Stress Associated with Simulated Transport, Changes Serum Biochemistry, Postmortem Muscle Metabolism, and Meat Quality of Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Bird Management and Experimental Design
2.3. Sample Collection
2.4. Parameter Determination
2.5. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
2.6. Statistical Analysis
3. Results
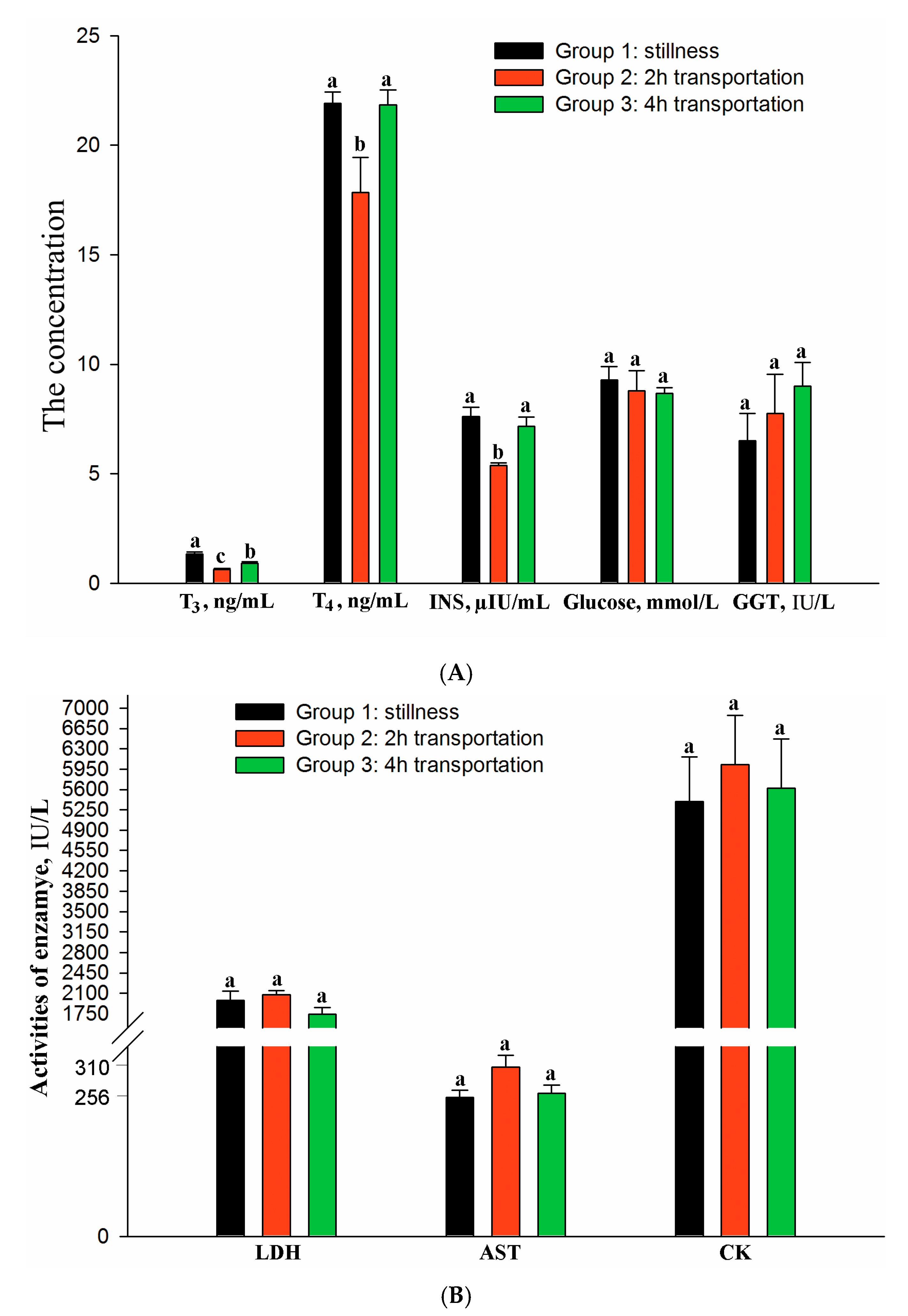
3.1. Blood Hormones and Metabolites
3.2. Meat Quality Characteristics
3.3. Activities of Key Enzymes from Breast and Thigh Muscle
3.4. Muscle Metabolites
3.5. HSP70 mRNA Transcription Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Becerril-Herrera, M.; Alonso-Spilsbury, M.; Ortega, M.E.; Guerrero-Legarreta, I.; Ramirez-Necoechea, R.; Roldan-Santiago, P.; Perez-Sato, M.; Soni-Guillermo, E.; Mota-Rojas, D. Changes in blood constituents of swine transported for 8 or 16 h to an Abattoir. Meat Sci. 2010, 86, 945–948. [Google Scholar] [CrossRef]
- Schtte, A. Transport tauglichkert von Schweinen. In Hygiene and Tierschutz Beim Tiertranspor; Hartung, J., Ed.; Proc. Deutsche Veterinrmedizinische Gesellschaft: Hannover, Germany, 1994; pp. 83–89. [Google Scholar]
- Yue, H.Y.; Zhang, L.; Wu, S.G.; Xu, L.; Zhang, H.J.; Qi, G.H. Effects of transport stress on blood metabolism, glycolytic potential, and meat quality in meat-type yellow-feathered chickens. Poult. Sci. 2010, 89, 413–419. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, H.Y.; Wu, S.G.; Xu, L.; Zhang, H.J.; Yan, H.J.; Cao, Y.L.; Gong, Y.S.; Qi, G.H. Transport stress in broilers. II. Superoxide production, adenosine phosphate concentrations, and mRNA levels of avian uncoupling protein, avian adenine nucleotide translocator, and avian peroxisome proliferator-activated receptor-gamma coactivator-1alpha in skeletal muscles. Poult. Sci. 2010, 89, 393–400. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, H.Y.; Zhang, H.J.; Xu, L.; Wu, S.G.; Yan, H.J.; Gong, Y.S.; Qi, G.H. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult. Sci 2009, 88, 2033–2041. [Google Scholar] [CrossRef]
- Al-Aqil, A.; Zulkifli, I. Changes in heat shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poult. Sci. 2009, 88, 1358–1364. [Google Scholar] [CrossRef]
- Jacobs, L.; Delezie, E.; Duchateau, L.; Goethals, K.; Ampe, B.; Buyse, J.; Tuyttens, F.A.M. Impact of transportation duration on stress responses in day-old chicks from young and old breeders. Res. Vet. Sci. 2017, 112, 172–176. [Google Scholar] [CrossRef]
- Satterlee, D.G.; Aguilera-Quintana, I.; Munn, B.J.; Krautmann, B.A. Vitamin C amelioration of the adrenal stress response in broiler chickens being prepared for slaughter. Comp. Biochem. Physiol. A Comp. Physiol. 1989, 94, 569–574. [Google Scholar]
- Todd, S.E.; Mellor, D.J.; Stafford, K.J.; Gregory, N.G.; Bruce, R.A.; Ward, R.N. Effects of food withdrawal and transport on 5- to 10-day-old calves. Res. Vet. Sci. 2000, 68, 125–134. [Google Scholar] [CrossRef]
- Buckham Sporer, K.R.; Burton, J.L.; Earley, B.; Crowe, M.A. Transportation stress in young bulls alters expression of neutrophil genes important for the regulation of apoptosis, tissue remodeling, margination, and anti-bacterial function. Vet. Immunol. Immunopathol. 2007, 118, 19–29. [Google Scholar] [CrossRef]
- Sherlock, L.; Wathes, C.M.; Cheng, Z.; Wathes, D.C. Differential hepatic gene expression in the broiler chicken in response to the combined stressors of food withdrawal, catching and transport at the end of production. Stress 2012, 15, 293–305. [Google Scholar] [CrossRef]
- Sun, F.; Zuo, Y.Z.; Ge, J.; Xia, J.; Li, X.N.; Lin, J.; Zhang, C.; Xu, H.L.; Li, J.L. Transport stress induces heart damage in newly hatched chicks via blocking the cytoprotective heat shock response and augmenting nitric oxide production. Poult. Sci. 2018, 97, 2638–2646. [Google Scholar] [CrossRef]
- Pan, L.; Ma, X.K.; Zhao, P.F.; Shang, Q.H.; Long, S.F.; Wu, Y.; Piao, X.S. Forsythia suspensa extract attenuates breast muscle oxidative injury induced by transport stress in broilers. Poult. Sci. 2018, 97, 1554–1563. [Google Scholar] [CrossRef]
- Mengert, U.; Fehlhaber, K. Effect of premortal stress on the endogenous microbial contamination of broiler carcasses. Berl. Munch. Tierarztl. Wochenschr. 1996, 109, 28–31. [Google Scholar]
- Hartung, J. Effects of transport on health of farm animals. Vet. Res. Commun. 2003, 27 (Suppl. S1), 525–527. [Google Scholar] [CrossRef]
- Brito Garcia, D.; JoséOliveira Silva, I.; Antônio Delfino Barbosa Filho, J.; Márcio Correa Vieira, F.; Tadeu dos Santos Dias, C. Evaluation of the effect of vibration in simulated condition of transport of broiler chickens. In Proceedings of the Livestock Environment VIII, Iguassu Falls, Brazil, 31 August–4 September 2008; p. 93. [Google Scholar]
- Compendio, J.D.Z.; Espina, D.M.; Ybañez, A.P. Simulated transportation stress: Its effect on the productivity and selected biochemical and haematological indices of laying hens. Philipp. J. Vet. Anim. Sci. 2016, 42, 91–101. [Google Scholar]
- Langer, R.O.d.S.; Simões, G.S.; Soares, A.L.; Oba, A.; Rossa, A.; Shimokomaki, M.; Ida, E.I. Broiler transportation conditions in a Brazilian commercial line and the occurrence of breast PSE (Pale, Soft, Exudative) meat and DFD-like (Dark, Firm, Dry) meat. Braz. Arch. Biol. Technol. 2010, 53, 1161–1167. [Google Scholar]
- Ghareeb, K.; Bohm, J. Stress indicators to pre-slaughter transportation of broiler chickens fed diets supplemented with a synbiotic. Int. J. Poult. Ence 2009, 8. [Google Scholar] [CrossRef]
- Tamzil, M.H.; Indarsih, B.; Jaya, I.N.S. Rest before slaughtering alleviates transportation stress and improves meat quality in broiler chickens. Int. J. Poult. Ence 2019, 18, 585–590. [Google Scholar]
- Zheng, A.; Luo, J.; Meng, K.; Li, J.; Zhang, S.; Li, K.; Liu, G.; Cai, H.; Bryden, W.L.; Yao, B. Proteome changes underpin improved meat quality and yield of chickens (Gallus gallus) fed the probiotic Enterococcus faecium. BMC Genom. 2014, 15, 1167. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Devore, J.; Peck, R. Statistics: The Exploration and Analysis of Data, 2nd ed.; Duxbury Press: Belmont, CA, USA, 1993. [Google Scholar]
- Carlisle, A.J.; Mitchell, M.A.; Hunter, R.R.; Duggan, J.A.; Randall, J.M. Physiological responses of broiler chickens to the vibrations experienced during road transportation. Br. Poult. Sci. 1998, 39, S48–S49. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Heymann, R.S.; Moatamed, F.; Schultz, J.J.; Sobel, D.; Brent, G.A. A mutant thyroid hormone receptor alpha antagonizes peroxisome proliferator-activated receptor alpha signaling in vivo and impairs fatty acid oxidation. Endocrinology 2007, 148, 1206–1217. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Bao, E.D.; Zhao, R.Q.; Lv, Q.X. Effect of transportation stress on heat shock protein 70 concentration and mRNA expression in heart and kidney tissues and serum enzyme activities and hormone concentrations of pigs. Am. J. Vet. Res. 2007, 68, 1145–1150. [Google Scholar] [CrossRef]
- Aguilera, G. Chapter 8—The hypothalamic–pituitary–adrenal axis and neuroendocrine responses to stress. In Handbook of Neuroendocrinology; Fink, G., Pfaff, D.W., Levine, J.E., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 175–196. [Google Scholar]
- Zhang, C.; Geng, Z.Y.; Chen, K.K.; Zhao, X.H.; Wang, C. L-theanine attenuates transport stress-induced impairment of meat quality of broilers through improving muscle antioxidant status. Poult. Sci. 2019, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.L.; Wang, X.F.; Zhu, X.D.; Gao, F.; Zhou, G.H. Attenuating effects of guanidinoacetic acid on preslaughter transport-induced muscle energy expenditure and rapid glycolysis of broilers. Poult. Sci. 2019, 1–10. [Google Scholar] [CrossRef]
- Xing, T.; Xu, X.L.; Zhou, G.H.; Wang, P.; Jiang, N.N. The effect of transportation of broilers during summer on the expression of heat shock protein 70, postmortem metabolism and meat quality. J. Anim. Sci. 2015, 93, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Woelfel, R.L.; Owens, C.M.; Hirschler, E.M.; Martinez-Dawson, R.; Sams, A.R. The characterization and incidence of pale, soft, and exudative broiler meat in a commercial processing plant. Poult. Sci. 2002, 81, 579–584. [Google Scholar] [CrossRef]
- Fanatico, A.C.; Cavitt, L.C.; Pillai, P.B.; Emmert, J.L.; Owens, C.M. Evaluation of slower-growing broiler genotypes grown with and without outdoor access: Meat quality. Poult. Sci. 2005, 84, 1785–1790. [Google Scholar]
- Perez, M.P.; Palacio, J.; Santolaria, M.P.; Acena, M.C.; Chacon, G.; Gascon, M.; Calvo, J.H.; Zaragoza, P.; Beltran, J.A.; Garcia-Belenguer, S. Effect of transport time on welfare and meat quality in pigs. Meat Sci. 2002, 61, 425–433. [Google Scholar]
- Ferreira, G.B.; Andrade, C.L.; Costa, F.; Freitas, M.Q.; Silva, T.J.; Santos, I.F. Effects of transport time and rest period on the quality of electrically stimulated male cattle carcasses. Meat Sci. 2006, 74, 459–466. [Google Scholar] [CrossRef]
- Ozyurt, B.; Iraz, M.; Koca, K.; Ozyurt, H.; Sahin, S. Protective effects of caffeic acid phenethyl ester on skeletal muscle ischemia-reperfusion injury in rats. Mol. Cell. Biochem. 2006, 292, 197–203. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. NMCD 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Zhao, X.H.; Chen, X.Y.; Yang, L.; Geng, Z.Y. Dietary resveratrol supplementation prevents transport-stress-impaired meat quality of broilers through maintaining muscle energy metabolism and antioxidant status. Poult. Sci. 2017, 96, 2219–2225. [Google Scholar] [CrossRef]
- Mayes, P.A. Gluconeogenesis and control of the blood glucose. In Harper’s Biochemistry, 24th ed.; Murray, R.K., Granner, D.K., Mayes, P.A., Rodwell, V.W., Eds.; Appleton and Lange: Stamford, CT, USA, 1996; pp. 153–162. [Google Scholar]
- Vosmerova, P.; Chloupek, J.; Bedanova, I.; Chloupek, P.; Kruzikova, K.; Blahova, J.; Vecerek, V. Changes in selected biochemical indices related to transport of broilers to slaughterhouse under different ambient temperatures. Poult. Sci. 2010, 89, 2719–2725. [Google Scholar] [CrossRef]
- Dadgar, S.; Crowe, T.G.; Classen, H.L.; Watts, J.M.; Shand, P.J. Broiler chicken thigh and breast muscle responses to cold stress during simulated transport before slaughter. Poult. Sci. 2012, 91, 1454–1464. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.L.; Gao, T.; Lin, M.; Wang, X.F.; Zhu, X.D.; Gao, F.; Zhou, G.H. Effects of dietary supplementation with creatine monohydrate during the finishing period on growth performance, carcass traits, meat quality and muscle glycolytic potential of broilers subjected to transport stress. Anim. Int. J. Anim. Biosci. 2014, 8, 1955–1962. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.B.; Pei, L.Y.; Bao, E.; Wang, Z.L.; Zhao, R.Q. Relationship between distribution, transcription level of HSPs mRNA and immunity tissue pathological lesion of transport stressed pigs. Sci. Agric. Sin. 2008, 41, 1832–1837. [Google Scholar]
- Xing, T.; Wang, M.F.; Han, M.Y.; Zhu, X.S.; Xu, X.L.; Zhou, G.H. Expression of heat shock protein 70 in transport-stressed broiler pectoralis major muscle and its relationship with meat quality. Anim. Int. J. Anim. Biosci. 2017, 11, 1599–1607. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhu, X.D.; Li, Y.J.; Liu, Y.; Li, J.L.; Gao, F.; Zhou, G.H.; Zhang, L. Effect of dietary creatine monohydrate supplementation on muscle lipid peroxidation and antioxidant capacity of transported broilers in summer. Poult. Sci. 2015, 94, 2797–2804. [Google Scholar] [CrossRef]
- Aditya, M.; Pragati, P.; Singh, H.S.; Girraj, G.; Baghel, R.P.S.; Pankaj, P.K. Effect of ascorbic acid supplementation on mRNA expression of HSP70 gene in broilers exposed to heat stress. Indian J. Poult. Sci. 2015, 50, 282–287. [Google Scholar]

| Ingredients | 0–3 Week | 4–6 Week |
|---|---|---|
| Maize | 62.20 | 59.20 |
| Soybean meal | 29.20 | 33.26 |
| Soybean oil | 1.40 | 2.71 |
| Fishmeal | 3.00 | 1.00 |
| Met | 0.10 | 0.03 |
| Lys | - | 0.01 |
| Dicalcium phosphate | 1.50 | 1.33 |
| Limestone | 1.30 | 1.17 |
| Sodium chloride | 0.30 | 0.30 |
| Vitamin–mineral premix 1 | 1.00 | 1.00 |
| Nutrient levels 2 | ||
| ME(MJ/kg) | 12.21 | 12.33 |
| CP, % | 21.00 | 20.00 |
| Ca, % | 1.00 | 0.90 |
| Available p, % | 0.45 | 0.43 |
| Lys, % | 1.12 | 1.00 |
| Met + Cys, % | 0.78 | 0.71 |
| Gene | Primer Sequence (5′–3′) | PCR Product (bp) | Melting Temperature (°C) |
|---|---|---|---|
| HSP70 | F: GCTTATGGTGCCGCTGTG R: TGGTGGTGTTACGCTTGATG | 151 | 55.7 |
| 18SrRNA | F: GACACGGACAGGATTGACAG R: CCAGAGTCTCGTTCGTTATCG | 120 | 55.6 |
| Item | Treatment | p-Value | Pooled SE | ||
|---|---|---|---|---|---|
| Stillness | 2 h Transportation | 4 h Transportation | |||
| Breast muscle | |||||
| Drip loss24, % | 0.54 a | 1.20 b | 1.47 c | 0.000 | 0.12 |
| pH 45 min | 6.31 a | 6.25 a | 6.03 b | 0.039 | 0.05 |
| pH 24 h | 5.99 a | 5.87 a | 5.31 b | 0.045 | 0.06 |
| Thigh muscle | |||||
| Drip loss24, % | 0.56 a | 1.11 b | 1.24 b | 0.000 | 0.09 |
| pH 45 min | 6.21 a | 6.19 a | 6.02 b | 0.003 | 0.08 |
| pH 24 h | 6.10 a | 6.16 a | 5.89 b | 0.002 | 0.05 |
| Treatment | NOS (U/mg Prot) | |
|---|---|---|
| in the Breast Muscle | in the Thigh Muscle | |
| Stillness | 0.67 | 0.48 a |
| 2 h transportation | 0.61 | 0.64 b |
| 4 h transportation | 0.70 | 0.38 a |
| p-value | 0.549 | 0.000 |
| Pooled SE | 0.03 | 0.03 |
| Item | Treatment | p-Value | Pooled SE | ||
|---|---|---|---|---|---|
| Stillness | 2 h Transportation | 4 h Transportation | |||
| Breast muscle | |||||
| LA 1 (mmol/mg prot) | 113.61 a | 121.09 b | 122.27 b | 0.008 | 2.71 |
| MDA 2 (nmol/mg prot) | 5.73 a | 6.22 b | 7.76 c | 0.000 | 0.25 |
| Glycogen (mg/g) | 5.97 b | 5.43 c | 4.16 a | 0.000 | 0.63 |
| Thigh muscle | |||||
| LA (mmol/mg prot) | 59.79 a | 64.20 b | 77.87 c | 0.000 | 2.29 |
| MDA (nmol/mg prot) | 3.41 a | 4.29 b | 4.96 c | 0.026 | 0.26 |
| Glycogen (mg/g) | 7.23 c | 5.53 b | 3.19 a | 0.000 | 0.38 |
| Treatment | The Relative Expression Level of HSP70 1 |
|---|---|
| Stillness | 1.00 |
| 2 h transportation | 0.96 |
| 4 h transportation | 0.97 |
| p-value | 0.533 |
| Pooled SE | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, A.; Lin, S.; Pirzado, S.A.; Chen, Z.; Chang, W.; Cai, H.; Liu, G. Stress Associated with Simulated Transport, Changes Serum Biochemistry, Postmortem Muscle Metabolism, and Meat Quality of Broilers. Animals 2020, 10, 1442. https://doi.org/10.3390/ani10081442
Zheng A, Lin S, Pirzado SA, Chen Z, Chang W, Cai H, Liu G. Stress Associated with Simulated Transport, Changes Serum Biochemistry, Postmortem Muscle Metabolism, and Meat Quality of Broilers. Animals. 2020; 10(8):1442. https://doi.org/10.3390/ani10081442
Chicago/Turabian StyleZheng, Aijuan, Shumei Lin, Shoaib Ahmed Pirzado, Zhimin Chen, Wenhuan Chang, Huiyi Cai, and Guohua Liu. 2020. "Stress Associated with Simulated Transport, Changes Serum Biochemistry, Postmortem Muscle Metabolism, and Meat Quality of Broilers" Animals 10, no. 8: 1442. https://doi.org/10.3390/ani10081442






