Serum Lipid, Amino Acid and Acylcarnitine Profiles of Obese Cats Supplemented with Dietary Choline and Fed to Maintenance Energy Requirements
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Diets
2.3. Experimental Design
2.4. Blood Collection and Analyses
2.5. Dual Energy X-ray Absorptiometry
2.6. Statistical Analyses
3. Results
3.1. Energy, Food and Choline Intake
3.2. Body Weight, Body Condition Score and Body Composition
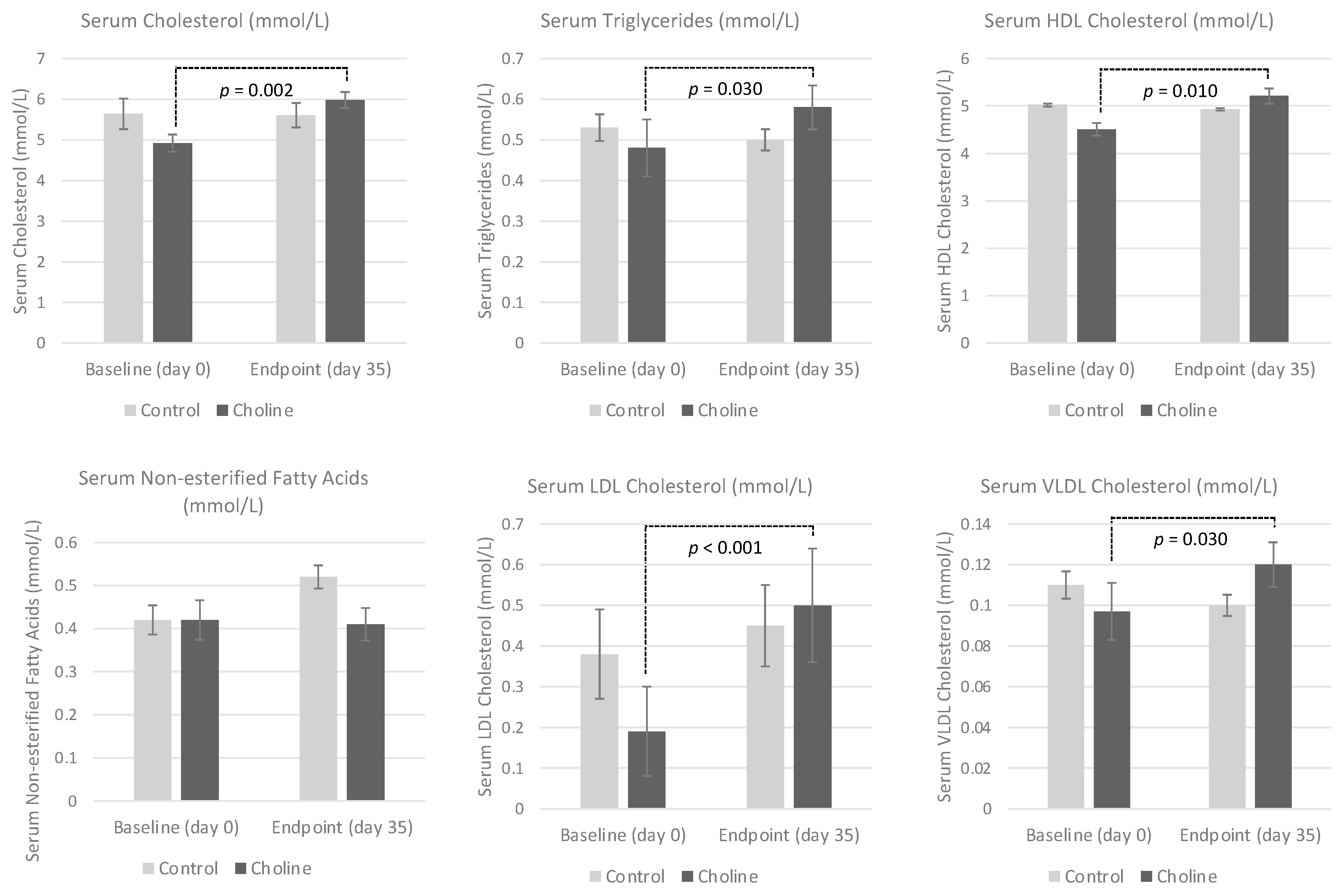
3.3. Serum Lipid Profile
3.4. Serum Liver Enzymes
3.5. Serum Creatinine and Blood Urea Nitrogen
3.6. Serum Glucose, Insulin and Leptin
3.7. Plasma Amino Acid Profile
3.8. Plasma Acylcarnitine Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scarlett, J.M.; Donoghue, S.; Saidla, J.; Wills, J. Overweight Cats: Prevalence and Risk Factors. Int. J. Obes. Relat. Metab. Disord. 1994, 18, S22–S28. [Google Scholar]
- Robertson, I.D. The Influence of Diet and Other Factors on Owner-Perceived Obesity in Privately Owned Cats from Metropolitan Perth, Western Australia. Prev. Vet. Med. 1999, 40, 75–85. [Google Scholar] [CrossRef]
- Öhlund, M.; Palmgren, M.; Holst, B.S. Overweight in Adult Cats: A Cross-Sectional Study. Acta Vet. Scand. 2018, 60, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Teng, K.T.; McGreevy, P.D.; Toribio, J.A.L.M.L.; Raubenheimer, D.; Kendall, K.; Dhand, N.K. Risk Factors for Underweight and Overweight in Cats in Metropolitan Sydney, Australia. Prev. Vet. Med. 2017, 144, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Vandendriessche, V.L.; Picavet, P.; Hesta, M. First Detailed Nutritional Survey in a Referral Companion Animal Population. J. Anim. Physiol. Anim. Nutr. 2017, 101, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Diez, M.; Picavet, P.; Ricci, R.; Dequenne, M.; Renard, M.; Bongartz, A.; Farnir, F.; Diez, M.; Picavet, P.; Renard, M.; et al. Health Screening to Identify Opportunities to Improve Preventive Medicine in Cats and Dogs. J. Small Anim. Pract. 2015, 56, 463–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cave, N.J.; Allan, F.J.; Schokkenbroek, S.L.; Metekohy, C.A.M.; Pfeiffer, D.U. A Cross-Sectional Study to Compare Changes in the Prevalence and Risk Factors for Feline Obesity between 1993 and 2007 in New Zealand. Prev. Vet. Med. 2012, 107, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Courcier, E.A.; O’Higgins, R.; Mellor, D.J.; Yam, P.S. Prevalence and Risk Factors for Feline Obesity in a First Opinion Practice in Glasgow, Scotland. J. Feline Med. Surg. 2010, 12, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Courcier, E.A.; Mellor, D.J.; Pendlebury, E.; Evans, C.; Yam, P.S. An Investigation into the Epidemiology of Feline Obesity in Great Britain: Results of a Cross-Sectional Study of 47 Companion Animal Practises. Vet. Rec. 2012, 171, 560. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.; Armstrong, P. Prevalence and Risk Factors for Obesity in Adult Cats from Private US Veterinary Practices. Int. J. Appl. Res. Vet. Med. 2005, 3, 4–6. [Google Scholar]
- Colliard, L.; Paragon, B.M.; Lemuet, B.; Bénet, J.J.; Blanchard, G. Prevalence and Risk Factors of Obesity in an Urban Population of Healthy Cats. J. Feline Med. Surg. 2009, 11, 135–140. [Google Scholar] [CrossRef]
- Teng, K.T.; McGreevy, P.D.; Toribio, J.A.L.M.L.; Raubenheimer, D.; Kendall, K.; Dhand, N.K. Associations of Body Condition Score with Health Conditions Related to Overweight and Obesity in Cats. J. Small Anim. Pract. 2018, 59, 603–615. [Google Scholar] [CrossRef]
- Christmann, U.; Bečvářová, I.; Werre, S.R.; Meyer, H.P. Effectiveness of a New Dietetic Weight Management Food to Achieve Weight Loss in Client-Owned Obese Cats. J. Feline Med. Surg. 2016, 18, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, M.; Ferguson, D.C. Effects of Neutering on Hormonal Concentrations and Energy Requirements in Male and Female Cats. Am. J. Vet. Res. 2002, 63, 634–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havel, P.J.; Ramsey, J.J.; Graham, J.L.; Kim, K.; Wei, A.; Lee, A.; Fascetti, A.J. Early Effects of Neutering on Energy Expenditure in Adult Male Cats. PLoS ONE 2014, 9, e89557. [Google Scholar] [CrossRef]
- Villaverde, C.; Ramsey, J.J.; Green, A.S.; Asami, D.K.; Yoo, S.; Fascetti, A.J. Energy Restriction Results in a Mass-Adjusted Decrease in Energy Expenditure in Cats That Is Maintained after Weight Regain. J. Nutr. 2018, 138, 856–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deagle, G.; Holden, S.L.; Biourge, V.; Morris, P.J.; German, A.J. Long-Term Follow-up after Weight Management in Obese Cats. J. Nutr. Sci. 2014, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Biourge, V.C.; Groff, J.M.; Munn, R.J.; Kirk, C.A.; Nyland, T.G.; Madeiros, V.A.; Morris, J.G.; Rogers, Q.R. Experimental Induction of Hepatic Lipidosis in Cats. Am. J. Vet. Res. 1994, 55, 1291–1302. [Google Scholar]
- National Research Council. Nutrient Requirements of Dogs and Cats; The National Academies Press: Washington, DC, USA, 2006; ISBN 978-0-309-08628-8. [Google Scholar]
- Brooks, D.; Churchill, J.; Fein, K.; Linder, D.; Michel, K.E.; Tudor, K.; Ward, E.; Witzel, A. 2014 AAHA Weight Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2013, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, P.J.; Blanchard, G. Hepatic Lipidosis in Cats. Vet. Clin. N. Am. Small 2009, 39, 599–616. [Google Scholar] [CrossRef]
- Center, S.A.; Crawford, M.A.; Guida, L.; Erb, H.N.; King, J. A Retrospective Study of 77 Cats With Severe Hepatic Lipidosis: 1975-1990. J. Vet. Intern. Med. 1993, 7, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Valtolina, C.; Favier, R.P. Feline Hepatic Lipidosis. Vet. Clin. N. Am. Small 2017, 47, 683–702. [Google Scholar] [CrossRef]
- Gagne, J.M.; Weiss, D.J.; Armstrong, P.J. Histopathologic Evaluation of Feline Inflammatory Liver Disease. Vet. Pathol. 1996, 33, 521–526. [Google Scholar] [CrossRef]
- Dimski, D.; Buffington, C.; Johnson, S.; Sherding, R.; Rosol, T. Serum Lipoprotein Concentrations and Hepatic Lesions in Obese Cats Undergoing Weight Loss. Am. J. Vet. Res. 1992, 53, 1259–1262. [Google Scholar]
- Verbrugghe, A.; Bakovic, M. Peculiarities of One-Carbon Metabolism in the Strict Carnivorous Cat and the Role in Feline Hepatic Lipidosis. Nutrients 2013, 5, 2811–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, B. Feline Hepatic Lipidosis: Pathophysiology, Clinical Signs, and Diagnosis. Compend. Contin. Educ. Pract. Vet. 2000, 22, 847–856. [Google Scholar]
- Kuzi, S.; Segev, G.; Kedar, S.; Yas, E.; Aroch, I. Prognostic Markers in Feline Hepatic Lipidosis: A Retrospective Study of 71 Cats. Vet. Rec. 2017, 181, 512. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.A.; Franklin, P.D.; Alexander, E.A.; Lamont, T.A.; Sheard, N.F.; Beiser, A. Choline, an Essential Nutrient for Humans. FASEB J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Canty, D.J.; Zeisel, S.H. Lecithin and Choline in Human Health and Disease. Nutr. Rev. 1994, 52, 327–339. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Xu, Z.R.; Feng, J. The Effect of Betaine and DL-Methionine on Growth Performance and Carcass Characteristics in Meat Ducks. Anim. Feed Sci. Tech. 2004, 116, 151–159. [Google Scholar] [CrossRef]
- Esteve-Garcia, E.; Mack, S.; de Freitas, A.R. The Effect of DL-Methionine and Betaine on Growth Performance and Carcass Characteristics in Broilers. Anim. Feed Sci. Tech. 2005, 87, 85–93. [Google Scholar] [CrossRef]
- Zhan, X.A.; Li, J.X.; Xu, Z.R.; Zhao, R.Q. Effects of Methionine and Betaine Supplementation on Growth Performance, Carcase Composition and Metabolism of Lipids in Male Broilers. Brit. Poult. Sci. 2006, 47, 576–580. [Google Scholar] [CrossRef]
- Fernández, C.; López-Saez, A.; Gallego, L.; De La Fuente, J.M. Effect of Source of Betaine on Growth Performance and Carcass Traits in Lambs. Anim. Feed Sci. Tech. 2000, 86, 71–82. [Google Scholar] [CrossRef]
- Lawrence, B.V.; Schinckel, A.P.; Adeola, O.; Cera, K.; Science, A. Impact of Betaine on Pig Finishing Performance and Carcass Composition. J. Anim. Sci. 2002, 80, 475–482. [Google Scholar] [CrossRef] [Green Version]
- McDevitt, R.M.; Mack, S.; Wallis, I.R. Can Betaine Partially Replace or Enhance the Effect of Methionine by Improving Broiler Growth and Carcase Characteristics? Brit. Poult. Sci. 2000, 41, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Xu, Z.R.; Li, W.F. Effects of Betaine on Growth Performance and Carcass Characteristics in Growing Pigs. Asian Austral. J. Anim. 2004, 17, 1700–1704. [Google Scholar] [CrossRef]
- Schenkel, L.C.; Sivanesan, S.; Zhang, J.; Wuyts, B.; Taylor, A.; Verbrugghe, A.; Bakovic, M. Choline Supplementation Restores Substrate Balance and Alleviates Complications of Pcyt2 Deficiency. J. Nutr. Biochem. 2015, 26, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Vance, D.E. The Active Synthesis of Phosphatidylcholine Is Required for Very Low Density Lipoprotein Secretion from Rat Hepatocytes. J. Biol. Chem. 1988, 263, 2998–3004. [Google Scholar] [CrossRef]
- Finkelstein, J. Methionine Metabolism in Mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef]
- Vance, D.E.; Ridgway, N.D. The Methylation of Phosphatidylethanolamine. Prog. Lipid Res. 1988, 27, 61–79. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Seim, H. Carnitine Metabolism and Its Regulation in Microorganisms and Mammals. Annu. Rev. Nutr. 2002, 18, 39–61. [Google Scholar] [CrossRef]
- Laflamme, D. Development and Validation of a Body Condition Score System for Cats: A Clinical Tool. Feline Pract. 1997, 25, 13–18. [Google Scholar]
- Horwitz, W.; Chichilo, P.; Reynolds, H. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 1970. [Google Scholar]
- American Oil Chemists’ Society; Firestone, D. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Champaign, IL, USA, 1997. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.H.; Cañas, E.Z.; Pérez, J.E. Comparison of Lipid Profile in Domestic Cat by Gender and Age. Bol. Cient. Cent. Mus. 2012, 16, 175–182. [Google Scholar]
- De Freitas, V.D.; Castilho, A.R.; da Conceição, L.A.V.; Sousa, V.R.F.; Mendonça, A.J.; da Silva, F.G.; Almeida, A.d.B.P.F. Metabolic Evaluation in Overweight and Obese Cats and Association with Blood Pressure. Cienc. Rural 2018, 48. [Google Scholar] [CrossRef] [Green Version]
- Strage, E.M.; Holst, B.S.; Nilsson, G.; Jones, B.; Lilliehöök, I. Validation of an Enzyme-Linked Immunosorbent Assay for Measurement of Feline Serum Insulin. Vet. Clin. Path. 2012, 41, 518–528. [Google Scholar] [CrossRef]
- Appleton, D.J.; Rand, J.S.; Sunvold, G.D. Basal Plasma Insulin and Homeostasis Model Assessment (HOMA) Are Indicators of Insulin Sensitivity in Cats. J. Feline Med. Surg. 2005, 7, 183–193. [Google Scholar] [CrossRef]
- Appleton, D.J.; Rand, J.S.; Sunvold, G.D. Plasma Leptin Concentrations in Cats: Reference Range, Effect of Weight Gain and Relationship with Adiposity as Measured by Dual Energy X-Ray Absorptiometry. J. Feline Med. Surg. 2000, 2, 191–199. [Google Scholar] [CrossRef]
- Rizzo, C.; Boenzi, S.; Wanders, R.J.; Duran, M.; Caruso, U.; Dionisi-Vici, C. Characteristic Acylcarnitine Profiles in Inherited Defects of Peroxisome Biogenesis: A Novel Tool for Screening Diagnosis Using Tandem Mass Spectrometry. Pediatr. Res. 2003, 53, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Vreken, P.; Van Lint, A.E.M.; Bootsma, A.H.; Overmars, H.; Wanders, R.J.A.; Van Gennip, A.H. Rapid diagnosis of organic acidemias and fatty-acid oxidation defects by quantitative electrospray tandem-MS acyl-carnitine analysis in plasma. In Current Views of Fatty Acid Oxidation and Ketogenesis; Springer: Boston, MA, USA, 2002; pp. 327–337. [Google Scholar]
- Bjørnvad, C.R.; Nielsen, M.E.; Hansen, S.E.M.; Nielsen, D.H. The Effect of Position on the Precision of Dual-Energy X-Ray Absorptiometry and Correlation with Body Condition Score in Dogs and Cats. J. Nutr. Sci. 2017, 6, e20. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H.; Blusztajn, J.K. Choline and Human Nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yan, J.; Caudill, M.A. Choline. In Handbook of Vitamins; Zempleni, J., Suttie, J.W., Gregory, J.F., Stover, P.J., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 491–513. [Google Scholar]
- Zeisel, S.H. Choline Deficiency. J. Nutr. Biochem. 1990, 1, 332–349. [Google Scholar] [CrossRef]
- Biourge, V.; Pion, P.; Lewis, J.; Morris, J.G.; Rogers, Q.R. Dietary Management of Idiopathic Feline Hepatic Lipidosis with a Liquid Diet Supplemented with Citrulline and Choline. J. Nutr. 1991, 121, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Vance, D.E.D.E. Phosphatidylcholine and Choline Homeostasis. J. Lipid. Res. 2008. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Vance, D.E. Reduction in VLDL, but Not HDL, in Plasma of Rats Deficient in Choline. Biochem. Cell Biol. 1990, 68, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, B.; Pani, P.; Schlunk, F.F. Choline-Deficiency Fatty Liver: Impaired Release of Hepatic Triglycerides. J. Lipid Res. 1968, 9, 437–446. [Google Scholar] [CrossRef]
- Hoffbauer, F.W.; Zaki, F.G. Fatty Liver due to Choline-Deficiency in the Primate. In Topical Problems in Diseases of the Liver; Karger Publishers: Basel, Switzerland, 1963; Volume 3, pp. 294–298. ISBN 978-3-8055-0841-4. [Google Scholar]
- Handler, P.; Bernheim, F. Choline Deficiency in the Hamster. Proc. Soc. Exp. Biol. Med. 1949, 72, 569–571. [Google Scholar] [CrossRef]
- Chahl, J.S.; Kratzing, C.C. Fatty Acid Composition of Tissue Lipids in Choline Deficient Rats. Q. J. Exp. Physiol. Cogn. Med. Sci. 1973, 58, 275–284. [Google Scholar] [CrossRef]
- Clark, M.H.; Larsen, R.; Lu, W.; Hoenig, M. Investigation of 1H MRS for Quantification of Hepatic Triglyceride in Lean and Obese Cats. Res. Vet. Sci. 2013, 95, 678–680. [Google Scholar] [CrossRef]
- Cole, L.K.; Vance, J.E.; Vance, D.E. Phosphatidylcholine Biosynthesis and Lipoprotein Metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 754–761. [Google Scholar] [CrossRef]
- Fielding, P.E.; Fielding, C.J. Dynamics of lipoprotein transport in the human circulatory system. In New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 2002; Volume 36, pp. 527–552. ISBN 0167-7306. [Google Scholar]
- Jordan, E.; Kley, S.; Le, N.-A.; Waldron, M.; Hoenig, M. Dyslipidemia in Obese Cats. Domest. Anim. Endocrinol. 2008, 35, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.E. Lipoprotein-Mediated Transport of Dietary and Synthesized Lipids and Lipid Abnormalities of Dogs and Cats. JAVMA J. Am. Vet. Med. Assocc. 2004, 224, 668–675. [Google Scholar] [CrossRef]
- Russell, J.C.; Proctor, S.D. Small Animal Models of Cardiovascular Disease: Tools for the Study of the Roles of Metabolic Syndrome, Dyslipidemia, and Atherosclerosis. Cardiovasc. Pathol. 2006, 15, 318–330. [Google Scholar] [CrossRef]
- Tinoco, J.; Shannon, A.; Lyman, R.L. Serum Lipids in Choline-Deficient Male and Female Rats. J. Lipid Res. 1964, 5, 57–62. [Google Scholar] [CrossRef]
- Wilgram, G.F.; Lewis, L.A.; Best, C.H. Effect of Choline and Cholesterol on Lipoprotein Patterns of Rats. Circ. Res. 1957, 5, 111–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, H.; Yu, L.; Wang, M.; Liu, S.; Sun, L.; Chen, Q. Effects of Supplementation of Rumen-Protected Choline on Growth Performance, Meat Quality and Gene Expression in Longissimus Dorsi Muscle of Lambs. Null 2015, 69, 340–350. [Google Scholar] [CrossRef]
- Lien, T.F.; Jan, D.F. The Effect on the Lipid Metabolism of Tsaiya Ducks When High Levels of Choline or Methionine Are Added to the Ducks’ Diet. Asian Austral. J. Anim. Sci. 1999, 12, 1090–1095. [Google Scholar] [CrossRef]
- Buchman, A.L.; Ament, M.E.; Sohel, M.; Dubin, M.; Jenden, D.J.; Roch, M.; Pownall, H.; Farley, W.; Awal, M.; Ahn, C. Choline Deficiency Causes Reversible Hepatic Abnormalities in Patients Receiving Parenteral Nutrition: Proof of a Human Choline Requirement: A Placebo-controlled Trial. J. Parenter. Enter. Nutr. 2001, 25, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Brink, E.J.; Katan, M.B.; Verhoef, P. Choline Supplemented as Phosphatidylcholine Decreases Fasting and Postmethionine-Loading Plasma Homocysteine Concentrations in Healthy Men. Am. J. Clin. Nutr. 2005, 82, 111–117. [Google Scholar] [CrossRef]
- Rahmani, M.G.; Kamalyan, R.G.; Dehghan-Banadaky, M.J.; Marmaryan, G.Y. The Effect of Oral Administration of Choline on Some Liver Function Characterized Blood Plasma Enzymes of Early Lactating Dairy Cows. Biol. J. Armen. 2012, 64, 83–86. [Google Scholar]
- Getty, C.M.; Dilger, R.N. Moderate Perinatal Choline Deficiency Elicits Altered Physiology and Metabolomic Profiles in the Piglet. PLoS ONE 2015, 10, e0133500. [Google Scholar] [CrossRef] [Green Version]
- Center, S.A. Feline Hepatic Lipidosis. Vet. Clin. N. Am. Small 2005, 35, 225–269. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.M.; Duncan, J.R.; Prasse, K.W. Alkaline Phosphatase, Leucine Aminopeptidase, and Alanine Aminotransferase Activities with Obstructive and Toxic Hepatic Disease in Cats. Am. J. Vet. Res. 1977, 38, 963–966. [Google Scholar]
- Holm, P.I.; Hustad, S.; Ueland, P.M.; Vollset, S.E.; Grotmol, T.; Schneede, J. Modulation of the Homocysteine-Betaine Relationship by Methylenetetrahydrofolate Reductase 677 C->T Genotypes and B-Vitamin Status in a Large-Scale Epidemiological Study. J. Clin. Endocrinol. Metab. 2007, 92, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Wu, R.D. Choline Oxidation and Choline Dehydrogenase. J. Protein Chem. 1986, 5, 193–200. [Google Scholar] [CrossRef]
- Ueland, P.M.; Holm, P.I.; Hustad, S. Betaine: A Key Modulator of One-Carbon Metabolism and Homocysteine Status. Clin. Chem. Lab. Med. 2005, 43, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M. Chapter 8—Amino Acids and Nitrogen Compounds. In Nutrient Metabolism, 2nd ed.; Kohlmeier, M., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 265–477. ISBN 978-0-12-387784-0. [Google Scholar]
- Barak, A.; Beckenhauer, H.; Uma, D. Betaine, Ethanol, and the Liver: A Review. Alcohol 1996, 13, 395–398. [Google Scholar] [CrossRef]
- Barak, A.J.; Beckenhauer, H.C.; Tuma, D.J. Use of S-Adenosylmethionine as an Index of Methionine Recycling in Rat Liver Slices. Anal. Biochem. 1982, 127, 372–375. [Google Scholar] [CrossRef]
- Poirier, L.A.; Grantham, P.H.; Rogers, A.E. The Effects of a Marginally Lipotrope-Deficient Diet on the Hepatic Levels of S-Adenosylmethionine and on the Urinary Metabolites of 2-Acetylaminofluorene in Rats. Cancer Res. 1977, 37, 744–748. [Google Scholar]
- Shivapurkar, N.; Poirier, L.A. Tissue Levels of S-Adenosylmethionine and S-Adenosylhomocysteine in Rats Fed Methyl-Deficient, Amino Acid-Defined Diets for One to Five Weeks. Carcinogenesis 1983, 4, 1051–1057. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Zola, T.; da Costa, K.-A.; Pomfret, E.A. Effect of Choline Deficiency on S-Adenosylmethionine and Methionine Concentrations in Rat Liver. Biochem. J. 1989, 259, 725–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stead, L.M.; Brosnan, J.T.; Brosnan, M.E.; Vance, D.E.; Jacobs, R.L. Is It Time to Reevaluate Methyl Balance in Humans? Am. J. Clin. Nutr. 2006, 83, 5–10. [Google Scholar] [CrossRef]
- Wei, Y.; Rector, R.; Thyfault, J.; Ibdath, J. Non-Alcoholic Fatty Liver Disease and Mitochondrial Dysfunction. World J. Gastroenterol. 2008, 14, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.S.; DeLany, J.P. Increased Levels of Plasma Acylcarnitines in Obesity and Type 2 Diabetes and Identification of a Marker of Glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [Green Version]
- Dahlhoff, C.; Worsch, S.; Sailer, M.; Hummel, B.A.; Fiamoncini, J.; Uebel, K.; Obeid, R.; Scherling, C.; Geisel, J.; Bader, B.L.; et al. Methyl-Donor Supplementation in Obese Mice Prevents the Progression of NAFLD, Activates AMPK and Decreases Acyl-Carnitine Levels. Mol. Metab. 2014, 3, 565–580. [Google Scholar] [CrossRef]
- Hoppel, C.L.; Genuth, S.M. Carnitine Metabolism in Normal-Weight and Obese Human Subjects during Fasting. Am. J. Physiol. Endocinol. Metab. 1980, 238, E409–E415. [Google Scholar] [CrossRef]
- Koves, T.R.; Li, P.; An, J.; Akimoto, T.; Slentz, D.; Ilkayeva, O.; Dohm, G.L.; Yan, Z.; Newgard, C.B.; Muoio, D.M. Peroxisome Proliferator-Activated Receptor-γ Co-Activator 1α-Mediated Metabolic Remodeling of Skeletal Myocytes Mimics Exercise Training and Reverses Lipid-Induced Mitochondrial Inefficiency. J. Biol. Chem. 2005, 280, 33588–33598. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature That Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Sivanesan, S.; Taylor, A.; Zhang, J.; Bakovic, M. Betaine and Choline Improve Lipid Homeostasis in Obesity by Participation in Mitochondrial Oxidative Demethylation. Front. Nutr. 2018, 5, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma Metabolomic Profile in Nonalcoholic Fatty Liver Disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampey, B.P.; Freemerman, A.J.; Zhang, J.; Kuan, P.-F.; Galanko, J.A.; O’Connell, T.M.; Ilkayeva, O.R.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; et al. Metabolomic Profiling Reveals Mitochondrial-Derived Lipid Biomarkers That Drive Obesity-Associated Inflammation. PLoS ONE 2012, 7, e38812. [Google Scholar] [CrossRef]
- Buchman, A.L.; Dubin, M.D.; Moukarzel, A.A.; Jenden, D.J.; Roch, M.; Rice, K.M.; Gornbein, J.; Ament, M.E. Choline Deficiency: A Cause of Hepatic Steatosis during Parenteral Nutrition That Can Be Reversed with Intravenous Choline Supplementation. Hepatology 1995, 22, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.D.; Hamp, T.J.; Reid, R.W.; Fischer, L.M.; Zeisel, S.H.; Fodor, A.A. Association between Composition of the Human Gastrointestinal Microbiome and Development of Fatty Liver with Choline Deficiency. Gastroenterology 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, V.; Singh, R.K.; Bakovic, M. The Impact of Choline Availability on Muscle Lipid Metabolism. Food Funct. 2011, 2, 53–62. [Google Scholar] [CrossRef]
- Schaeffer, M.C.; Rogers, Q.R.; Morris, J.G. The Choline Requirement of the Growing Kitten in the Presence of Just Adequate Dietary Methionine. Nutr. Res. 1982, 2, 289–299. [Google Scholar] [CrossRef]
- Grant, C.E.; Chan, J.; Shoveller, A.K.; Bakovic, M.; Blois, S.; Fascetti, A.J.; Yu, J.Z.; Verbrugghe, A. Theoretical Intake of Amino Acids and Vitamins in Obese Cats Undergoing Energy Restriction Using Veterinary Therapeutic Diets for Weight Loss Compared to over the Counter Diets. BMC Vet. Res. 2021. submitted. [Google Scholar]
- Grant, C.E.; Shoveller, A.K.; Blois, S.; Bakovic, M.; Monteith, G.; Verbrugghe, A. Dietary Intake of Amino Acids and Vitamins Compared to NRC Requirements in Obese Cats Undergoing Energy Restriction for Weight Loss. BMC Vet. Res. 2020, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.A.; Villaverde, C.; Fascetti, A.J.; Larsen, J.A. Evaluation of the Nutritional Adequacy of Recipes for Home-Prepared Maintenance Diets for Cats. JAVMA J. Am. Vet. Med. Assocc. 2019, 254, 1172–1179. [Google Scholar] [CrossRef]
- Blanchard, G.; Paragon, B.M.; Serougne, C.; Lutton, C.; Ferezou, J.; Milliat, F.; Lutton, C. Plasma Lipids, Lipoprotein Composition and Profile during Induction and Treatment of Hepatic Lipidosis in Cats and the Metabolic Effect of One Daily Meal in Healthy Cats. J. Anim. Physiol. Anim. Nutr. 2004, 88, 73–87. [Google Scholar] [CrossRef] [PubMed]

| Control Diet | High Choline Diet | ||
|---|---|---|---|
| Moisture | % as fed | 5.60 | 5.05 |
| Protein | % DM | 35.53 | 36.70 |
| Fat | % DM | 19.81 | 16.75 |
| Ash | % DM | 6.43 | 6.47 |
| Crude Fibre | % DM | 2.75 | 3.58 |
| NFE 1 | % DM | 35.48 | 36.50 |
| ME 2 | kcal/100g | 414.31 | 400.43 |
| Chloride | % DM | 0.77 | 1.33 |
| Sodium | % DM | 0.55 | 0.59 |
| Choline | mg/100 g | 458.69 | 1895.73 |
| Cobalamine (B12) | μg/100g | 8.47 | 10.53 |
| Folate (B9) | mg/100g | 0.32 | 0.42 |
| Pyridoxine (B6) | mg/100g | 1.02 | 1.37 |
| Methionine | % DM | 0.94 | 0.94 |
| Cysteine | % DM | 0.48 | 0.47 |
| Control Diet | High Choline Diet | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 5 Weeks | Baseline | 5 Weeks | Time | Diet | T × D | |
| BCS | 8.7 ± 0.2 | 8.5 ± 0.2 | 9.0 ± 0.2 | 8.8 ± 0.2 | 0.343 | 0.387 | 0.453 |
| BW/kg | 7.1 ± 0.5 | 7.1 ± 0.5 | 7.6 ± 0.4 | 7.6 ± 0.3 | 0.915 | 0.491 | 0.409 |
| DEXA SCAN | |||||||
| Area/cm2 | 578.3 ± 28.5 | 574.5 ± 23.1 | 600.8 ± 20.7 | 586.5 ± 19.2 | 0.075 | 0.607 | 0.272 |
| BMC/g | 167.7 ± 12.4 | 167.6 ± 11.2 | 167.4 ± 6.6 | 164.6 ± 6.4 | 0.180 | 0.901 | 0.191 |
| BMD g/cm2 | 167.7 ± 12.4 | 167.6 ± 11.2 | 167.4 ± 6.6 | 164.6 ± 6.4 | 0.180 | 0.901 | 0.191 |
| FM/g | 2784.8 ± 295.5 | 2919.5 ± 283.5 | 3034.7 ± 292.6 | 2995.0 ± 357.4 | 0.497 | 0.714 | 0.225 |
| LBM/g | 4427.2 ± 327.7 | 4300.8 ± 340.5 | 4710.1 ± 146.0 | 4730.2 ± 209.1 | 0.371 | 0.365 | 0.226 |
| TM/g | 7211.9 ± 500.6 | 7220.3 ± 497.0 | 7744.8 ± 345.1 | 7725.2 ± 347.8 | 0.847 | 0.635 | 0.412 |
| BF/% | 38.4 ± 2.7 | 40.3 ± 2.6 | 38.7 ± 2.5 | 38.2 ± 3.4 | 0.394 | 0.827 | 0.161 |
| Control Diet | High Choline Diet | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 5 Weeks | Baseline | 5 Weeks | Time | Diet | T × D | |
| CHOL | 5.64 ± 0.384 | 5.61 ± 0.296 | 4.92 ± 0.210 a | 5.98 ± 0.200 b | 0.017 | 0.634 | 0.013 |
| TAG | 0.533 ± 0.033 | 0.500 ± 0.026 | 0.483 ± 0.070 a | 0.583 ± 0.054 b | 0.260 | 0.799 | 0.038 |
| HDL-C | 5.02 ± 0.304 | 4.93 ± 0.246 | 4.51 ± 0.132 a | 5.21 ± 0.164 b | 0.077 | 0.679 | 0.029 |
| NEFA | 0.417 ± 0.034 | 0.515 ± 0.027 | 0.417 ± 0.046 | 0.4133 ± 0.038 | 0.110 | 0.279 | 0.090 |
| LDL-C | 0.378 ± 0.106 | 0.453 ± 0.101 | 0.194 ± 0.107 a | 0.503 ± 0.144 b | 0.001 | 0.683 | 0.014 |
| VLDL-C | 0.242 ± 0.015 | 0.227 ± 0.012 | 0.220 ± 0.032 a | 0.265 ± 0.025 b | 0.260 | 0.799 | 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbrugghe, A.; Rankovic, A.; Armstrong, S.; Santarossa, A.; Kirby, G.M.; Bakovic, M. Serum Lipid, Amino Acid and Acylcarnitine Profiles of Obese Cats Supplemented with Dietary Choline and Fed to Maintenance Energy Requirements. Animals 2021, 11, 2196. https://doi.org/10.3390/ani11082196
Verbrugghe A, Rankovic A, Armstrong S, Santarossa A, Kirby GM, Bakovic M. Serum Lipid, Amino Acid and Acylcarnitine Profiles of Obese Cats Supplemented with Dietary Choline and Fed to Maintenance Energy Requirements. Animals. 2021; 11(8):2196. https://doi.org/10.3390/ani11082196
Chicago/Turabian StyleVerbrugghe, Adronie, Alexandra Rankovic, Shafeeq Armstrong, Amanda Santarossa, Gordon M. Kirby, and Marica Bakovic. 2021. "Serum Lipid, Amino Acid and Acylcarnitine Profiles of Obese Cats Supplemented with Dietary Choline and Fed to Maintenance Energy Requirements" Animals 11, no. 8: 2196. https://doi.org/10.3390/ani11082196






