Using RNA-Seq to Identify Reference Genes of the Transition from Brown to White Adipose Tissue in Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. RNA Extraction and cDNA Synthesis
2.4. Statistical Analysis of the Reference Genes from RNA-Seq Data
2.5. Use Bio-Rad CFX Real-Time Quantitative Instruments for qPCR
2.6. Analysis of the Expression Stability for Candidate Reference Genes
2.7. Validation of Selected Reference Genes
3. Results
3.1. Reference Gene Selection during the Transformation from Goat BAT to WAT Using RNA-Seq Data
3.2. RNA Purity, Primer Verification and Amplification Efficiency
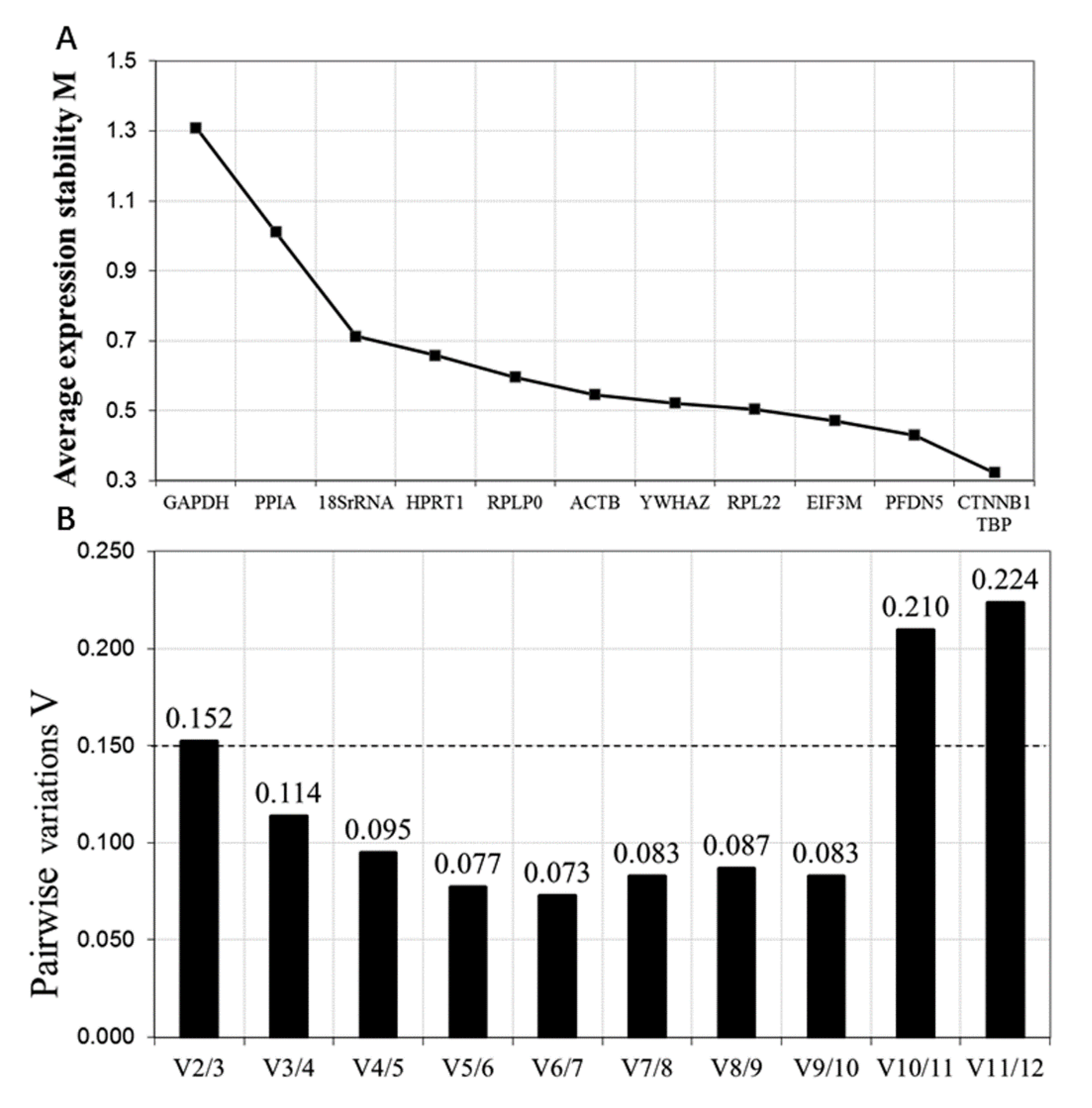
3.3. Expression Stability of the Reference Genes by geNorm Analysis
3.4. Expression Stability of the Reference Genes by NormFinder Analysis
3.5. Expression Stability of the Reference Genes by BestKeeper Analysis
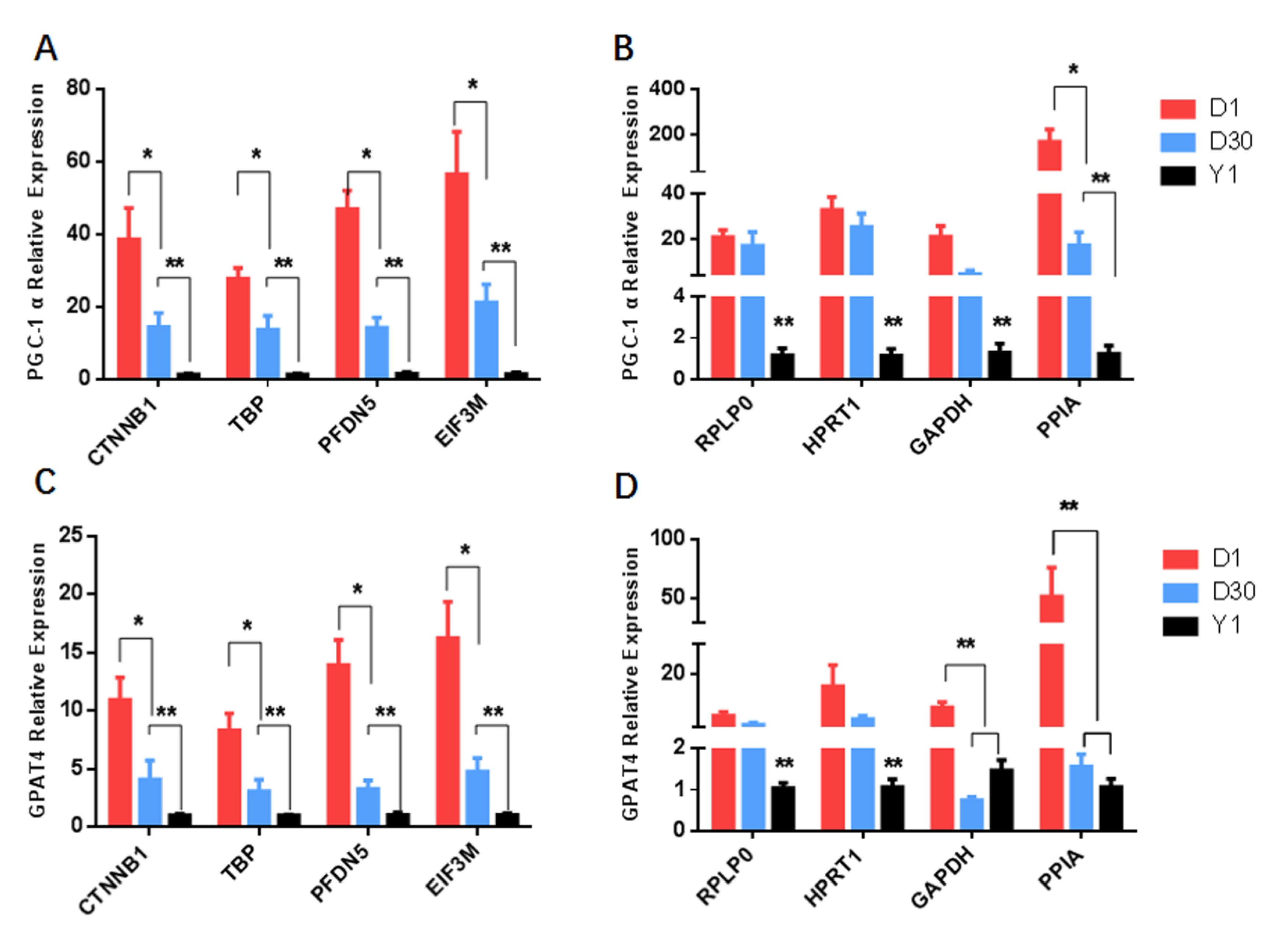
3.6. Correcting the Stability of Candidate Reference Genes with Target Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Helga, S.; Emoke, B.; Laura, R.; Fabrizio, C. The Adipose Tissue in Farm Animals: A Proteomic Approach. Curr. Protein Pept. Sci. 2014, 15, 146–155. [Google Scholar]
- Peirce, V.; Carobbio, S.; Vidal-Puig, A. The Different Shades of Fat. Nature 2014, 510, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; A Virtanen, K.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symonds, M.; Pope, M.; Budge, H. The Ontogeny of Brown Adipose Tissue. Annu. Rev. Nutr. 2015, 35, 295–320. [Google Scholar] [CrossRef] [PubMed]
- Louveau, I.; Perruchot, M.-H.; Bonnet, M.; Gondret, F. Invited Review: Pre- and Postnatal Adipose Tissue Development in Farm Animals: From Stem Cells to Adipocyte Physiology. Anim. Int. J. Anim. Biosci. 2016, 10, 1839–1847. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine that Drives Brown-Fat-Like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Fenzl, A.; Kiefer, F.W. Brown Adipose Tissue and Thermogenesis. Horm. Mol. Boil. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The Effects of Acute and Chronic Exercise on PGC-1α, Irisin and Browning of Subcutaneous Adipose Tissue in Humans. FEBS J. 2013, 281, 739–749. [Google Scholar] [CrossRef]
- Niu, G.; Yang, Y.; Zhang, Y.; Hua, C.; Wang, Z.; Tang, Z.-L.; Li, K. Identifying Suitable Reference Genes for Gene Expression Analysis in Developing Skeletal Muscle in Pigs. PeerJ 2016, 4, e2428. [Google Scholar] [CrossRef]
- Zhuang, H.; Fu, Y.; He, W.; Wang, L.; Wei, Y.-H. Selection of Appropriate Reference Genes for Quantitative Real-Time PCR in Oxytropis Ochrocephala Bunge Using Transcriptome Datasets under Abiotic Stress Treatments. Front. Plant Sci. 2015, 6, 475. [Google Scholar] [CrossRef] [Green Version]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do Successful Gene Expression Analysis Using Real-Time PCR. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, H.J.M.; Fehrmann, R.S.N.; De Bont, E.S.J.M.; Hofstra, R.M.; Gerbens, F.; Kamps, W.A.; De Vries, E.G.; Van Der Zee, A.G.J.; Meerman, G.J.T.; Ter Elst, A. Evidence Based Selection of Housekeeping Genes. PLoS ONE 2007, 2, e898. [Google Scholar] [CrossRef] [Green Version]
- Fink, T.; Lund, P.; Pilgaard, L.; Rasmussen, J.G.; Duroux, M.; Zachar, V. Instability of Standard PCR Reference Genes in Adipose-Derived Stem Cells during Propagation, Differentiation and Hypoxic Exposure. BMC Mol. Boil. 2008, 9, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basse, A.L.; Dixen, K.; Yadav, R.; Tygesen, M.P.; Qvortrup, K.; Kristiansen, K.; Quistorff, B.; Gupta, R.; Wang, J.; Hansen, J.B. Global Gene Expression Profiling of Brown to White Adipose Tissue Transformation in Sheep Reveals Novel Transcriptional Components Linked to Adipose Remodeling. BMC Genom. 2015, 16, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yang, X.; Zhu, Y.; Zhan, S.; Chao, Z.; Zhong, T.; Guo, J.; Wang, Y.; Li, L.; Zhang, H. Genome-Wide Identification and Characterization of Long Noncoding RNAs of Brown to White Adipose Tissue Transformation in Goats. Cells 2019, 8, 904. [Google Scholar] [CrossRef] [Green Version]
- Fuller-Jackson, J.-P.; Henry, B.A. Adipose and Skeletal Muscle Thermogenesis: Studies from Large Animals. J. Endocrinol. 2018, 237, R99–R115. [Google Scholar] [CrossRef] [Green Version]
- Symonds, M.; Pope, M.; Budge, H. Adipose Tissue Development during Early Life: Novel Insights into Energy Balance from Small and Large Mammals. Proc. Nutr. Soc. 2012, 71, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Nedergaard, J.; Cannon, B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Deng, B.; Zhang, R.; Qin, X.; Zhang, J.; Zhao, J. Dietary Sea Buckthorn Pomace Induces Beige Adipocyte Formation in Inguinal White Adipose Tissue in Lambs. Animals 2019, 9, 193. [Google Scholar] [CrossRef] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Boil. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of Stable Housekeeping Genes, Differentially Regulated Target Genes and Sample Integrity: BestKeeper–Excel-based Tool Using Pair-Wise Correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.X.; Hao, D.; Wang, J.; Peng, W.W.; Yan, Y.; Cao, H.X.; Sun, F.; Chen, H. Cold Exposure Induces the Acquisition of Brown Adipocyte Gene Expression Profiles in Cattle Inguinal Fat Normalized with a New Set of Reference Genes for qrt-pcr. Res. Vet. Sci. 2017, 114, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.J.; Rios, L.; Trivedi, P.; D’Souza, K.; Cowie, A.; Nzirorera, C.; Webster, D.; Brunt, K.; Legare, J.-F.; Hassan, A.; et al. Validation of Optimal Reference Genes for Quantitative Real Time PCR in Muscle and Adipose Tissue for Obesity and Diabetes Research. Sci. Rep. 2017, 7, 3612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taube, M.; Andersson-Assarsson, J.C.; Lindberg, K.; Pereira, M.J.; Gäbel, M.; Svensson, M.K.; Eriksson, J.W.; Svensson, P.-A. Evaluation of Reference Genes for Gene Expression Studies in Human Brown Adipose Tissue. Adipocyte 2015, 4, 280–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafpanah, M.J.; Sadeghi, M.; Bakhtiarizadeh, M.R. Reference Genes Selection for Quantitative Real-Time PCR Using RankAggreg Method in Different Tissues of Capra hircus. PLoS ONE 2013, 8, e83041. [Google Scholar] [CrossRef]
- Nazari, F.; Parham, A.; Maleki, A.F. GAPDH, β-actin and β2-microglobulin, as Three Common Reference Genes, are not Reliable for Gene Expression Studies in Equine Adipose- and Marrow-Derived Mesenchymal Stem Cells. J. Anim. Sci. Technol. 2015, 57, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, X.-D.; Liu, X.; Li, Y.-S.; Ding, J.-P.; Zhang, Y.; Zhang, X.-R. Reference Gene Screening for Analyzing Gene Expression Across Goat Tissue. Asian-Australas. J. Anim. Sci. 2013, 26, 1665–1671. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Seale, P. Control of Brown and Beige Fat Development. Nat. Rev. Mol. Cell Boil. 2016, 17, 691–702. [Google Scholar] [CrossRef]
- Almeida-Oliveira, F.; Leandro, J.G.B.; Ausina, P.; Sola-Penna, M.; Majerowicz, D. Reference Genes for Quantitative PCR in the Adipose Tissue of Mice with Metabolic Disease. Biomed. Pharmacother. 2017, 88, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, T.; Gregoire, F.; Delforge, V.; Delporte, C.; Perret, J. Murine 3T3-L1 Adipocyte Cell Differentiation Model: Validated Reference Genes for qPCR Gene Expression Analysis. PLoS ONE 2012, 7, e37517. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Bernard, L.; Bes, S.; Leroux, C. Selection of Reference Genes for Quantitative Real-Time PCR Normalisation in Adipose Tissue, Muscle, Liver and Mammary Gland from Ruminants. Animal 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Lin, Y.; Liao, H.; Wang, Y. Selection of Reference Genes for Gene Expression Studies Related to Intramuscular Fat Deposition in Capra hircus Skeletal Muscle. PLoS ONE 2015, 10, e0121280. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.E.; Grevengoed, T.J.; Klett, E.L.; Coleman, R.A. Glycerol-3-Phosphate Acyltransferase Isoform-4 (GPAT4) Limits Oxidation of Exogenous Fatty Acids in Brown Adipocytes. J. Boil. Chem. 2015, 290, 15112–15120. [Google Scholar] [CrossRef] [Green Version]
| Gene Symbol | GenBank No. | Sequence 5′–3′ | Tm(°C) | Size (bp) | Eff (%) |
|---|---|---|---|---|---|
| PPIA | XM_018047035.1 | AAGTCCCGAAGACAGCAGAA | 60 | 209 | 90.8 |
| GATGCCAGGACCTGTATGCT | |||||
| GAPDH | XM_005680968.3 | GCAAGTTCCACGGCACAG | 61.3 | 249 | 95.7 |
| GGTTCACGCCCATCACAA | |||||
| 18S rRNA | DQ149973 | TAATCCCGCCGAACCCCATT | 61.3 | 125 | 93.3 |
| GGTGTGTACAAAGGGCAGG | |||||
| YWHAZ | XM_018058314.1 | ACTACTATCGCTACTTGGCTGAG | 61.3 | 84 | 99.1 |
| CTTCTTGTTATGCTTGCTGTGA | |||||
| ACTB | XM_018039831.1 | CCTGCGGCATTCACGAAACTAC | 61.3 | 87 | 97.4 |
| ACAGCACCGTGTTGGCGTAGAG | |||||
| TBP | XM_018053502.1 | TCGCCAAGAATAGTGTGCTG | 61.3 | 202 | 95.7 |
| CCGTAAGGCATCATTGGACT | |||||
| HPRT1 | XM_012167243.2 | CGAGATGTGATGAAGGAGATGG | 60 | 186 | 96.3 |
| GCCTGTTGACTGGTCGTTAC | |||||
| EIF3M | XM_018059285.1 | CTGTGCGAGAAACTGGTCAA | 60 | 164 | 95.7 |
| ATATACTGGATGGCCCCACA | |||||
| PFDN5 | XM_005679909.1 | GCTTATTGACGTGGGAACT | 60 | 120 | 98.1 |
| TGCAGAGCTGGCTGGATT | |||||
| CTNNB1 | XM_018066894.1 | CACAGTTCGATGCTGCTCAT | 61.3 | 161 | 99.3 |
| CTGGTCTTCGTCATTCAGCA | |||||
| RPLP0 | XM_005709526 | TTCTCCTTCGGGCTGGTCA | 60 | 104 | 94.5 |
| TCCAGGAAGCGGGAATGC | |||||
| RPL22 | XM_005690753.3 | CGGTGTTGTAACAATCGA | 60 | 209 | 91.9 |
| CCTCATCTTCCTCCTCTTC | |||||
| GPAT4 | XM_018041983.1 | GGAGTCTCCTTTGGTATCCG | 61.4 | 128 | 96.8 |
| CCATTGGTGTAGGGCTTGTA | |||||
| PGC-1α | NM_001285631.1 | TAAAGCCAACCAAGATAACCC | 61.4 | 242 | 92.2 |
| CACCAAACAGCCGAAGACT |
| Gene Name | Stability Value | Rank Order |
|---|---|---|
| PFDN5 | 0.13 | 1 |
| EIF3M | 0.14 | 2 |
| CTNNB1 | 0.14 | 3 |
| TBP | 0.14 | 4 |
| ACTB | 0.14 | 5 |
| YWHAZ | 0.16 | 6 |
| 18SrRNA | 0.18 | 7 |
| RPL22 | 0.19 | 8 |
| RPLP0 | 0.21 | 9 |
| HPRT1 | 0.21 | 10 |
| GAPDH | 0.23 | 11 |
| PPIA | 0.23 | 12 |
| Gene Name | Coeff. of Corr. [r] | Std Dev [±CT] | CV [% CT] | Rank Order |
|---|---|---|---|---|
| TBP | 0.90 | 0.52 | 2.22 | 1 |
| CTNNB1 | 0.83 | 0.51 | 2.52 | 2 |
| 18SrRNA | 0.81 | 0.97 | 3.69 | 3 |
| PFDN5 | 0.78 | 0.46 | 2.65 | 4 |
| EIF3M | 0.78 | 0.50 | 2.51 | 5 |
| ACTB | 0.74 | 0.47 | 2.60 | 6 |
| YWHAZ | 0.66 | 0.41 | 2.05 | 7 |
| RPLP0 | 0.61 | 0.58 | 3.51 | 8 |
| HPRT1 | 0.51 | 0.54 | 2.39 | 9 |
| RPL22 | −0.01 | 0.33 | 1.18 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Chen, X.; Song, T.; Zhang, X.; Zhan, S.; Cao, J.; Zhong, T.; Guo, J.; Li, L.; Zhang, H.; et al. Using RNA-Seq to Identify Reference Genes of the Transition from Brown to White Adipose Tissue in Goats. Animals 2020, 10, 1626. https://doi.org/10.3390/ani10091626
Wang L, Chen X, Song T, Zhang X, Zhan S, Cao J, Zhong T, Guo J, Li L, Zhang H, et al. Using RNA-Seq to Identify Reference Genes of the Transition from Brown to White Adipose Tissue in Goats. Animals. 2020; 10(9):1626. https://doi.org/10.3390/ani10091626
Chicago/Turabian StyleWang, Linjie, Xingyue Chen, Tianzeng Song, Xujia Zhang, Siyuan Zhan, Jiaxue Cao, Tao Zhong, Jiazhong Guo, Li Li, Hongping Zhang, and et al. 2020. "Using RNA-Seq to Identify Reference Genes of the Transition from Brown to White Adipose Tissue in Goats" Animals 10, no. 9: 1626. https://doi.org/10.3390/ani10091626





