossRef]
- Hoffmann, I.; From, T.; Boerma, D. Ecosystem Services Provided by Livestock Species and Breeds, with Special Consideration to the Contributions of Small-Scale Livestock Keepers and Pastoralists; FAO Background Study Paper No. 66 Rev. 1; FAO: Geneva, Switzerland, 2014. [Google Scholar]
- Magan, J.B.; O′Callaghan, T.F.; Kelly, A.L.; McCarthy, N.A. Compositional and functional properties of milk and dairy products derived from cows fed pasture or concentrate-based diets. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2769–2800. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Ross, R.P.; Hill, C.; Fitzgerald, G.F.; Stanton, C. Milk intelligence: Mining milk for bioactive substances associated with human health. Int. Dairy J. 2011, 21, 377–401. [Google Scholar] [CrossRef]
- European Union Commission Delegated Regulation (EU) No 528/2014. Off. J. Eur. Union 2014, L179/23, 2012–2014.
- Joint Research Centre, Institute for Prospective Technological Studies; Gomez y Paloma, S.; Guri, F.; Santini, F. Labelling of Agricultural and Food Products of Mountain Farming; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Butler, G.; Nielsen, J.H.; Slots, T.; Seal, C.; Eyre, M.D.; Sanderson, R.; Leifert, C. Fatty acid and fat-soluble antioxidant concentrations in milk from high- and low-input conventional and organic systems: Seasonal variation. J. Sci. Food Agric. 2008, 88, 1431–1441. [Google Scholar] [CrossRef]
- Serrapica, F.; Masucci, F.; Di Francia, A.; Napolitano, F.; Braghieri, A.; Esposito, G.; Romano, R. Seasonal variation of chemical composition, fatty acid profile, and sensory properties of a mountain pecorino cheese. Foods 2020, 9, 1091. [Google Scholar] [CrossRef]
- Collomb, M.; Bütikofer, U.; Sieber, R.; Jeangros, B.; Bosset, J.O. Composition of fatty acids in cow’s milk fat produced in the lowlands, mountains and highlands of Switzerland using high-resolution gas chromatography. Int. Dairy J. 2002, 12, 649–659. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Doreau, M. Effect of different types of forages, animal fat or marine oils in cow’s diet on milk fat secretion and composition, especially conjugated linoleic acid (CLA) and polyunsaturated fatty acids. Livest. Prod. Sci. 2001, 70, 31–48. [Google Scholar] [CrossRef]
- Chilliard, Y.; Glasser, F.; Ferlay, A.; Bernard, L.; Rouel, J.; Doreau, M. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Eur. J. Lipid Sci. Technol. 2007, 109, 828–855. [Google Scholar] [CrossRef]
- Schwendel, B.H.; Morel, P.C.H.; Wester, T.J.; Tavendale, M.H.; Deadman, C.; Fong, B.; Shadbolt, N.M.; Thatcher, A.; Otter, D.E. Fatty acid profile differs between organic and conventionally produced cow milk independent of season or milking time. J. Dairy Sci. 2015, 98, 1411–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craninx, M.; Steen, A.; Van Laar, H.; Van Nespen, T.; Martin-Tereso, J.; De Baets, B.; Fievez, V. Effect of lactation stage on the odd- and branched-chain milk fatty acids of dairy cattle under grazing and indoor conditions. J. Dairy Sci. 2008, 91, 2662–2677. [Google Scholar] [CrossRef] [Green Version]
- Povolo, M.; Pelizzola, V.; Passolungo, L.; Biazzi, E.; Tava, A.; Contarini, G. Characterization of two Agrostis-Festuca alpine pastures and their influence on cheese composition. J. Agric. Food Chem. 2013, 61, 447–455. [Google Scholar] [CrossRef]
- Povolo, M.; Pelizzola, V.; Lombardi, G.; Tava, A.; Contarini, G. Hydrocarbon and fatty acid composition of cheese as affected by the pasture vegetation type. J. Agric. Food Chem. 2011, 60, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed. Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Bas, P.; Archimède, H.; Rouzeau, A.; Sauvant, D. Fatty acid composition of mixed-rumen bacteria: Effect of concentration and type of forage. J. Dairy Sci. 2003, 86, 2940–2948. [Google Scholar] [CrossRef] [Green Version]
- Capuano, E.; Van Der Veer, G.; Boerrigter-Eenling, R.; Elgersma, A.; Rademaker, J.; Sterian, A.; Van Ruth, S.M. Verification of fresh grass feeding, pasture grazing and organic farming by cows farm milk fatty acid profile. Food Chem. 2014, 164, 234–241. [Google Scholar] [CrossRef]
- Białek, A.; Białek, M.; Lepionka, T.; Czerwonka, M.; Czauderna, M. Chemometric analysis of fatty acids profile of ripening chesses. Molecules 2020, 25, 1814. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. Preparation of derivatives of fatty acids. In Lipid Analysis: Isolation, Separation and Structural Analysis of Lipids, 3rd ed.; Christie, W.W., Ed.; The Oily Press: Bridgwater, UK, 2003; pp. 205–215. ISBN 0-9531949-5-7. [Google Scholar]
- Ulberth, F.; Gabernig, R.G.; Schrammel, F. Flame-Ionization Detector Response to Methyl, Ethyl, Propyl, and Butyl Esters of Fatty Acids. J. Am. Oil Chem. Soc. 1999, 76, 263–266. [Google Scholar] [CrossRef]
- Smolinska, A.; Engel, J.; Szymanska, E.; Buydens, L.; Blanchet, L. General Framing of Low-, Mid-, and High-Level Data Fusion with Examples in the Life Sciences. Data Handl. Sci. Technol. 2019, 31, 51–79. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorlier, A.; Lonati, M.; Renna, M.; Lussiana, C.; Lombardi, G.; Battaglini, L.M. Changes in pasture and cow milk compositions during a summer transhumance in the western Italian Alps. J. Appl. Bot. Food Qual. 2012, 85, 216–223. [Google Scholar]
- Peiretti, P.G.; Gai, F.; Alonzi, S.; Battelli, G.; Tassone, S. Characterization of Alpine highland pastures located at different altitudes: Forage evaluation, chemical composition, in vitro digestibility, fatty acid, and terpene contents. Plant Biosyst. 2017, 151, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Bovolenta, S.; Saccà, E.; Ventura, W.; Piasentier, E. Effect of type and level of supplement on performance of dairy cows grazing on alpine pasture. Ital. J. Anim. Sci. 2002, 1, 255–263. [Google Scholar] [CrossRef]
- Ravetto Enri, S.; Renna, M.; Probo, M.; Lussiana, C.; Battaglini, L.M.; Lonati, M.; Lombardi, G. Relationships between botanical and chemical composition of forages: A multivariate approach to grasslands in the Western Italian Alps. J. Sci. Food Agric. 2017, 97, 1252–1259. [Google Scholar] [CrossRef]
- Leiber, F.; Kreuzer, M.; Nigg, D.; Wettstein, H.R.; Scheeder, M.R.L. A study on the causes for the elevated n-3 fatty acids in cows’ milk of alpine origin. Lipids 2005, 40, 191–202. [Google Scholar] [CrossRef]
- Collomb, M.; Bütikofer, U.; Spahni, M.; Jeangros, B.; Bosset, J.O. Fatty acid and glyceride composition of cow’s milk fat in high- and lowland regions. Sci. Aliment. 1999, 19, 97–110. [Google Scholar]
- Revello Chion, A.; Tabacco, E.; Giaccone, D.; Peiretti, P.G.; Battelli, G.; Borreani, G. Variation of fatty acid and terpene profiles in mountain milk and “Toma piemontese” cheese as affected by diet composition in different seasons. Food Chem. 2010, 121, 393–399. [Google Scholar] [CrossRef]
- Lock, A.L.; Garnsworthy, P.C. Seasonal variation in milk conjugated linoleic acid and δ9-desaturase activity in dairy cows. Livest. Prod. Sci. 2003, 79, 47–59. [Google Scholar] [CrossRef]
- Hanus, O.; Samkova, E.; Křížova, L.; Hasoňova, L.; Kala, R. Role of fatty acids in milk fat and the influence of selected factors on their variability—A review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fievez, V.; Colman, E.; Castro-Montoya, J.M.; Stefanov, I.; Vlaeminck, B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function-An update. Anim. Feed Sci. Technol. 2012, 172, 51–65. [Google Scholar] [CrossRef]
- Kraft, J.; Collomb, M.; Möckel, P.; Sieber, R.; Jahreis, G. Differences in CLA isomer distribution of cow’s milk lipids. Lipids 2003, 38, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Ran-Ressler, R.R.; Bae, S.; Lawrence, P.; Wang, D.H.; Thomas Brenna, J. Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr. 2014, 112, 565–572. [Google Scholar] [CrossRef]
- Benbrook, C.M.; Davis, D.R.; Heins, B.J.; Latif, M.A.; Leifert, C.; Peterman, L.; Butler, G.; Faergeman, O.; Abel-Caines, S.; Baranski, M. Enhancing the fatty acid profile of milk through forage-based rations, with nutrition modeling of diet outcomes. Food Sci. Nutr. 2018, 6, 681–700. [Google Scholar] [CrossRef]
- Collomb, M.; Bisig, W.; Bütikofer, U.; Sieber, R.; Bregy, M.; Etter, L. Fatty acid composition of mountain milk from Switzerland: Comparison of organic and integrated farming systems. Int. Dairy J. 2008, 18, 976–982. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Shingfield, K.J.; Lee, M.R.F.; Scollan, N.D. Increasing the concentrations of beneficial polyunsaturated fatty acids in milk produced by dairy cows in high-forage systems. Anim. Feed Sci. Technol. 2006, 131, 168–206. [Google Scholar] [CrossRef]
- Doreau, M.; Ferlay, A. Digestion and utilisation of fatty acids by ruminants. Anim. Feed Sci. Technol. 1994, 45, 379–396. [Google Scholar] [CrossRef]
- Chilliard, Y.; Rouel, J.; Ferlay, A.; Bernard, L.; Gaborit, P.; Raynal-Ljutovac, K.; Lauret, A.; Leroux, C. Optimising Goat’s Milk and Cheese Fatty Acid Composition; Woodhead Publishing Limited: Shaftesbury, UK, 2006; ISBN 9781855739659. [Google Scholar]
- Elgersma, A. Grazing increases the unsaturated fatty acid concentration of milk from grass-fed cows: A review of the contributing factors, challenges and future perspectives. Eur. J. Lipid Sci. Technol. 2015, 117, 1345–1369. [Google Scholar] [CrossRef]
- Davis, H.; Chatzidimitriou, E.; Leifert, C.; Butler, G. Evidence that forage-fed cows can enhance milk quality. Sustainability 2020, 12, 3688. [Google Scholar] [CrossRef]
- Slots, T.; Butler, G.; Leifert, C.; Kristensen, T.; Skibsted, L.H.; Nielsen, J.H. Potentials to differentiate milk composition by different feeding strategies. J. Dairy Sci. 2009, 92, 2057–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.N.; O’Donovan, M.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef] [PubMed]
| Summer Diets | |||||
|---|---|---|---|---|---|
| AS | BS | ||||
| Pasture (Mixed Pools) | Fresh Grass | TMR | Concentrate B | Total Daily Ration | |
| %DM | 38.13 ± 6.71 | 18.79 ± 2.39 | 63.55 | 88.7 | 51.95 ± 0.76 |
| CP %DM | 12.53 ± 0.75 | 16.12 ± 1.54 | 15.78 | 16.22 | 15.93 ± 0.49 |
| EE %DM | 2.67 ± 0.21 | 2.88 ± 0.25 | 2.26 | 2.97 | 2.53 ± 0.08 |
| Ash% DM | 5.11 ± 0.86 | 12.65 ± 3.60 | 7.01 | 7.02 | 8.80 ± 0.56 |
| NDF %DM | 67.26 ± 6.96 | 60.21 ± 2.67 | 36.04 | 19.84 | 42.03 ± 0.85 |
| ADF %DM | 36.63 ± 1.79 | 40.97 ± 2.50 | 23.93 | 6.6 | 27.55 ± 0.85 |
| F:C Ratio | 100 | 56:44 | |||
| C16:0 | 11.75 ± 0.69 | 12.07 ± 2.09 | 13.94 | 22.51 | 14.23 ± 0.66 |
| C16:1 | 1.58 ± 0.16 | 2.11 ± 0.42 | 0.37 | 0.31 | 0.91 ± 0.13 |
| C18:0 | 1.97 ± 0.35 | 1.75 ± 0.43 | 3.33 | 5.19 | 3.02 ± 0.13 |
| C18:1 | 12.91 ± 2.80 | 4.43 ± 0.98 | 26.55 | 36.67 | 20.58 ± 0.31 |
| C18:2 | 21.46 ± 1.19 | 16.28 ± 2.69 | 37.86 | 23.07 | 29.50 ± 0.85 |
| C18:3 | 27.80 ± 6.51 | 38.27 ± 5.67 | 3.91 | 1.87 | 14.59 ± 1.80 |
| Winter Diets | |||||
| AW | BW | ||||
| Total Daily Ration | TMR | Concentrate B | Total Daily Ration | ||
| %DM | 61.67 | 71.53 | 88.7 | 73.28 | |
| CP %DM | 14.69 | 13.74 | 15.25 | 13.89 | |
| EE %DM | 1.59 | 2.34 | 4.45 | 2.56 | |
| Ash% DM | 9.05 | 7.73 | 7.17 | 7.67 | |
| NDF %DM | 58.48 | 46.55 | 20.93 | 43.94 | |
| ADF %DM | 34.42 | 29.79 | 7.72 | 27.54 | |
| F:C Ratio | 65:35 | 50:50 | |||
| C16:0 | 13.51 | 13.26 | 23.14 | 14.27 | |
| C16:1 | 0.55 | 0.44 | 0.19 | 0.41 | |
| C18:0 | 2.34 | 3.28 | 5.78 | 3.54 | |
| C18:1 | 25.19 | 25.41 | 39.98 | 26.90 | |
| C18:2 | 47.75 | 44.76 | 20.38 | 42.27 | |
| C18:3 | 5.68 | 7.49 | 0.97 | 6.82 | |
| FA | AS | BS | AW | BW | sign | ||
|---|---|---|---|---|---|---|---|
| F | S | S × F | |||||
| Saturated Fatty Acids (SFAs) | |||||||
| 4:0 | 5.11 ± 0.67 | 4.91 ± 0.22 | 4.96 ± 0.17 | 4.89 ± 0.24 | ns | ns | ns |
| 6:0 | 2.46 ± 0.37 B | 2.85 ± 0.36 A | 2.57 ± 0.07 AB | 2.70 ± 0.10 AB | ns | * | ns |
| 8:0 | 1.35 ± 0.27 B | 1.75 ± 0.19 A | 1.53 ± 0.05 AB | 1.68 ± 0.05 A | ns | *** | * |
| 10:0 | 2.44 ± 0.67 C | 3.76 ± 0.30 A | 3.03 ± 0.20 B | 3.54 ± 0.12 AB | ns | *** | ** |
| 12:0 | 2.70 ± 0.79 B | 4.18 ± 0.23 A | 3.35 ± 0.28 C | 3.92 ± 0.14 AB | ns | *** | ** |
| 14:0 | 9.16 ± 1.54 C | 12.31 ± 0.15 A | 10.65 ± 0.69 B | 11.62 ± 0.30 AB | ns | *** | ** |
| 16:0 | 27.42 ± 4.77 | 29.23 ± 0.91 | 26.40 ± 0.78 | 28.59 ± 0.41 | ns | ns | ns |
| 18:0 | 11.21 ± 2.63 A | 8.20 ± 0.41 B | 11.18 ± 0.77 A | 10.31 ± 0.50 A | * | *** | * |
| 20:0 | 0.20 ± 0.05 A | 0.16 ± 0.05 AB | 0.19 ± 0.01 AB | 0.15 ± 0.01 B | ns | ** | ns |
| 22:0 | 0.10 ± 0.04 A | 0.06 ± 0.02 B | 0.09 ± 0.01 AB | 0.06 ± 0.00 B | ns | *** | ns |
| 24:0 | 0.07 ± 0.03 A | 0.04 ± 0.02 B | 0.06 ± 0.01 AB | 0.04 ± 0.00 B | ns | ** | ns |
| Total SFAs | 62.21 ± 5.32 B | 67.43 ± 0.85 A | 64.01 ± 1.07 AB | 67.51 ± 0.53 A | ns | *** | ns |
| Odd- and Branched-Chain Fatty Acids (OBCFAs) | |||||||
| 13:0 | 0.08 ± 0.02 B | 0.11 ± 0.02 A | 0.08 ± 0.01 B | 0.10 ± 0.01 AB | ns | ** | ns |
| 15:0 | 0.78 ± 0.21 B | 1.10 ± 0.06 A | 1.14 ± 0.07 A | 1.03 ± 0.03 A | ** | * | *** |
| 17:0 | 0.74 ± 0.09 A | 0.52 ± 0.01 B | 0.66 ± 0.06 A | 0.51 ± 0.02 B | ns | *** | ns |
| 17:1 | 0.09 ± 0.04 C | 0.05 ± 0.01 D | 0.26 ± 0.04 A | 0.17 ± 0.01 B | *** | *** | * |
| 21:0 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.05 ± 0.06 | 0.03 ± 0.00 | ns | ns | ns |
| Total OCFAs | 1.73 ± 0.30 B | 1.80 ± 0.06 B | 2.19 ± 0.15 A | 1.83 ± 0.04 B | *** | * | ** |
| iso13 | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | ns | ns | ns |
| iso14 | 0.19 ± 0.04 A | 0.13 ± 0.01 B | 0.19 ± 0.02 A | 0.17 ± 0.00 A | * | *** | * |
| iso15 | 0.34 ± 0.07 A | 0.24 ± 0.01 B | 0.31 ± 0.02 A | 0.23 ± 0.01 B | ns | *** | ns |
| anteiso15 | 1.37 ± 0.27 A | 0.53 ± 0.02 B | 0.65 ± 0.04 B | 0.52 ± 0.01 B | *** | *** | *** |
| iso16 | 0.39 ± 0.08 A | 0.27 ± 0.01 B | 0.41 ± 0.04 A | 0.30 ± 0.01 B | ns | *** | ns |
| iso17 | 0.03 ± 0.06 B | 0.02 ± 0.00 B | 0.01 ± 0.00 B | 0.23 ± 0.03 A | *** | *** | *** |
| anteiso17 | 0.87 ± 0.11 A | 0.26 ± 0.14 D | 0.58 ± 0.06 B | 0.39 ± 0.02 C | * | *** | *** |
| Total BCFAs | 3.24 ± 0.50 A | 1.50 ± 0.13 C | 2.20 ± 0.16 B | 1.88 ± 0.02 B | ** | *** | *** |
| Total OBCFAs | 4.97 ± 0.78 A | 3.31 ± 0.11 C | 4.39 ± 0.19 B | 3.71 ± 0.04 C | ns | *** | ** |
| Monounsaturated Fatty Acids (MUFAs) | |||||||
| 14:1 | 0.89 ± 0.36 AB | 1.14 ± 0.09 A | 0.87 ± 0.10 B | 0.96 ± 0.05 AB | ns | * | ns |
| trans, 16:1 | 0.10 ± 0.09 | 0.12 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.00 | ns | ns | ns |
| cis, 16:1 | 0.64 ± 0.50 B | 1.55 ± 0.29 A | 1.52 ± 0.18 A | 1.24 ± 0.15 A | * | ** | *** |
| trans9, 18:1 | 0.31 ± 0.07 | 0.39 ± 0.15 | 0.37 ± 0.03 | 0.34 ± 0.03 | ns | ns | ns |
| trans11, 18:1 | 1.97 ± 1.73 | 2.03 ± 0.33 | 1.26 ± 0.21 | 1.32 ± 0.011 | ns | ns | ns |
| cis9, 18:1 | 24.64 ± 3.59 A | 17.88 ± 0.57 B | 22.22 ± 0.93 A | 19.31 ± 0.45 B | ns | *** | ** |
| cis11, 18:1 | 0.56 ± 0.16 A | 0.55 ± 0.05 A | 0.50 ± 0.08 AB | 0.40 ± 0.02 B | ** | ns | ns |
| 20:1 n9 | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | ns | ns | ns |
| 22:1 n9 | 0.03 ± 0.02 | 0.04 ± 0.03 | 0.03 ± 0.01 | 0.03 ± 0.00 | ns | ns | ns |
| Total MUFAs | 29.21 ± 4.34 A | 23.75 ± 0.98 B | 26.89 ± 0.88 A | 23.71 ± 0.46 B | ns | *** | ns |
| Polyunsaturated Fatty Acids (PUFAs) | |||||||
| t9t12, 18:2 n6 | 0.01 ± 0.01 A | 0.05 ± 0.08 A | 0.23 ± 0.02 B | 0.21 ± 0.02 B | *** | ns | ns |
| c9t12, 18:2 n6 | 0.02 ± 0.01 B | 0.03 ± 0.01 AB | 0.04 ± 0.02 A | 0.04 ± 0.02 AB | ** | ns | ns |
| t9c12, 18:2 n6 | 0.04 ± 0.03 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.00 | ns | ns | ns |
| c9c12, 18:2 n6 | 1.38 ± 0.49 C | 2.73 ± 0.32 A | 2.25 ± 0.15 B | 2.80 ± 0.08 A | ** | *** | ** |
| 18:3 n6 | 0.02 ± 0.01 AB | 0.03 ± 0.02 A | 0.02 ± 0.01 B | 0.0 ± 0.01 B | * | ns | ns |
| 18:3 n3 | 0.62 ± 0.17 | 0.79 ± 0.19 | 0.73 ± 0.13 | 0.70 ± 0.03 | ns | ns | ns |
| c9t11–18:2 | 1.12 ± 0.71 AB | 1.31 ± 0.12 A | 0.78 ± 0.12 B | 0.73 ± 0.04 B | ** | ns | ns |
| 20:2 | 0.02 ± 0.01 | 0.05 ± 0.03 | 0.03 ± 0.00 | 0.03 ± 0.00 | ns | ns | ns |
| 20:3 n6 | 0.07 ± 0.02 B | 0.13 ± 0.05 A | 0.10 ± 0.01 AB | 0.10 ± 0.00 AB | ns | ** | ** |
| 20:4 n6 | 0.13 ± 0.02 B | 0.16 ± 0.01 B | 0.14 ± 0.01 B | 0.14 ± 0.00 AB | ns | ** | ** |
| 20:3 n3 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | ns | ns | ns |
| 20:5 n3 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.05 ± 0.00 | ns | ns | ns |
| 22:5 n3 | 0.09 ± 0.06 | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.08 ± 0.01 | ns | ns | ns |
| Total PUFAs | 3.60 ± 0.93 C | 5.49 ± 0.29 A | 4.51 ± 0.24 B | 4.93 ± 0.12 AB | ns | *** | ** |
| Total n3 PUFAs | 0.79 ± 0.21 | 0.96 ± 0.24 | 0.90 ± 0.14 | 0.84 ± 0.03 | ns | ns | ns |
| Total n6 PUFAs | 1.67 ± 0.50 C | 3.17 ± 0.22 AB | 2.80 ± 0.16 B | 3.33 ± 0.11 A | *** | *** | *** |
| Indexes | |||||||
| PUFAs/SFAs | 0.06 ± 0.02 B | 0.08 ± 0.00 A | 0.07 ± 0.00 AB | 0.07 ± 0.00 AB | ns | ** | * |
| n6/n3 | 2.20 ± 0.80 C | 3.46 ± 0.69 AB | 3.16 ± 0.48 B | 3.98 ± 0.15 A | ** | *** | ns |
| DI 1 | 0.09 ± 0.02 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 00 | ns | ns | ns |
| FA | Diet.NDF | Diet.ADF | F:C Ratio | |||
|---|---|---|---|---|---|---|
| Correlation Coefficient | sign | Correlation Coefficient | sign | Correlation Coefficient | sign | |
| 6:0 | −0.4632 | ** | −0.5716 | ** | −0.4585 | ** |
| 8:0 | −0.699 | *** | −0.7464 | *** | −0.6903 | *** |
| 10:0 | −0.8094 | *** | −0.8296 | *** | −0.7797 | *** |
| 12:0 | −0.8076 | *** | −0.8107 | *** | −0.7515 | *** |
| 14:0 | −0.8101 | *** | −0.8306 | *** | −0.7626 | *** |
| 16:0 | −0.4064 | * | −0.5001 | ** | ns | |
| 18:0 | 0.535 | ** | 0.6184 | ** | 0.3569 | * |
| 20:0 | 0.6408 | ** | 0.7656 | *** | 0.579 | ** |
| 22:0 | 0.5596 | ** | 0.7062 | *** | 0.573 | ** |
| 24:0 | 0.4687 | ** | 0.6019 | ** | 0.521 | ** |
| SFA | −0.686 | *** | −0.746 | *** | −0.5952 | ** |
| 13:0 | −0.5719 | ** | −0.521 | ** | −0.4094 | * |
| 15:0 | −0.516 | ** | −0.3702 | * | −0.6746 | *** |
| 17:0 | 0.794 | *** | 0.8788 | *** | 0.7981 | *** |
| iso14 | 0.5696 | ** | 0.6079 | ** | 0.4239 | * |
| iso15 | 0.7487 | *** | 0.8459 | *** | 0.7178 | *** |
| anteiso15 | 0.7539 | *** | 0.7586 | *** | 0.927 | *** |
| iso16 | 0.7349 | *** | 0.7689 | *** | 0.517 | ** |
| iso17 | −0.478 | ** | −0.5356 | ** | −0.4515 | * |
| anteiso17 | 0.8764 | *** | 0.8825 | *** | 0.8641 | *** |
| BCFAs | 0.8184 | *** | 0.8302 | *** | 0.881 | *** |
| OBCFAs | 0.7803 | *** | 0.8489 | *** | 0.755 | *** |
| 14:1 | −0.4955 | ** | −0.5035 | ** | ns | |
| cis, 16:1 | −0.6133 | ** | −0.4999 | ** | −0.6802 | *** |
| cis9, 18:1 | 0.8656 | *** | 0.8404 | *** | 0.7397 | *** |
| cis11, 18:1 | ns | ns | 0.376 | * | ||
| MUFAs | 0.761 | *** | 0.7962 | *** | 0.6888 | *** |
| t9t12, 18:2 n6 | ns | ns | −0.5682 | ** | ||
| c9t12, 18:2 n6 | ns | ns | −0.418 | * | ||
| c9c12, 18:2 n6 | −0.7971 | *** | −0.8484 | *** | −0.9023 | *** |
| 20:0 | −0.6655 | *** | −0.6302 | ** | −0.6721 | *** |
| 20:3 n6 | −0.6809 | *** | −0.6755 | *** | −0.7917 | *** |
| 20:4 n6 | −0.462 | ** | −0.5687 | ** | −0.4728 | ** |
| 20:5 n3 | 0.5192 | ** | 0.528 | ** | 0.3586 | * |
| PUFAs | −0.7067 | *** | −0.6561 | *** | −0.749 | *** |
| PUFAs/SFAs | −0.4921 | ** | −0.4132 | * | −0.5477 | ** |
| n6 | −0.7798 | *** | −0.8173 | *** | −0.9273 | *** |
| n6/n3 | −0.7108 | *** | −0.7938 | *** | −0.8208 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, A.; Bellagamba, F.; Savoini, G.; Moretti, V.M.; Cattaneo, D. Characterization of Fat Quality in Cow Milk from Alpine Farms as Influenced by Seasonal Variations of Diets. Animals 2022, 12, 515. https://doi.org/10.3390/ani12040515
Lopez A, Bellagamba F, Savoini G, Moretti VM, Cattaneo D. Characterization of Fat Quality in Cow Milk from Alpine Farms as Influenced by Seasonal Variations of Diets. Animals. 2022; 12(4):515. https://doi.org/10.3390/ani12040515
Chicago/Turabian StyleLopez, Annalaura, Federica Bellagamba, Giovanni Savoini, Vittorio Maria Moretti, and Donata Cattaneo. 2022. "Characterization of Fat Quality in Cow Milk from Alpine Farms as Influenced by Seasonal Variations of Diets" Animals 12, no. 4: 515. https://doi.org/10.3390/ani12040515








