Ability of Garlic and Ginger Oil to Reduce Salmonella in Post-Harvest Poultry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Salmonella Strains and Culture Conditions
2.2. Preparation of Garlic and Ginger Solutions
2.3. Antimicrobial Activity against S. infantis on Chicken Skin
2.4. Determination of Sub-Inhibitory Concentration
2.5. Impact of Garlic and Ginger on Quorum Sensing Activity of Salmonella
2.6. Changes in Salmonella Gene Expression following Garlic and Ginger Exposure
2.7. Statistical Analysis
3. Results
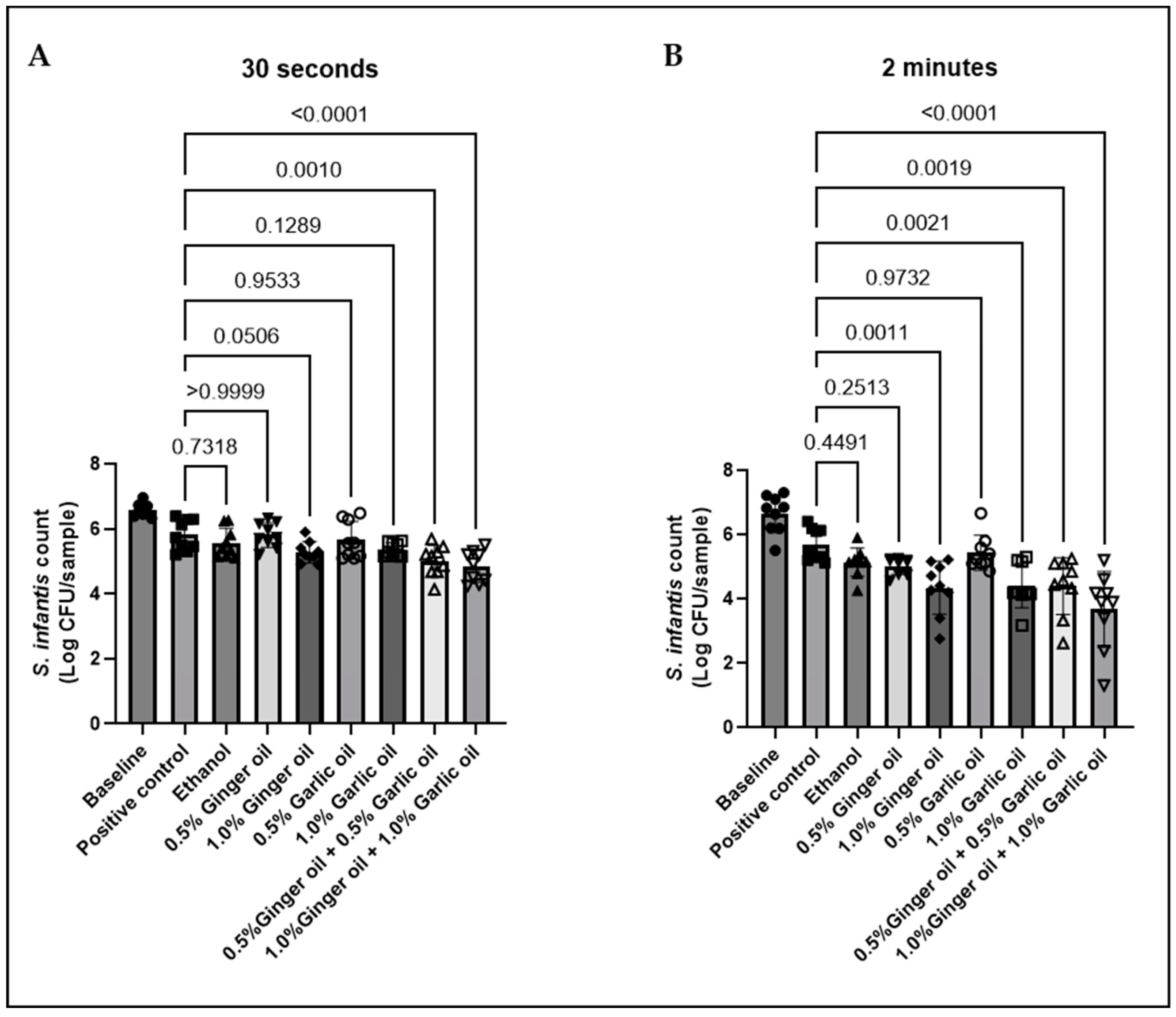
3.1. Antimicrobial Activity of Garlic and Ginger Oil in the Scalding Tank Environment
3.2. Effect of Various Concentrations of Garlic and Ginger Oil on Salmonella Growth
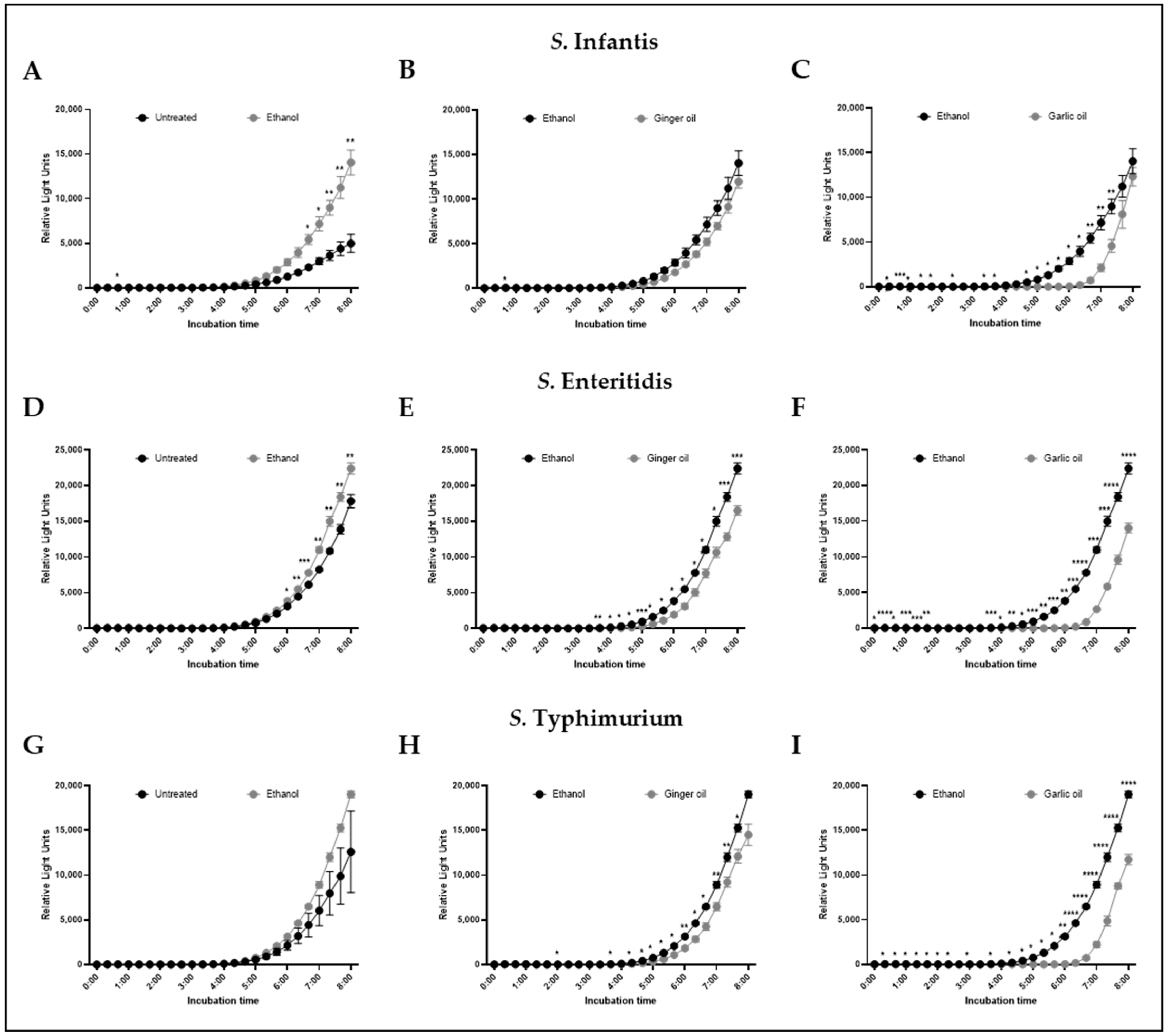
3.3. Inhibition of Quorum Sensing by Garlic and Ginger Oil
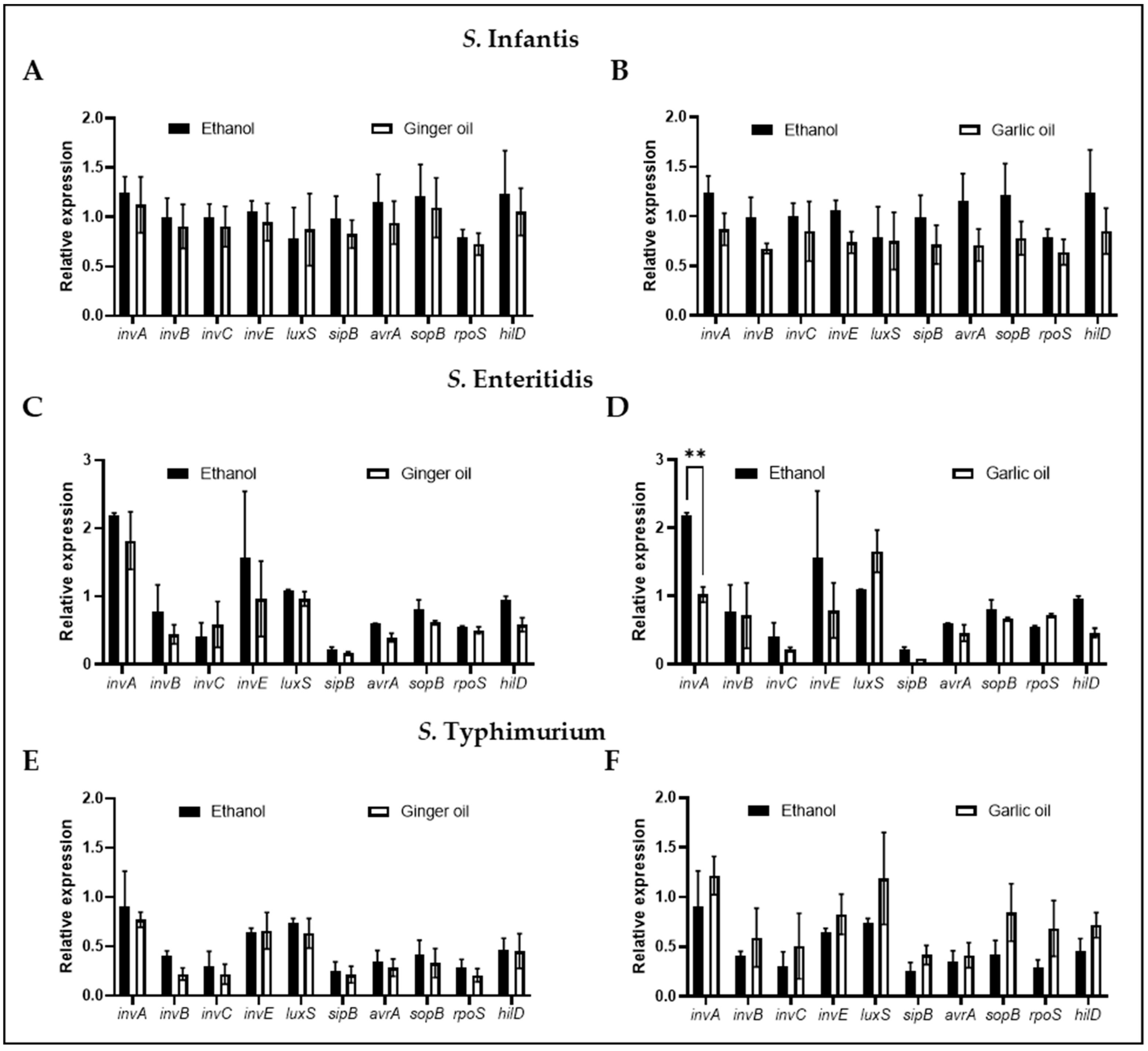
3.4. Effect of Garlic and Ginger on Salmonella Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- CDC. Salmonella. Available online: https://www.cdc.gov/Salmonella/index.html (accessed on 4 April 2022).
- Amalaradjou, M.A. Pre-harvest Approaches to Improve Poultry Meat Safety. In Food Safety in Poultry Meat Production; Springer: Berlin/Heidelberg, Germany, 2019; pp. 95–122. [Google Scholar]
- Lahellec, C.; Colin, P. Relationship between serotypes of Salmonellae from hatcheries and rearing farms and those from processed poultry carcases. Br. Poult. Sci. 1985, 26, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.; Sarlin, L.; Caldwell, D.; Yezak, C., Jr.; Hume, M.; Corrier, D.; Deloach, J.; Hargis, B. Effect of feed withdrawal on the incidence of Salmonella in the crops and ceca of market age broiler chickens. Poult. Sci. 1997, 76, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Leboveic, A.; Burke, K.; Biswas, D. Post-harvest approaches to improve poultry meat safety. In Food safety in Poultry Meat Production; Springer: Berlin/Heidelberg, Germany, 2019; pp. 123–138. [Google Scholar]
- Berry, E.D.; Wells, J.E. Reducing foodborne pathogen persistence and transmission in animal production environments: Challenges and opportunities. Preharvest Food Saf. 2018, 2018, 177–203. [Google Scholar] [CrossRef]
- Foley, S.L.; Nayak, R.; Hanning, I.B.; Johnson, T.J.; Han, J.; Ricke, S.C. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 2011, 77, 4273–4279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Pornsukarom, S.; Thakur, S. Antibiotic usage in poultry production and antimicrobial-resistant Salmonella in poultry. In Food Safety in Poultry Meat Production; Springer: Berlin/Heidelberg, Germany, 2019; pp. 47–66. [Google Scholar]
- Medeiros, M.A.N.; Oliveira, D.C.N.d.; Rodrigues, D.d.P.; Freitas, D.R.C.d. Prevalence and antimicrobial resistance of Salmonella in chicken carcasses at retail in 15 Brazilian cities. Rev. Panam. Salud. Publica. 2011, 30, 555–560. [Google Scholar] [CrossRef]
- Wagle, B.R.; Donoghue, A.M.; Jesudhasan, P.R. Select phytochemicals reduce Campylobacter jejuni in postharvest poultry and modulate the virulence attributes of C. jejuni. Front. Microbiol. 2021, 12, 725087. [Google Scholar] [CrossRef]
- Upadhyay, A.; Arsi, K.; Upadhyaya, I.; Donoghue, A.M.; Donoghue, D.J. Natural and environmentally friendly strategies for controlling Campylobacter jejuni colonization in poultry, survival in poultry products and infection in humans. In Food Safety in Poultry Meat Production; Springer: Berlin/Heidelberg, Germany, 2019; pp. 67–93. [Google Scholar]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Lillehoj, E.P.; Bravo, D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr. 2013, 109, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jang, S.I.; Kim, D.K.; Ionescu, C.; Bravo, D.; Lillehoj, H.S. Effect of dietary Curcuma, Capsicum, and Lentinus on enhancing local immunity against Eimeria acervulina infection. J. Poult. Sci. 2009, 47, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Castillo, S.; Heredia, N.; Arechiga-Carvajal, E.; García, S. Citrus extracts as inhibitors of quorum sensing, biofilm formation and motility of Campylobacter jejuni. Food Biotechnol. 2014, 28, 106–122. [Google Scholar] [CrossRef]
- Kovács, J.K.; Felső, P.; Makszin, L.; Pápai, Z.; Horváth, G.; Ábrahám, H.; Palkovics, T.; Böszörményi, A.; Emődy, L.; Schneider, G. Antimicrobial and virulence-modulating effects of clove essential oil on the foodborne pathogen Campylobacter jejuni. Appl. Environ. Microbiol. 2016, 82, 6158–6166. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Rasco, B.A.; Jabal, J.M.; Aston, D.E.; Lin, M.; Konkel, M.E. Investigating antibacterial effects of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni by using Fourier transform infrared spectroscopy, Raman spectroscopy, and electron microscopy. Appl. Environ. Microbiol. 2011, 77, 5257–5269. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Samuelson, D.R.; Rasco, B.A.; Konkel, M.E. Antimicrobial effect of diallyl sulphide on Campylobacter jejuni biofilms. J. Antimicrob. Chemother. 2012, 67, 1915–1926. [Google Scholar] [CrossRef]
- Murali, N.; Kumar-Phillips, G.; Rath, N.; Marcy, J.; Slavik, M. Effect of marinating chicken meat with lemon, green tea and turmeric against foodborne bacterial pathogens. Int. J. Poult. Sci. 2012, 11, 326. [Google Scholar] [CrossRef] [Green Version]
- Wagle, B.; Arsi, K.; Upadhyay, A.; Shrestha, S.; Venkitanarayanan, K.; Donoghue, A.; Donoghue, D. β-resorcylic acid, a phytophenolic compound, reduces Campylobacter jejuni in postharvest poultry. J. Food Prot. 2017, 80, 1243–1251. [Google Scholar] [CrossRef]
- Wagle, B.; Shrestha, S.; Arsi, K.; Upadhyaya, I.; Donoghue, A.; Donoghue, D. Pectin or chitosan coating fortified with eugenol reduces Campylobacter jejunion chicken wingettes and modulates expression of critical survival genes. Poult. Sci. 2019, 98, 1461–1471. [Google Scholar] [CrossRef]
- Wagle, B.R.; Arsi, K.; Shrestha, S.; Upadhyay, A.; Upadhyaya, I.; Bhargava, K.; Donoghue, A.; Donoghue, D.J. Eugenol as an antimicrobial wash treatment reduces Campylobacter jejuni in postharvest poultry. J. Food Saf. 2019, 39, e12704. [Google Scholar] [CrossRef]
- Rivlin, R.S. Historical perspective on the use of garlic. J. Nutr. 2001, 131, 951S–954S. [Google Scholar] [CrossRef] [Green Version]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Karuppiah, P.; Rajaram, S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pac. J. Trop. Biomed. 2012, 2, 597–601. [Google Scholar] [CrossRef]
- Yang, C.; Li, L.; Yang, L.; Lv, H.; Wang, S.; Sun, G. Anti-obesity and Hypolipidemic effects of garlic oil and onion oil in rats fed a high-fat diet. Nutr. Metab. 2018, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R. Anti tubercular activity of garlic oil. Indian J. Pathol. Microbiol. 1998, 41, 131. [Google Scholar] [PubMed]
- Viswanathan, V.; Phadatare, A.; Mukne, A. Antimycobacterial and antibacterial activity of Allium sativum bulbs. Indian J. Pharm. Sci. 2014, 76, 256. [Google Scholar] [PubMed]
- O’Gara, E.A.; Hill, D.J.; Maslin, D.J. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 2000, 66, 2269–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avato, P.; Tursi, F.; Vitali, C.; Miccolis, V.; Candido, V. Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine 2000, 7, 239–243. [Google Scholar] [CrossRef]
- Casella, S.; Leonardi, M.; Melai, B.; Fratini, F.; Pistelli, L. The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytother. Res. 2013, 27, 380–383. [Google Scholar] [CrossRef]
- Ross, Z.; O’Gara, E.A.; Hill, D.J.; Sleightholme, H.; Maslin, D.J. Antimicrobial properties of garlic oil against human enteric bacteria: Evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl. Environ. Microbiol. 2001, 67, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Mnayer, D.; Fabiano-Tixier, A.-S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules 2014, 19, 20034–20053. [Google Scholar] [CrossRef] [Green Version]
- Tsao, S.-M.; Yin, M.-C. In vitro activity of garlic oil and four diallyl sulphides against antibiotic-resistant Pseudomonas aeruginosa and Klebsiella pneumoniae. J. Antimicrob. Chemother. 2001, 47, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Robyn, J.; Rasschaert, G.; Hermans, D.; Pasmans, F.; Heyndrickx, M. Is allicin able to reduce Campylobacter jejuni colonization in broilers when added to drinking water? Poult. Sci. 2013, 92, 1408–1418. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Nikolić, M.; Vasić, S.; Đurđević, J.; Stefanović, O.; Čomić, L. Antibacterial and anti-biofilm activity of ginger (Zingiber officinale (Roscoe)) ethanolic extract. Kragujevac J. Sci. 2014, 39, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998, 26, 118–122. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Rall, V.L.M.; Fernandes, A.A.H.; Ushimaru, P.I.; da Silva Probst, I.; Fernandes, A., Jr. Essential oils against foodborne pathogens and spoilage bacteria in minced meat. Foodborne Pathog. Dis. 2009, 6, 725–728. [Google Scholar] [CrossRef]
- World Health Organization. Risk Assessments of Salmonella in Eggs and Broiler Chickens: Interpretive Summary; Food and Agriculture Organization: Roma, Italy, 2002; Volume 1.
- Chandran, D.; Emran, T.B.; Nainu, F.; Sharun, K.; Kumar, M.; Mitra, S.; Chakraborty, S.; Mohapatra, R.K.; Tuli, H.S.; Dhama, K. Beneficial Effects of Dietary Allium sativum (Garlic) Supplementation on Health and Production of Poultry: A Mini-Review. Toxicol. Rep. 2022, 9, 821–824. [Google Scholar]
- Dieumou, F.; Teguia, A.; Kuiate, J.; Tamokou, J.; Fonge, N.; Dongmo, M. Effects of ginger (Zingiber officinale) and garlic (Allium sativum) essential oils on growth performance and gut microbial population of broiler chickens. Livest. Res. Rural Dev. 2009, 21, 23–32. [Google Scholar]
- Khan, R.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Javdani, M.; Qureshi, M.; Laudadio, V. Potential applications of ginger (Zingiber officinale) in poultry diets. Worlds Poult. Sci. J. 2012, 68, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Sharadamma, K.; Radhakrishna, P. Effects of a garlic active based growth promoter on growth performance and specific pathogenic intestinal microbial counts of broiler chicks. Int. J. Poult. Sci. 2010, 9, 244–246. [Google Scholar] [CrossRef] [Green Version]
- Munir, M.T. Effect of garlic on the health and performance of broilers. Veterinaria 2015, 3, 32–39. [Google Scholar]
- Sadeghi, A.; Izadi, W.; Shawrang, P.; Chamani, M.; Amin, A.M. A comparison of the effects of dietary ginger powder and Avilamycin on growth performance and intestinal Salmonella count of challenged broiler chickens. Iranian J App. Anim. Sci. 2013, 3, 769–775. [Google Scholar]
- Tavakol, M.; Khodaei, H.; Nasr, J. Ginger powder effect on the immune system in broilers exposed to Salmonella infection. Der. Pharma. Chemica. 2015, 7, 266–269. [Google Scholar]
- De Moura Oliveira, K.A.; Santos-Mendonça, R.C.; De Miranda Gomide, L.A.; Vanetti, M.C.D. Aqueous garlic extract and microbiological quality of refrigerated poultry meat. J. Food Process. Preserv. 2005, 29, 98–108. [Google Scholar] [CrossRef]
- Sudrashan, S.; Fairoze, N.; Wildfred, S.; Shekar, R. Effect of aqueous extract and essential oils of ginger and garlic as immunostimulant in chicken meat. Res. J. Poult. Sci. 2010, 3, 58–61. [Google Scholar]
- Nejad, A.S.M.; Shabani, S.; Bayat, M.; Hosseini, S.E. Antibacterial effect of garlic aqueous extract on Staphylococcus aureus in hamburger. Jundishapur J. Microbiol. 2014, 7, e13134. [Google Scholar]
- Upadhyay, A.; Arsi, K.; Wagle, B.R.; Upadhyaya, I.; Shrestha, S.; Donoghue, A.M.; Donoghue, D.J. Trans-cinnamaldehyde, carvacrol, and eugenol reduce Campylobacter jejuni colonization factors and expression of virulence genes in vitro. Front. Microbiol. 2017, 8, 713. [Google Scholar] [CrossRef] [Green Version]
- Bassler, B.L.; Wright, M.; Silverman, M.R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: Sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 1994, 13, 273–286. [Google Scholar] [CrossRef]
- Plummer, P.J. LuxS and quorum-sensing in Campylobacter. Front. Cell. Infect. 2012, 2, 22. [Google Scholar] [CrossRef]

| Gene with Accession No. | Product Size | Primer | Sequence (5′-3′) |
|---|---|---|---|
| 16S-rRNA (NC_003197.2) | 90 bp | F | 5′-TTGTACACACCGCCCGTCAC-3′ |
| R | 5′-AAAGTGGTAAGCGCCCTCCC-3′ | ||
| invA (NC_003197.2) | 94 bp | F | 5′-CCGATTTGAAGGCCGGTATTA-3′ |
| R | 5′-ACCGTCAAAGGAACCGTAAAG-3′ | ||
| invB (NC_003197.2) | 92 bp | F | 5′-AAGTATCTGTATCAGCGTCAAGG-3′ |
| R | 5′-TCATAAGCCCGCTGTTGTAATA-3′ | ||
| invC (NC_003197.2) | 103 bp | F | 5′-GTTATCCCGCCTCCGTATTC-3′ |
| R | 5′-GCTTTCCAGCAGTACCGTATAA-3′ | ||
| invE (NC_003197.2) | 82 bp | F | 5′-GTCAGGCGCGTAGCTTATT-3′ |
| R | 5′-CACGATCTCTTCCAGGTCTTTAC-3′ | ||
| luxS (NC_003197.2) | 122 bp | F | 5′-TGCTGAAAGTGCAGGATCAA-3′ |
| R | 5′-GCACATCACGCTCCAGAATA-3′ | ||
| sipB (NC_003197.2) | 121 bp | F | 5′-CGACGGGAGTGTCGTTTATT-3′ |
| R | 5′-CTTATCGACGCCTAATCCTTCC-3′ | ||
| avrA (NC_003197.2) | 135 bp | F | 5′-GTCCATGAGCTTGTTTCCTCTA-3′ |
| R | 5′-CCGATGTCTTTCCGTCCATAA-3′ | ||
| sopB (NC_003197.2) | 128 bp | F | 5′-CACTCGCTGCATAACCTCTATAA-3′ |
| R | 5′-GTCCGCTTTAACTTTGGCTAAC-3′ | ||
| rpoS (AF184104.1) | 103 bp | F | 5′-GATAACGACCTGGCTGAAGAA-3′ |
| R | 5′-ACAGTGGTGAATACCCAATCTC-3′ | ||
| hilD (NC_003197.2) | 97 bp | F | 5′-GGCGCTCTCTATGCACTTATC-3′ |
| R | 5′-GCAGGAAAGTCAGGCGTATAG-3′ |
| Treatment | Salmonella-Positive (Positive Samples/Total Samples) | |
|---|---|---|
| 0 h | 2 h | |
| Positive Control | 15/15 | 15/15 |
| Ethanol | 7/15 | 0/15 |
| 0.5% Ginger oil | 15/15 | 7/15 |
| 1.0% Ginger oil | 1/15 | 0/15 |
| 0.5% Garlic oil | 13/15 | 5/15 |
| 1.0% Garlic oil | 2/15 | 0/15 |
| 0.5% Ginger oil + 0.5% Garlic oil | 1/15 | 0/15 |
| 1.0% Ginger oil + 1.0% Garlic oil | 0/15 | 0/15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, K.; Assumpcao, A.L.F.V.; Arsi, K.; Donoghue, A.; Jesudhasan, P.R.R. Ability of Garlic and Ginger Oil to Reduce Salmonella in Post-Harvest Poultry. Animals 2022, 12, 2974. https://doi.org/10.3390/ani12212974
Robinson K, Assumpcao ALFV, Arsi K, Donoghue A, Jesudhasan PRR. Ability of Garlic and Ginger Oil to Reduce Salmonella in Post-Harvest Poultry. Animals. 2022; 12(21):2974. https://doi.org/10.3390/ani12212974
Chicago/Turabian StyleRobinson, Kelsy, Anna L. F. V. Assumpcao, Komala Arsi, Annie Donoghue, and Palmy R. R. Jesudhasan. 2022. "Ability of Garlic and Ginger Oil to Reduce Salmonella in Post-Harvest Poultry" Animals 12, no. 21: 2974. https://doi.org/10.3390/ani12212974






