Wheat Germ Oil and Propolis Decrease Parasite Burden and Restore Marked Histopathological Changes in Liver and Lung in Mice with Chronic Toxoplasmosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Materials
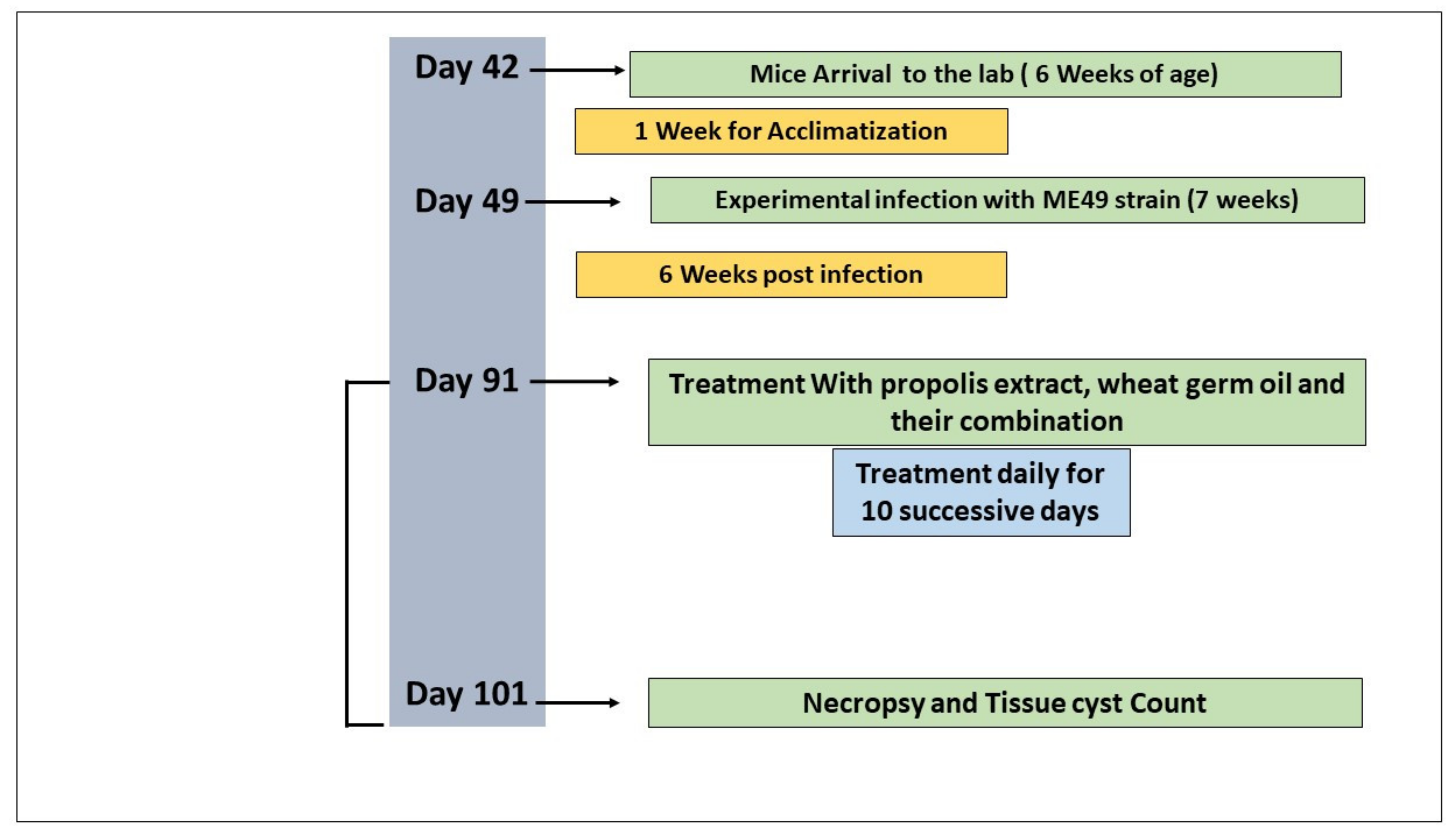
2.3. Experimental Procedure
2.4. Parasitological Assessment
2.5. Molecular Identification
2.6. Histopathological Examination
2.7. Histopathologic Scoring
2.8. Statistical Analysis
3. Results
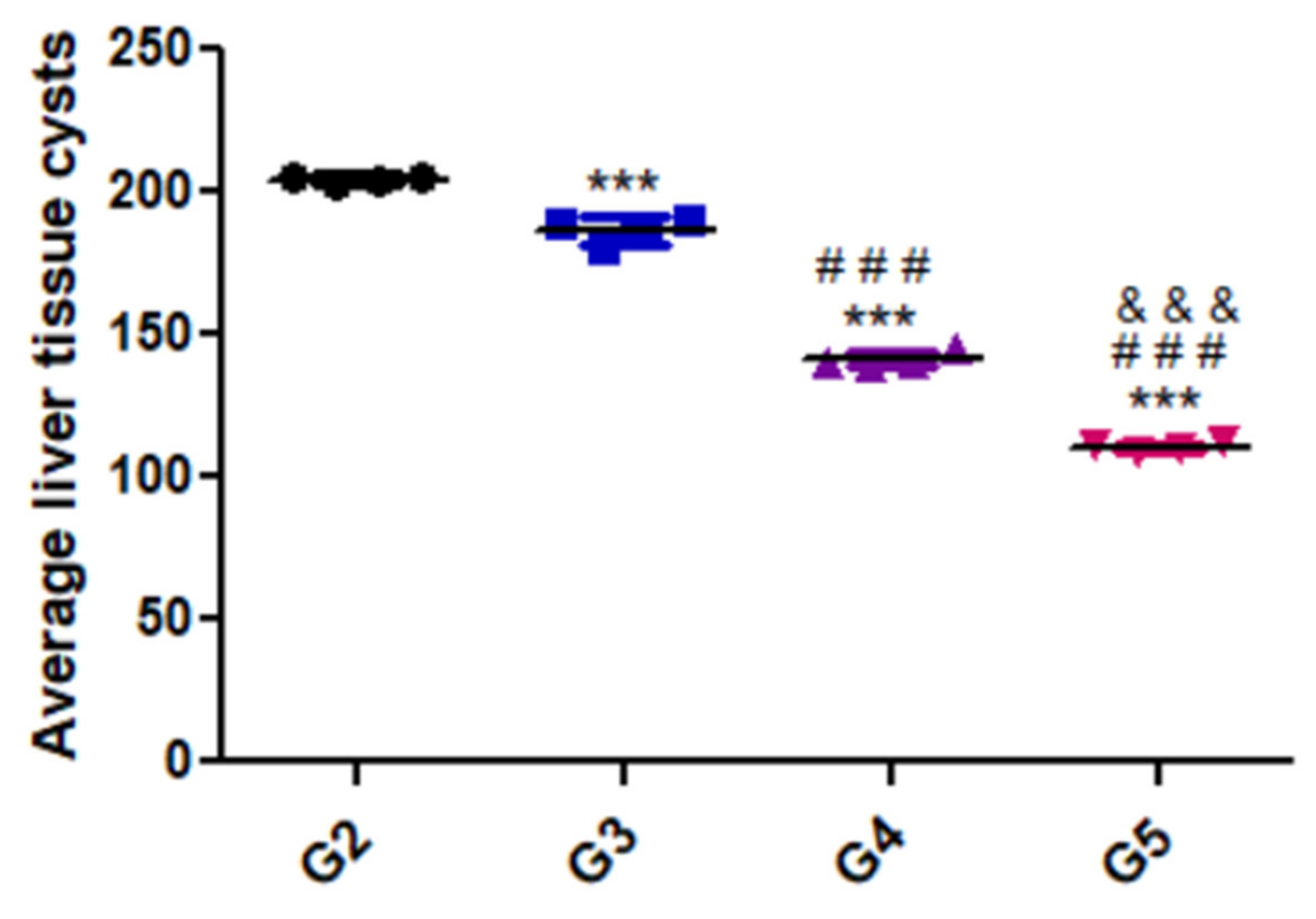
3.1. Parasitic Load
3.2. Molecular Results
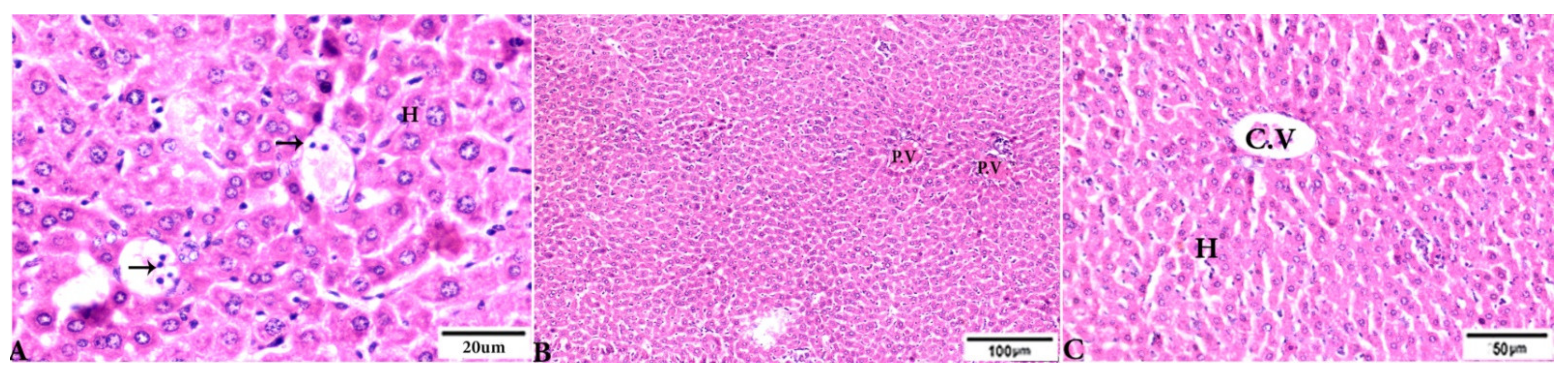
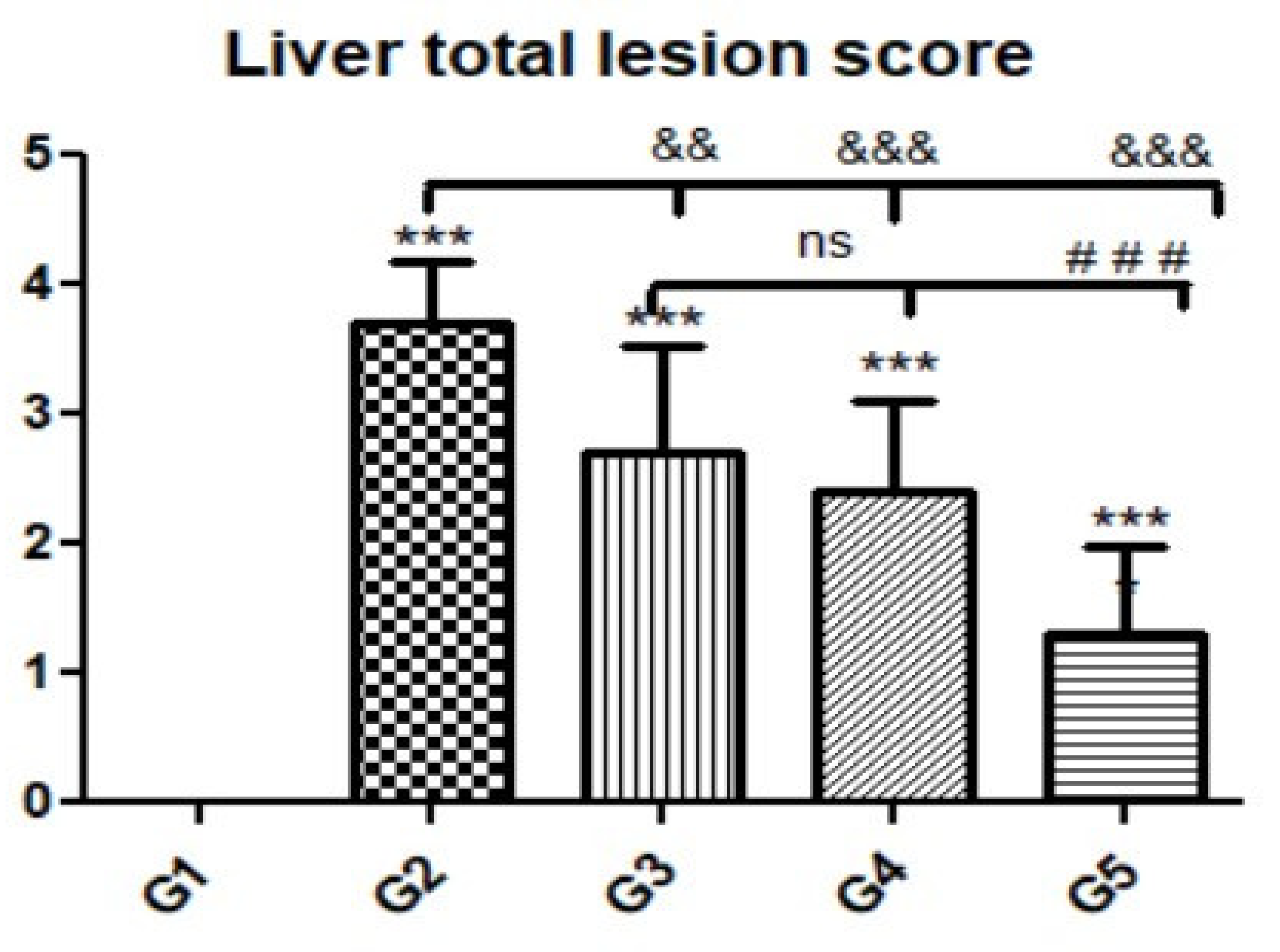
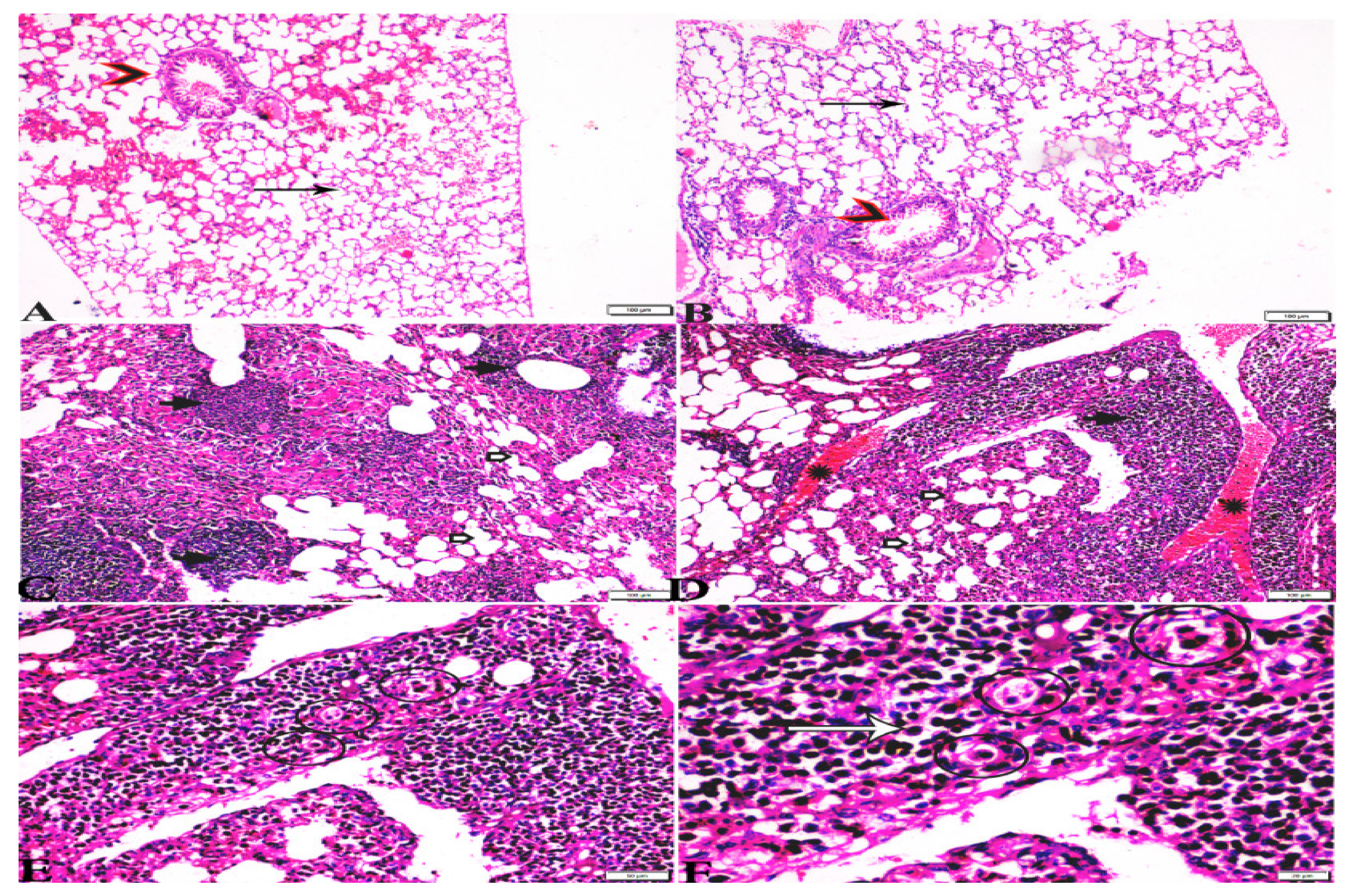
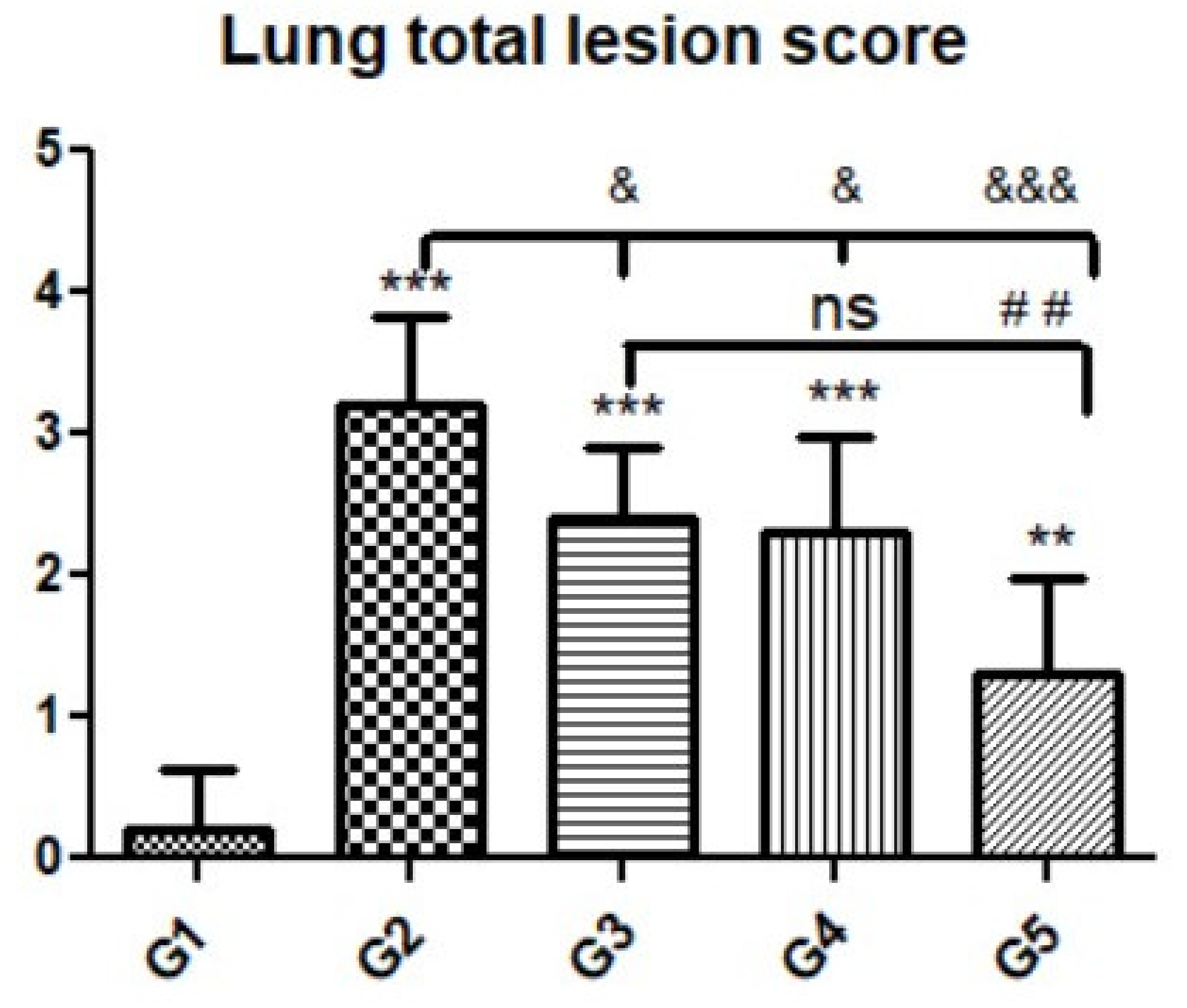
3.3. Histopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, D.E.; Chirukandoth, S.; Dubey, J.P. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 2005, 6, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, diagnosis, and prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Chen, Z.; Li, H.L.; Zheng, H.; He, S.; Lin, R.Q.; Zhu, X.Q. Toxoplasma gondii infection in humans in China. Parasit. Vectors 2011, 4, 165. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.D. The Protozoan Phylum Apicomplexa; CRC Press: Boca Raton, FL, USA, 2018; Volume 2. [Google Scholar]
- Frenkel, J.K. Biology of Toxoplasma gondii. In Congenital Toxoplasmosis; Springer: Paris, France, 2000; pp. 9–25. [Google Scholar]
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar] [CrossRef]
- Belluco, S.; Simonato, G.; Mancin, M.; Pietrobelli, M.; Ricci, A. Toxoplasma gondii infection and food consumption: A systematic review and meta-analysis of case-controlled studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 3085–3096. [Google Scholar] [CrossRef]
- Evans, R. Life cycle and animal infection. In Human Toxoplasmosis; Ho-Yen, D.O., Joss, A.W.L., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 26–55. [Google Scholar]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef]
- Lass, A.; Kontogeorgos, I.; Ma, L.; Zhang, X.; Li, X.; Karanis, P. Investigation of Toxoplasma gondii in wastewater and surface water in the Qinghai-Tibet Plateau, China using real-time PCR and multilocus genotyping. Sci. Rep. 2022, 12, 5428. [Google Scholar] [CrossRef]
- Matignon, M.; Bonnefoy, F.; Lang, P.; Grimbert, P. Organ transplantation and blood transfusion. Transfus. Clin. Biol. 2011, 18, 70–78. [Google Scholar] [CrossRef]
- Bowie, W.R.; King, A.S.; Werker, D.H.; Isaac-Renton, J.L.; Bell, A.; Eng, S.B.; Marion, S.A. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet 1997, 350, 173–177. [Google Scholar] [CrossRef]
- Ho-Yen, D.O.; Joss, A.W. Human Toxoplasmosis; Oxford University Press: New York, NY, USA, 1992. [Google Scholar]
- Miller, C.M.; Boulter, N.R.; Ikin, R.J.; Smith, N.C. The immunobiology of the innate response to Toxoplasma gondii. Int. J. Parasitol. 2009, 39, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Boothroyd, J.C.; Kovacs, J.A. Toxoplasma gondii. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3122–3153.e3127. [Google Scholar]
- Goldstein, E.J.; Montoya, J.G.; Remington, J.S. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 2008, 47, 554–566. [Google Scholar]
- Montoya, J.G.; Bennett, J.E.; Dolin, R.F. (Eds.) Toxoplasma gondii, 5th ed.; Churchill Livingstone: London, UK, 2000; pp. 2858–2888. [Google Scholar]
- Liu, Q.; Wang, Z.-D.; Huang, S.-Y.; Zhu, X.-Q. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasit. Vectors 2015, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.C.; Olivera, G.C.; Barragan, A. Early passage of Toxoplasma gondii across the blood–brain barrier. Trends Parasitol. 2022, 38, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Dubey, J.P. Toxoplasma gondii as a parasite in food: Analysis and control. In Preharvest Food Safety; Microbiol Spectrum 4: PFS-0011-2015; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Guevara, R.B.; Fox, B.A.; Falla, A.; Bzik, D.J. Toxoplasma gondii intravacuolar-network-associated dense granule proteins regulate maturation of the cyst matrix and cyst wall. MSphere 2019, 4, e00487-19. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Tonkal, A. In vitro antitrichomonal effect of Nigella sativa aqueous extract and wheat germ agglutinin. J. Med. Sci 2009, 16, 17–34. [Google Scholar] [CrossRef]
- Etewa, S.E.; El-Maaty, D.A.A.; Hamza, R.S.; Metwaly, A.S.; Sarhan, M.H.; Abdel-Rahman, S.A.; Fathy, G.M.; El-Shafey, M.A. Assessment of spiramycin-loaded chitosan nanoparticles treatment on acute and chronic toxoplasmosis in mice. J. Parasit. Dis. 2018, 42, 102–113. [Google Scholar] [CrossRef]
- Grant, J.; Mahanty, S.; Khadir, A.; MacLean, J.; Kokoskin, E.; Yeager, B.; Joseph, L.; Diaz, J.; Gotuzzo, E.; Mainville, N.; et al. Wheat germ supplement reduces cyst and trophozoite passage in people with giardiasis. Am. J. Trop. Med. 2001, 65, 705–710. [Google Scholar] [CrossRef]
- Blumenthal, C.; Stone, P.; Gras, P.; Bekes, F.; Clarke, B.; Barlow, E.; Appels, R.; Wrigley, C. Heat-shock protein 70 and dough-quality changes resulting from heat stress during grain filling in wheat. Cereal Chem. 1998, 75, 43–50. [Google Scholar] [CrossRef]
- Ismail, K.; El Kadery, A.; Sabry, N.; Mohammad, O. A study on the Amoebicidal effect of Nigella sativa aqueous and alcoholic extracts and wheat germ agglutinin on pathogenic Acanthamoeba. J. Clin. Med. Ther. 2018, 3, 12. [Google Scholar]
- Moustafa, M.A. Role of wheat germ agglutinin (WGA) in treatment of experimental cryptosporidiosis. J. Egypt. Soc. Parasitol. 2003, 33, 443–456. [Google Scholar] [PubMed]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid. Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Saarnio, S.; Julkunen-Tiitto, R. Phenolic Compounds of Propolis from the Boreal Coniferous Zone. J. Apic. Sci. 2012, 56, 13–22. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Ferreres, F.; García-Viguera, C.; Bankova, V.S.; De Castro, S.L.; Dantas, A.P.; Valente, P.H.; Paulino, N. Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 2001, 74, 105–112. [Google Scholar] [CrossRef]
- Siheri, W.; Ebiloma, G.; Igoli, J.; Gray, A.; Biddau, M.; Akrachalanont, P.; Alenezi, S.; Alwashih, M.; Edrada-Ebel, R.; Muller, S.; et al. Isolation of a Novel Flavanonol and an Alkylresorcinol with Highly Potent Anti-Trypanosomal Activity from Libyan propolis. Molecules 2019, 24, 1041. [Google Scholar] [CrossRef] [PubMed]
- De L Paula, L.A.; Cândido, A.; Santos, M.F.C.; Caffrey, C.R.; Bastos, J.K.; Ambrósio, S.R.; Magalhães, L.G. Antiparasitic Properties of Propolis Extracts and Their Compounds. Chem Biodivers 2021, 18, e2100310. [Google Scholar] [CrossRef]
- Rebouças-Silva, J.; Celes, F.S.; Lima, J.B.; Barud, H.S.; de Oliveira, C.I.; Berretta, A.A.; Borges, V.M. Parasite Killing of Leishmania (V) braziliensis by Standardized Propolis Extracts. Evid. Based Complement. Altern. Med. 2017, 2017, 6067172. [Google Scholar] [CrossRef]
- Alotaibi, A.; Ebiloma, G.; Williams, R.; Alenezi, S.; Donachie, A.; Guillaume, S.; Igoli, J.; Fearnley, J.; De Koning, H.; Watson, D. European propolis is highly active against trypanosomatids including Crithidia fasciculata. Sci. Rep. 2019, 9, 11364. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Soufy, H.; El-Beih, N.M.; Nasr, S.M.; Abd El-Aziz, T.H.; Khalil, F.A.M.; Ahmed, Y.F.; Abou Zeina, H.A.A. Effect of Egyptian propolis on cryptosporidiosis in immunosuppressed rats with special emphasis on oocysts shedding, leukogram, protein profile and ileum histopathology. Asian Pac. J. Trop. Dis. 2017, 10, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Mohsen, E.; Abd El Nasser, G. Sensory metabolites profiling in Myristica fragrans (Nutmeg) organs and in response to roasting as analyzed via chemometric tools. Lwt 2018, 97, 684–692. [Google Scholar] [CrossRef]
- Farag, M.A.; Ammar, N.M.; El Gendy, A.N.; Mohsen, E. Effect of grilling as processing method on Zea mays (corn) metabolites composition as analyzed via SPME GC-MS and chemometrics. J. Food Process. Preserv. 2019, 43, e14165. [Google Scholar] [CrossRef]
- El Gendy, A.N.G.; Tavarini, S.; Conte, G.; Pistelli, L.; Hendawy, S.F.; Omer, E.A.; Angelini, L.G. Yield and qualitative characterisation of seeds of Amaranthus hypochondriacus L. and Amaranthus cruentus L. grown in central Italy. Ital. J. Agron. 2018, 13, 63–73. [Google Scholar] [CrossRef]
- Selem, R.F.; Rashed, G.A.; Barakat, A.M.A.; Elfadaly, H.M.A.; Hussien, B.E.-S.T.; Mohamed, H.M.; Nageeb, M.M.; Moharm, A.F. Effect of the anticancer drug tamoxifen on chronic toxoplasmosis in experimentally infected rats. Afr. J. Pharm. Pharmacol. 2019, 13, 259–265. [Google Scholar]
- El Fakhry, Y.; Achbarou, A.; Desportes-Livage, I.; Mazier, D. Encephalitozoon intestinalis: Humoral responses in interferon-γ receptor knockout mice infected with a microsporidium pathogenic in AIDS patients. Exp. Parasitol. 1998, 89, 113–121. [Google Scholar] [CrossRef]
- El-Shafey, A.A.; Hegab, M.; Seliem, M.M.; Barakat, A.; Mostafa, N.E.; Abdel-Maksoud, H.A.; Abdelhameed, R.M. Curcumin@ metal organic frameworks nano-composite for treatment of chronic toxoplasmosis. J. Mater. Sci. Mater. Med. 2020, 31, 90. [Google Scholar] [CrossRef]
- Hegazi, A.; Toaleb, N.; El Fadaly, H.; Abdel-Rahman, E.; Barakat, A. In vivo-cellular and humoral immune response for evaluation of propolis effect on chronic toxoplasmosis in rats. Adv. Anim. Vet. Sci. 2021, 9, 1045–1052. [Google Scholar] [CrossRef]
- Karabacak, M.; Kanbur, M.; Eraslan, G.; Sarıca, Z.S. The antioxidant effect of wheat germ oil on subchronic coumaphos exposure in mice. Ecotoxicol. Environ. Saf. 2011, 74, 2119–2125. [Google Scholar] [CrossRef]
- Barakat, A.M.; Elfadaly, H.A.M.; Selem, R.F.; Madboli, A.E.-N.A.; El-Razik, A.; Abd El-Hamid, K.; Hassan, E.A.; Alghamdi, A.H.; Elmahallawy, E.K. Tamoxifen increased the parasite burden and induced a series of histopathological and immunohistocehmical changes during chronic toxoplasmosis in experimentally infected Mice. Front. Microbiol. 2021, 13, 1648. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Huang, B.; Chen, Y.; Li, S.; Yan, J.; Zheng, H.; Huang, S.; Shen, J.; Lun, Z.-R.; Wang, Y. Upregulated expression of Tim-3 involved in the process of toxoplasmic encephalitis in mouse model. Parasitol. Res. 2013, 112, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- James, P.M.; Coltman, D.W.; Murray, B.W.; Hamelin, R.C.; Sperling, F.A. Spatial genetic structure of a symbiotic beetle-fungal system: Toward multi-taxa integrated landscape genetics. PLoS ONE 2011, 6, e25359. [Google Scholar]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Salman, K.H.; Ali, F.A.Z.; Elhanbaly, R. Effect of cultured white soft cheese on the histopathological changes in the kidneys and liver of albino rats. Sci Rep 2022, 12, 2564. [Google Scholar] [CrossRef]
- Chew, W.K.; Segarra, I.; Ambu, S.; Mak, J.W. Significant reduction of brain cysts caused by Toxoplasma gondii after treatment with spiramycin coadministered with metronidazole in a mouse model of chronic toxoplasmosis. Antimicrob. Agents Chemother. 2012, 56, 1762–1768. [Google Scholar] [CrossRef]
- Al-Ghandour, A.M.F.; Ahmed, H.K.; Salem, A.E.; Tealeb, A.-S.M.; Mohamed, R.M.; Yousef, A.M. Efficacy of olibanum and propolis medicinal extracts versus metronidazole in Giardia lamblia experimentally infected mice. Microbes Infect. Dis. 2020, 1, 209–220. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; El Fadaly, H.A.M.; Soror, A.H.; Ali, F.A.Z.; Abd El-Razik, K.A.; Soliman, Y.A.; Alkhaldi, A.A.M.; Albezrah, N.K.A.; Barakat, A.M. Novel insights on the potential activity of propolis and wheat germ oil against chronic toxoplasmosis in experimentally infected mice. Biomed. Pharm. 2022, 156, 113811. [Google Scholar] [CrossRef]
- Hagras, N.A.-e.; Mogahed, N.M.F.H.; Sheta, E.; Darwish, A.A.-e.; El-Hawary, M.A.; Hamed, M.T.; Elwakil, B.H. The powerful synergistic effect of spiramycin/propolis loaded chitosan/alginate nanoparticles on acute murine toxoplasmosis. PLoS Negl. Trop. Dis. 2022, 16, e0010268. [Google Scholar] [CrossRef]
- Touzani, S.; Embaslat, W.; Imtara, H. In Vitro Evaluation of the Potential Use of Propolis as a Multitarget Therapeutic Product: Physicochemical Properties, Chemical Composition, and Immunomodulatory, Antibacterial, and Anticancer Properties. Biomed. Res. Int. 2019, 2019, 4836378. [Google Scholar] [CrossRef]
- Asfaram, S.; Fakhar, M.; Keighobadi, M.; Akhtari, J. Promising anti-protozoan activities of propolis (bee glue) as natural product: A review. Acta Parasitol. 2021, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Antwi, C.A.; Amisigo, C.M.; Adjimani, J.P.; Gwira, T.M. In vitro activity and mode of action of phenolic compounds on Leishmania donovani. PLoS Negl. Trop. Dis. 2019, 13, e0007206. [Google Scholar] [CrossRef] [PubMed]
- Embley, M.; der Giezen, M.v.; Horner, D.S.; Dyal, P.L.; Foster, P. Mitochondria and hydrogenosomes are two forms of the same fundamental organelle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 191–203. [Google Scholar] [CrossRef]
- Tsen, C.C. Amino acid composition and biological value of cereal germs. In Amino Acid Composition and Biological Value of Cereal Proteins; Springer: Berlin/Heidelberg, Germany, 1985; pp. 453–466. [Google Scholar]
- Lee, Y.-L.; Cesario, T.; Wang, Y.; Shanbrom, E.; Thrupp, L. Antibacterial activity of vegetables and juices. Nutrition 2003, 19, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Oluwatosin, K.Y.; Justine, T.E. Studies of phytochemical constituents and anti-trypanosomal properties of fermented wheat germ and garlic bulbs extract on Trypanosoma brucei –infected rats. J. Med. Plant Res. 2010, 4, 2016–2020. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; Martínez, A.S.; Rodriguez-Granger, J.; Hoyos-Mallecot, Y.; Agil, A.; Mari, J.M.N.; Fernández, J.G. Diagnosis of leishmaniasis. J. Infect. Dev. Ctries. 2014, 8, 961–972. [Google Scholar] [CrossRef] [PubMed]
- El-Newishy, A.M.A.; Salem, L.M.A.; Barakat, A.M.; Elmahallawy, E.K.A. Zoonotic Importance of Toxoplasma gondii Tissue Cysts in Chickens. Benha Vet. Med. J. 2012, 23, 53–60. [Google Scholar]
- Dunn, J.D.; Ravindran, S.; Kim, S.-K.; Boothroyd, J.C. The Toxoplasma gondii dense granule protein GRA7 is phosphorylated upon invasion and forms an unexpected association with the rhoptry proteins ROP2 and ROP4. Infect. Immun. 2008, 76, 5853–5861. [Google Scholar] [CrossRef]
- Garg, D.; Madan, N.; Qaqish, O.; Nagarakanti, S.; Patel, V. Pulmonary toxoplasmosis diagnosed on transbronchial lung biopsy in a mechanically ventilated patient. Case Rep. Infect. Dis. 2020, 2020, 9710182. [Google Scholar] [CrossRef]
- Desoubeaux, G.; Cabanne, É.; Franck-Martel, C.; Gombert, M.; Gyan, E.; Lissandre, S.; Renaud, M.; Monjanel, H.; Dartigeas, C.; Bailly, É. Pulmonary toxoplasmosis in immunocompromised patients with interstitial pneumonia: A single-centre prospective study assessing PCR-based diagnosis. J. Clin. Pathol. 2016, 69, 726–730. [Google Scholar] [CrossRef]




| P29 Q-f | CAGCATGGATAAGGCATCTG |
| P29 Q-r | GTTGCTCCTCTGTTAGTTCC |
| Groups | Sample Number | CT Value | Concentration (µg/µL) | Significance |
|---|---|---|---|---|
| G1 (noninfected, nontreated) | C- | No Ct | None | - |
| G2 (infected, nontreated) | 1 | 23.22 | 0.0284 | - |
| 2 | 19.11 | 0.0361 | ||
| 3 | 20.06 | 0.0069 | ||
| G3 | 4 | 25.28 | 0.1459 | Nonsignificant |
| 5 | 26.55 | 0.0106 | ||
| 6 | 28.54 | 0.0553 | ||
| G4 | 7 | 29.25 | 0.1740 | Nonsignificant |
| 8 | 32.13 | 0.1126 | ||
| 9 | 31.34 | 0.1330 | ||
| G5 | 10 | 33.37 | 0.1568 | Significant (p < 0.05) |
| 11 | 36.13 | 0.1056 | ||
| 12 | 36.26 | 0.2080 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, A.M.; Fadaly, H.A.M.E.; Gareh, A.; Abd El-Razik, K.A.; Ali, F.A.Z.; Saleh, A.A.; Sadek, S.A.S.; Dahran, N.; El-Gendy, A.E.-N.G.; El-Khadragy, M.F.; et al. Wheat Germ Oil and Propolis Decrease Parasite Burden and Restore Marked Histopathological Changes in Liver and Lung in Mice with Chronic Toxoplasmosis. Animals 2022, 12, 3069. https://doi.org/10.3390/ani12223069
Barakat AM, Fadaly HAME, Gareh A, Abd El-Razik KA, Ali FAZ, Saleh AA, Sadek SAS, Dahran N, El-Gendy AE-NG, El-Khadragy MF, et al. Wheat Germ Oil and Propolis Decrease Parasite Burden and Restore Marked Histopathological Changes in Liver and Lung in Mice with Chronic Toxoplasmosis. Animals. 2022; 12(22):3069. https://doi.org/10.3390/ani12223069
Chicago/Turabian StyleBarakat, Ashraf Mohamed, Hassan Ali Mohamed El Fadaly, Ahmed Gareh, Khaled A. Abd El-Razik, Fatma Abo Zakaib Ali, Amira A. Saleh, Sabry A. S. Sadek, Naief Dahran, Abd El-Nasser G. El-Gendy, Manal F. El-Khadragy, and et al. 2022. "Wheat Germ Oil and Propolis Decrease Parasite Burden and Restore Marked Histopathological Changes in Liver and Lung in Mice with Chronic Toxoplasmosis" Animals 12, no. 22: 3069. https://doi.org/10.3390/ani12223069
APA StyleBarakat, A. M., Fadaly, H. A. M. E., Gareh, A., Abd El-Razik, K. A., Ali, F. A. Z., Saleh, A. A., Sadek, S. A. S., Dahran, N., El-Gendy, A. E.-N. G., El-Khadragy, M. F., & Elmahallawy, E. K. (2022). Wheat Germ Oil and Propolis Decrease Parasite Burden and Restore Marked Histopathological Changes in Liver and Lung in Mice with Chronic Toxoplasmosis. Animals, 12(22), 3069. https://doi.org/10.3390/ani12223069








