Initial Characterization of Male Southern Stingray (Hypanus americanus) Reproductive Parameters and Preliminary Investigation of Sperm Cryopreservation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
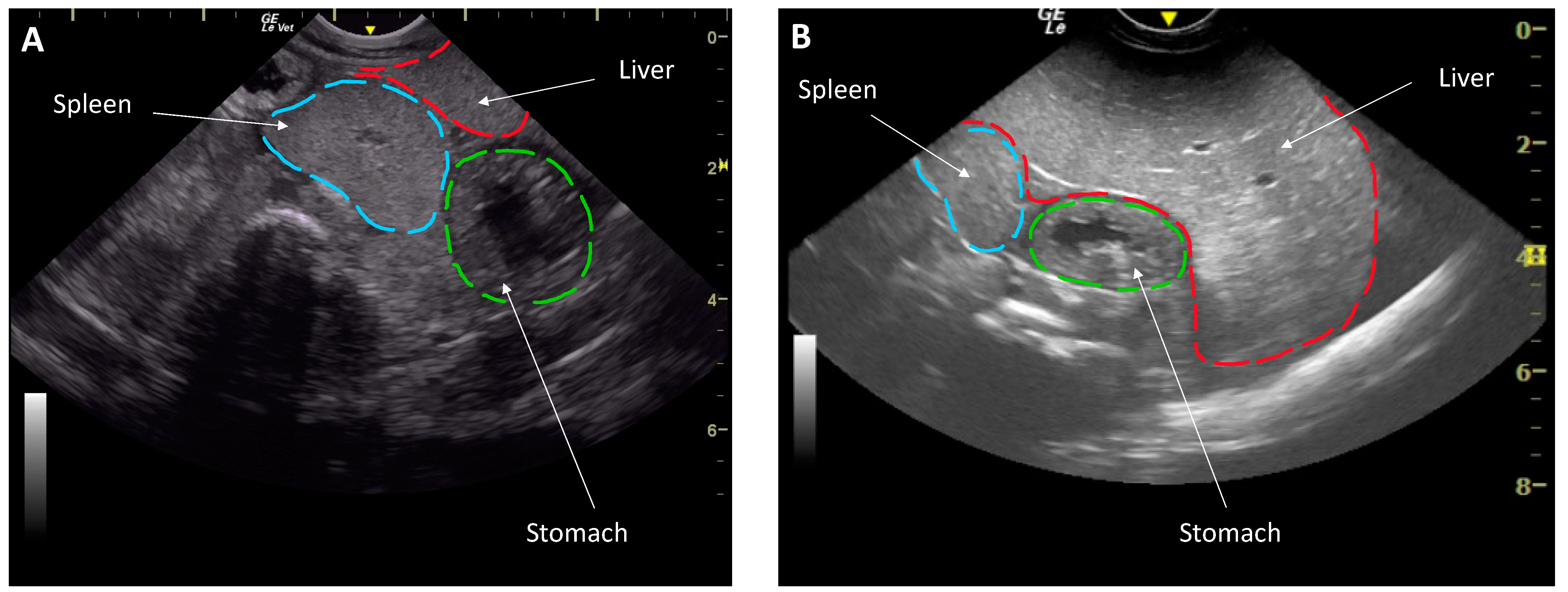
2.2. Body Condition Assessment
2.3. Semen Collection, Assessments and Cryopreservation
2.3.1. Media Preparation
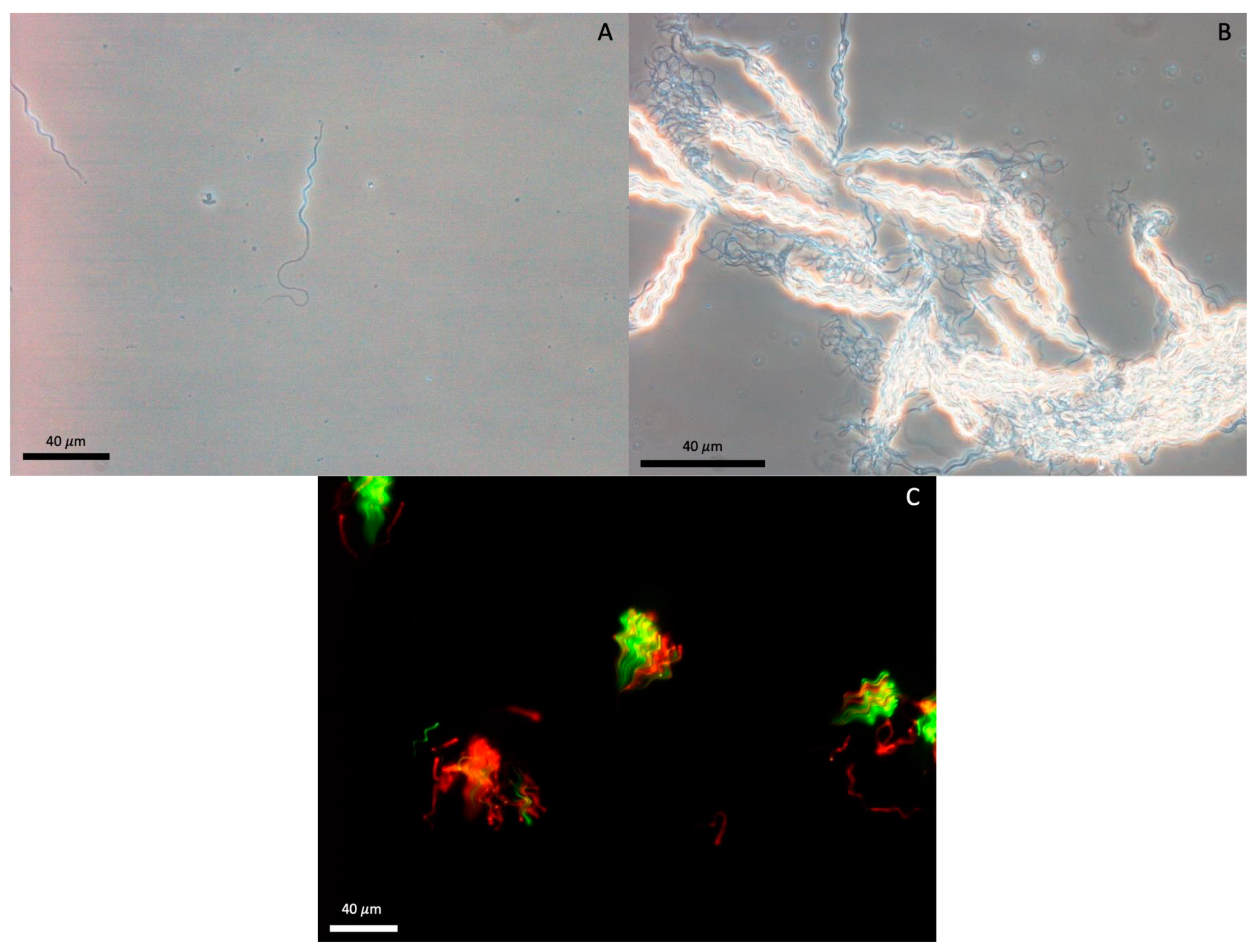
2.3.2. Semen Collection and Analysis
2.3.3. Sperm Cryopreservation
2.3.4. Post-Thaw Analysis
2.4. Total Testosterone Enzyme-Linked Immunoassay
2.5. Statistical Analysis
3. Results
3.1. Body Condition
3.2. Semen and Sperm Characteristics
3.3. Sperm Cryopreservation
3.4. Testosterone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. Elife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elisio, M.; Awruch, C.A.; Massa, A.M.; Macchi, G.J.; Somoza, G.M. Effects of temperature on the reproductive physiology of female elasmobranchs: The case of the narrownose smooth-hound shark (Mustelus schmitti). Gen. Comp. Endocrinol. 2019, 284, 113242. [Google Scholar] [CrossRef] [PubMed]
- Wyffels, J.T.; George, R.; Adams, L.; Adams, C.; Clauss, T.; Newton, A.; Hyatt, M.W.; Yach, C.; Penfold, L.M. Testosterone and semen seasonality for the sand tiger shark Carcharias taurus. Biol. Reprod. 2020, 102, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.G.; Lynch, C.; Santymire, R.M.; Marinari, P.E.; Wildt, D.E. Recovery of gene diversity using long-term cryopreserved spermatozoa and artificial insemination in the endangered black-footed ferret. Anim. Conserv. 2016, 19, 102–111. [Google Scholar] [CrossRef]
- Asturiano, J.F.; Cabrita, E.; Horváth, Á. Progress, challenges and perspectives on fish gamete cryopreservation: A mini-review. Gen. Comp. Endocrinol. 2017, 245, 69–76. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, E.; Pérez-Jiménez, J.C.; Mendoza-Carranza, M. Reproductive parameters of the southern stingray (Dasyatis americana) in the southern gulf of Mexico. Lat. Am. J. Aquat. Res. 2012, 40, 335–344. [Google Scholar] [CrossRef]
- Mylniczenko, N.D.; Sumigama, S.; Wyffels, J.T.; Wheaton, C.J.; Guttridge, T.L.; DiRocco, S.; Penfold, L.M. Ultrasounographic and hormonal characterization of reproductive health and disease in wild, semiwild, and aquarium-housed southern stingrays (Hypanus americanus). Am. J. Vet. Res. 2019, 80, 931–942. [Google Scholar] [CrossRef]
- Chapman, D.D.; Corcoran, M.J.; Harvey, G.M.; Malan, S.; Shivji, M.S. Mating behavior of southern stingrays, Dasyatis americana (Dasyatidae). Environ. Biol. Fishes 2003, 68, 241–245. [Google Scholar] [CrossRef]
- Dzyuba, V.; Ninhaus-Silveira, A.; Kahanec, M.; Veríssimo-Silveira, R.; Rodina, M.; Holt, W.V.; Dzyuba, B. Sperm motility in ocellate river stingrays: Evidence for post-testicular sperm maturation and capacitation in Chondrichthyes. J. Zool. 2019, 307, 9–16. [Google Scholar] [CrossRef]
- Morales-Gamba, R.D.; Caldas, J.S.; Godoy, L.; Marcon, J.L. Sperm characterization of the Amazonian freshwater cururu stingray Potamotrygon wallacei (Potamotryogonidae): Basic knowledge for reproduction and conservation plans. Zygote 2019, 27, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, N.; Sulikowski, J. Killing for conservation: The need for alternatives to lethal sampling of apex predatory sharks. Endanger. Species Res. 2011, 14, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Hammerschlag, N.; Skubel, R.A.; Sulikowski, J.; Irschick, D.J.; Gallagher, A.J. A Comparison of Reproductive and Energetic States in a Marine Apex Predator (the Tiger Shark, Galeocerdo cuvier ). Physiol. Biochem. Zool. 2018, 91, 933–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussey, N.; Cocks, D.; Dudley, S.; McCarthy, I.; Wintner, S. The condition conundrum: Application of multiple condition indices to the dusky shark Carcharhinus obscurus. Mar. Ecol. Prog. Ser. 2009, 380, 199–212. [Google Scholar] [CrossRef]
- Mylniczenko, N.D.; Penfold, L.M.; Guttridge, T.L.; Wyffels, J.T. Ultrasound comparison of the liver to spleen as an evalustion of liver and body condition in elasmobranchs. In Proceedings of the American Association of Zoo Veterinarians, Prague, Czech Republic, 6 October 2018. [Google Scholar]
- Penfold, L.M.; Wyffels, J.T. Reproductive Science in Sharks and Rays. In Reproductive Science in Animal Conservation; Comizzoli, P., Brown, J.L., Holt, W.V., Eds.; Springer Nature: Basingstoke, UK, 2019; Volume 1200, pp. 465–488. ISBN 9783030236335. [Google Scholar]
- Daly, J.; Jones, R. The use of reproductive technologies in breeding programs for elasmobranchs in aquaria. In The Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Murray, M., Ezcurra, J., Eds.; Special Publication of the Ohio Biological Survey: Columbus, OH, USA, 2017; pp. 363–374. ISBN 978-0-86727-166-9. [Google Scholar]
- Daly, J.; Holland, M.K.; Galloway, D.B. Preliminary Investigations on Sperm Cryopreservation of a Stingray, the Sparsely Spotted Stingaree; Tiersch, T.R., Green, C.C., Eds.; World Aquaculture Society: Baton Rouge, LA, USA, 2011. [Google Scholar]
- García-Salinas, P.; Gallego, V.; Asturiano, J.F. Development of Sperm Cryopreservation Protocols for Sharks and Rays: New Tools for Elasmobranch Conservation. Front. Mar. Sci. 2021, 8, 941. [Google Scholar] [CrossRef]
- Wyffels, J.T.; Adams, L.M.; Bulman, F.; Fustukjian, A.; Hyatt, M.W.; Feldheim, K.A.; Penfold, L.M. Artificial insemination and parthenogenesis in the whitespotted bamboo shark Chiloscyllium plagiosum. Sci. Rep. 2021, 11, 9966. [Google Scholar] [CrossRef] [PubMed]
- Penfold, L.; Harnal, V.; Lynch, W.; Bird, D.; Derrickson, S.; Wildt, D.E. Characterization of Northern pintail (Anas acuta) ejaculate and the effect of sperm preservation on fertility. Reproduction 2001, 121, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Roser, J.F.; Hughes, J.P. Seasonal Effects on Seminal Quality, Plasma Hormone Concentrations, and GnRH-Induced LH Response in Fertile and Subfertile Stallions. J. Androl. 1992, 13, 214–223. [Google Scholar] [PubMed]
- Heupel, M.R.; Whittier, J.M.; Bennett, M.B. Plasma Steroid Hormone Profiles and Reproductive Biology of the Epaulette Shark, Hemiscyllium ocellatum. J. Exp. Zool. 1999, 284, 586–594. [Google Scholar] [CrossRef]
- Howard, J.; Bush, M.; Wildt, D.E. Teratospermia in Domestic Cats Compromises Penetration of Zona-Free Hamster Ova and Cat Zonae Pellucidae. J. Androl. 1991, 12, 36–45. [Google Scholar]
- O’Brien, J.K.; Robeck, T.R. Preservation of beluga (Delphinapterus leucas) spermatozoa using a trehalose-based cryodiluent and directional freezing technology. Reprod. Fertil. Dev. 2010, 22, 653. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mossman, J.A.; Pearson, J.T.; Moore, H.D.; Pacey, A.A. Variation in mean human sperm length is linked with semen characteristics. Hum. Reprod. 2013, 28, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Robeck, T.R.; Gearhart, S.A.; Steinman, K.J.; Katsumata, E.; Loureiro, J.D.; O’Brien, J.K. In vitro sperm characterization and development of a sperm cryopreservation method using directional solidification in the killer whale (Orcinus orca). Theriogenology 2011, 76, 267–279. [Google Scholar] [CrossRef]
- Daochai, C.; Keschumras, N.; Chansue, N.; Haetrakul, T. Preliminary of intra-vagina artificial insemination using fresh semen in Ocellate river stingray (Potamotrygon motoro). Thai J. Vet. Med. 2020, 50, 383–385. [Google Scholar]
- Garner, D.L.; Johnson, L.A. Viability Assessment of Mammalian Sperm Using SYBR-14 and Propidium Iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef]
- Varela Junior, A.S.; Corcini, C.D.; Gheller, S.M.M.; Jardim, R.D.; Lucia, T.; Streit, D.P.; Figueiredo, M.R.C. Use of amides as cryoprotectants in extenders for frozen sperm of tambaqui, Colossoma macropomum. Theriogenology 2012, 78, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Curry, M. Cryopreservation of semen from domestic livestock. J. Reprod. Fertil. 2000, 5, 46–52. [Google Scholar] [CrossRef]
- Parks, J.E.; Lynch, D.V. Lipid Composition and Thermotropic Phase Behavior of Boar, Bull, Stallion, and Rooster Sperm Membranes. Cryobiology 1992, 29, 255–266. [Google Scholar] [CrossRef]
- Wolfe, J.; Bryant, G. Cellular cryobiology: Thermodynamic and mechanical effects. Int. J. Refrig. 2001, 24, 438–450. [Google Scholar] [CrossRef]
- Gao, D.Y.; Liu, J.; Liu, C.; Mcgann, L.E.; Watson, P.F.; Kleinhans, F.W.; Mazur, P.; Critser, E.S.; Critser, J.K. Andrology: Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum. Reprod. 1995, 10, 1109–1122. [Google Scholar] [CrossRef]
- Moura, T.; Serra-Pereira, B.; Gordo, L.S.; Figueiredo, I. Sperm storage in males and females of the deepwater shark Portuguese dogfish with notes on oviducal gland microscopic organization. J. Zool. 2011, 283, 210–219. [Google Scholar] [CrossRef]
- Drake, A. Observations on bull sperm rotation. Biol. Reprod. 1974, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Cortese, D.; Wan, K.Y. Control of Helical Navigation by Three-Dimensional Flagellar Beating. Phys. Rev. Lett. 2021, 126, 88003. [Google Scholar] [CrossRef]
- Abaigar, T.; Holt, W.V.; Harrison, R.A.P.; del Barrio, G. Sperm Subpopulations in Boar (Sus scrofa) and Gazelle (Gazella dama mhorr) Semen as Revealed by Pattern Analysis of Computer-Assisted Motility Assessments. Biol. Reprod. 1999, 60, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Minamikawa, S.; Morisawa, M. Acquisition, Initiation and Maintenance of Sperm Motility in the Shark, Triakis scyllia. Comp. Biochem. Physiol. 1996, 113A, 387–392. [Google Scholar] [CrossRef]
- Chatchavalvanich, K.; Thongpan, A.; Nakai, M. Structure of the testis and genital duct of freshwater stingray, Himantura signifer (Elasmobranchii: Myliobatiformes: Dasyatidae). Ichthyol. Res. 2005, 52, 123–131. [Google Scholar] [CrossRef]
- Hau, M. Regulation of male traits by testosterone: Implications for the evolution of vertebrate life histories. BioEssays 2007, 29, 133–144. [Google Scholar] [CrossRef]
- Tricas, T.C.; Maruska, K.P.; Rasmussen, L.E. Annual Cycles of Steroid Hormone Production, Gonad Development, and Reproductive Behavior in the Atlantic Stingray. Gen. Comp. Endocrinol. 2000, 118, 209–225. [Google Scholar] [CrossRef] [Green Version]
- Mull, C.G.; Lowe, C.G.; Young, K.A. Photoperiod and water temperature regulation of seasonal reproduction in male round stingrays (Urobatis halleri). Comp. Biochem. Physiol. Part A 2008, 151, 717–725. [Google Scholar] [CrossRef]
- Ortavant, R.; Mauleon, P.; Thibult, C. Photoperiodic Control of Gonadal and Hypophyseal Activity in Domestic Mammals. Ann. N. Y. Acad. Sci. 1963, 177, 157–193. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, S.G.; Gallagher, A.J.; Merly, L.; Hammerschlag, N. Variation of body condition and plasma energy substrates with life stage, sex, and season in wild-sampled nurse sharks Ginglymostoma cirratum. J. Fish Biol. 2021, 98, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Wyffels, J.T.; Penfold, L.M. Relationship between thyroid and testosterone hormones in aquarium and wild sand tiger sharks. In Proceedings of the 6th Annual Conference of the International Society of Wildlife Endocrinology, Kruger National Park, South Africa, 14 October 2019. [Google Scholar]
- Wheaton, C.J.; Mylniczenko, N.D. Challenges, pitfalls and surprises: Measuring stress, reproductive and thyroid hormones in Elasmobranchs. In Proceedings of the 18th International Congress of Comparative Endocrinology, Banff National Park, AB, Canada, 4–9 June 2017. [Google Scholar]

| Parameter | March n = 7 | June n = 7 |
|---|---|---|
| Volume (mL) | 0.29 ± 0.1 a | 2.12 ± 0.45 b |
| pH | 7.43 ±0.27 | 8.06 ± 0.04 |
| Osmolarity (mOsm) | 776 ± 66.9 | 857 ± 38.9 |
| Total motility; raw (%) | 5.71 ± 2.77 a | 51.4 ± 14.3 b |
| Status; raw (0–5) | 0.71 ± 0.36 | 1.5 ± 0.46 |
| Total motility; in ASW (%) | 32.9 ± 12.0 | 33.6 ± 15.5 |
| Status; in ASW (0–5) | 1.21 ± 0.46 | 1.57 ± 0.57 |
| Concentration (×106 sperm/mL) | 1296 ± 353 a | 371 ± 104 b |
| Total sperm count (×106) | 280 ± 88.4 | 284 ± 46.2 |
| Plasma total testosterone (ng/mL) | 8.0 ± 7.2 a | 97.3 ± 11.3 b |
| Extender | Total Motility (%) | Status (0–5) |
|---|---|---|
| ER Fraction A | 43.3 ± 17.6 | 2.17 ± 0.83 |
| ER-GLY | 36.7 ± 17.6 | 1.83 ± 0.72 |
| ER-DMSO | 23.3 ± 13.3 | 2.0 ± 0.76 |
| ER-GLY/MF | 36.7 ± 17.6 | 1.83 ± 0.73 |
| Cryoprotecting Agent | |||||||
|---|---|---|---|---|---|---|---|
| GLY | DMSO | GLY/MF | |||||
| Time (h) | Motility (%) | PMI (%) | Motility (%) | PMI (%) | Motility (%) | PMI (%) | |
| Modified slow freeze | 0 | 16.3 ± 7.47 0–35 | 26.3 ± 9.21 5–50 | 9 ± 8.67 0–35 | 23.8 ± 19.1 0–80 | 6.25 ± 3.75 0–15 | 28.8 ± 17.6 0–80 |
| 1 | 4 ± 2.27 0–10 | 8.75 ± 4.27 0–20 | 6.5 ± 6.17 0–25 | 10 ± 6.12 0–25 | 1.5 ± 1.19 0–5 | 8.75 ± 3.15 0–15 | |
| 4 | 2.5 ± 1.44 0–5 | 6.25 ± 3.15 0–15 | 2.5 ± 2.5 0–10 | 6.25 ± 3.75 0–15 | 1.5 ± 1.19 0–5 | 6.25 ± 2.39 0–10 | |
| Conventional cryopreservation | 0 | 1.5 ± 1.19 0–5 | 5 ± 3.34 0–15 | 6.75 ± 6.09 0–25 | 30 ± 18.4 0–80 | 1.5 ± 1.19 0–5 | 4 ± 3.67 0–15 |
| 1 | 0.25 ± 0.25 0–1 | 0.25 ± 0.25 0–1 | 1.25 ± 1.25 0–5 | 2.5 ± 1.44 0–5 | 1.5 ± 1.19 0–5 | 2.75 ± 2.43 0–10 | |
| 4 | 0.25 ± 0.25 0–1 | 0.25 ± 0.25 0–1 | 0 0 | 1.5 ±1.19 0–5 | 0.25 ± 0.25 0–1 | 2.75 ± 2.43 0–10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gillis, J.D.; Penfold, L.M.; Mylniczenko, N.D. Initial Characterization of Male Southern Stingray (Hypanus americanus) Reproductive Parameters and Preliminary Investigation of Sperm Cryopreservation. Animals 2021, 11, 2716. https://doi.org/10.3390/ani11092716
Gillis JD, Penfold LM, Mylniczenko ND. Initial Characterization of Male Southern Stingray (Hypanus americanus) Reproductive Parameters and Preliminary Investigation of Sperm Cryopreservation. Animals. 2021; 11(9):2716. https://doi.org/10.3390/ani11092716
Chicago/Turabian StyleGillis, James D., Linda M. Penfold, and Natalie D. Mylniczenko. 2021. "Initial Characterization of Male Southern Stingray (Hypanus americanus) Reproductive Parameters and Preliminary Investigation of Sperm Cryopreservation" Animals 11, no. 9: 2716. https://doi.org/10.3390/ani11092716






