The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium
Abstract
:Simple Summary
Abstract
1. Background
2. Materials and Methods
2.1. Animals and Tissue Collection
2.2. In Vitro Endometrial Explant Culture
2.3. Viability of Endometrial Explants
2.4. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.5. Western Blot Analysis
2.6. Zymography
2.7. Statistical Analysis
3. Results
3.1. Validation of the Viability of Long-Term Endometrial Explant Culture
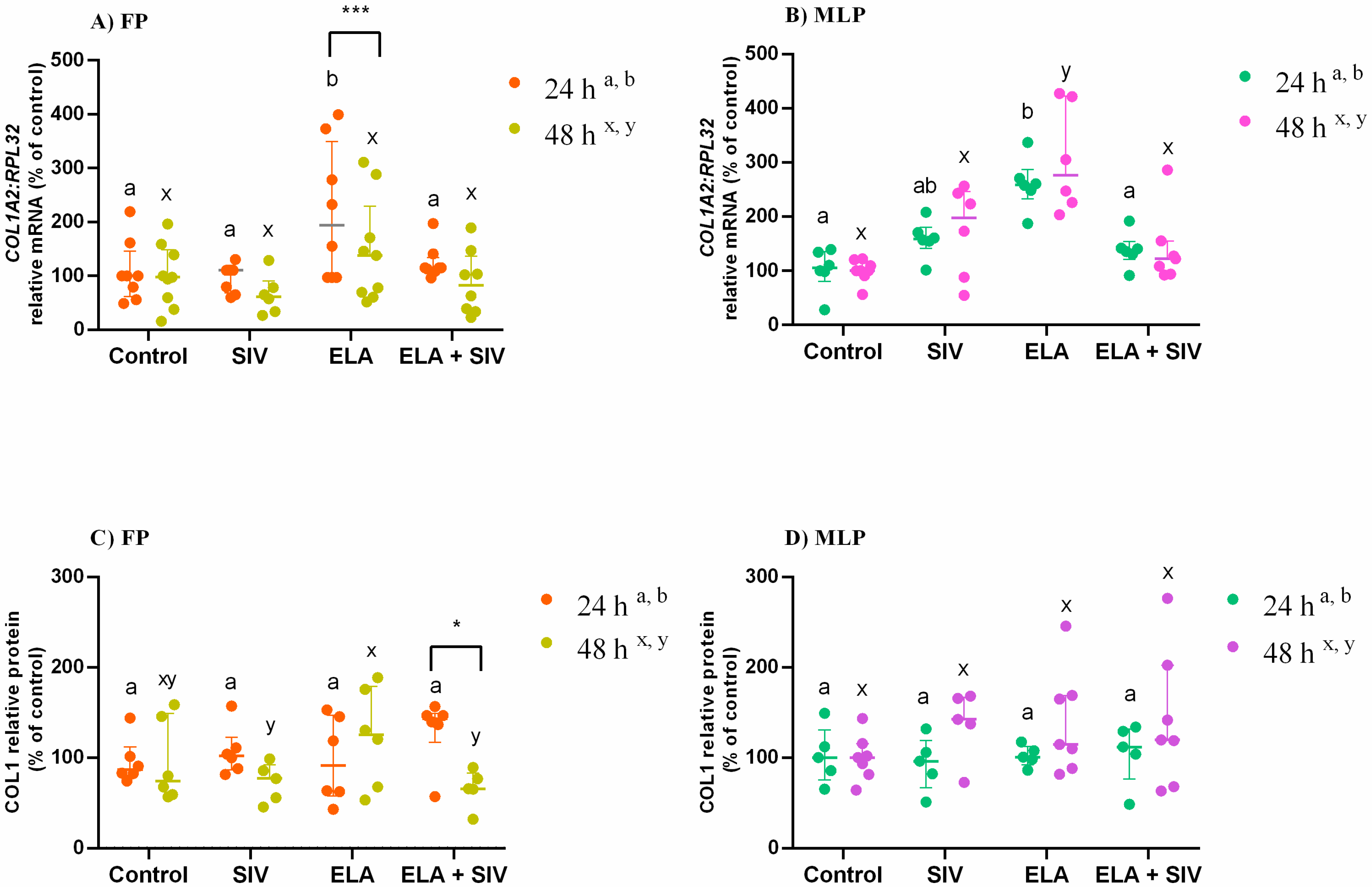
3.2. Inhibitory Effect of Sivelestat on ELA-Induced COL1
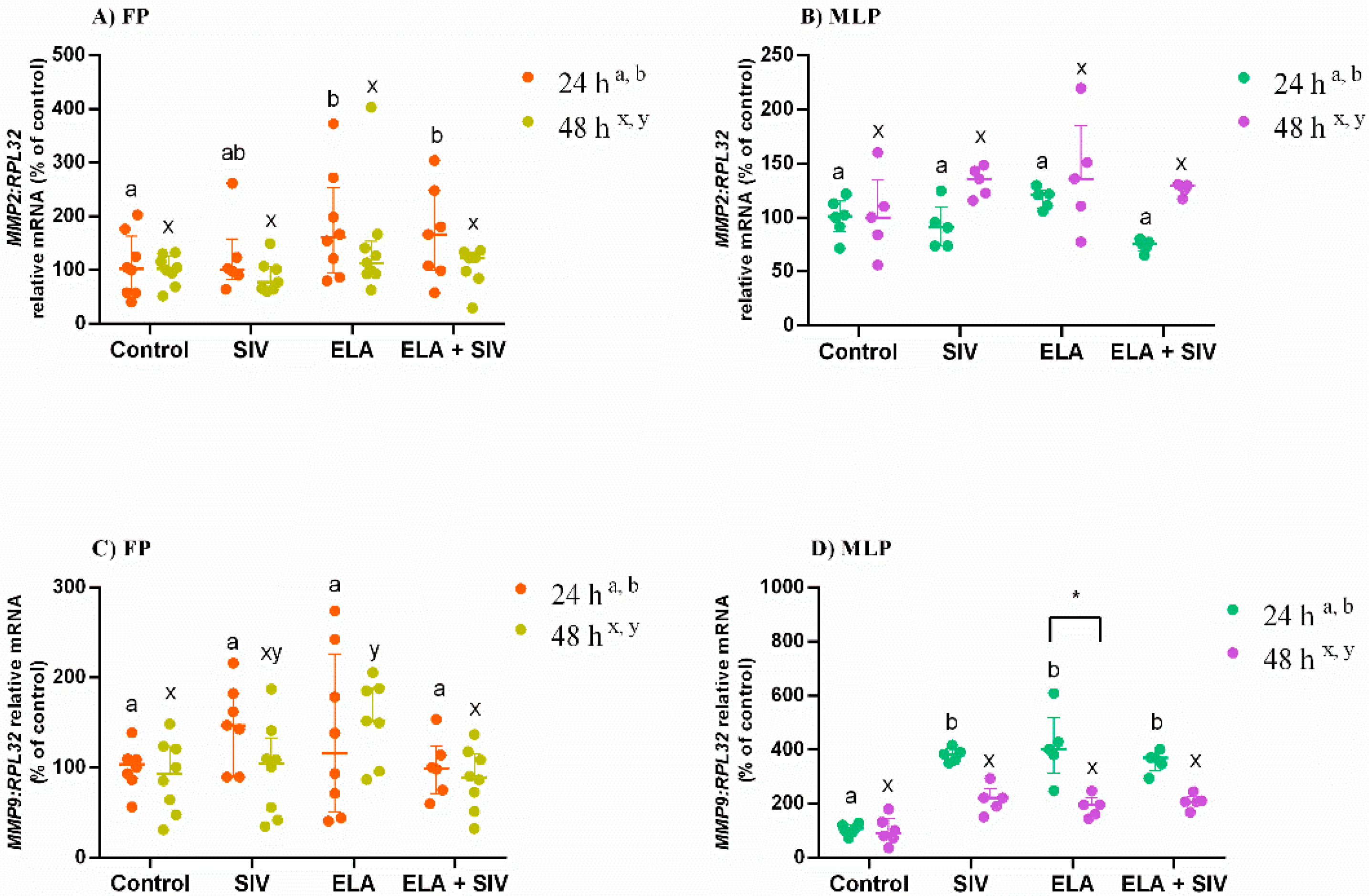
3.3. The Effect of ELA and SIV on MMP Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kotilainen, T.; Huhtinen, M.; Katila, T. Sperm-induced leukocytosis in the equine uterus. Theriogenology 1994, 41, 629–636. [Google Scholar] [CrossRef]
- Katila, T. Onset and Duration of Uterine Inflammatory Response of Mares after Insemination with Fresh Semen. Boil. Reprod. 1995, 52, 515–517. [Google Scholar] [CrossRef]
- Troedsson, M.H. Function of uterine and blood-derived polymorphonuclear neutrophils in mares susceptible and resistant to chronic uterine infection: Phagocytosis and chemotaxis. Boil. Reprod. 1993, 49, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troedsson, M.H. Breeding-Induced Endometritis in Mares. Veter Clin. N. Am. Equine Pract. 2006, 22, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Rebordão, M.; Carneiro, C.; Alexandre-Pires, G.; Brito, P.; Pereira, C.; Nunes, T.; Galvão, A.; Leitao, A.; Vilela, C.; Dias, G.M.L.F. Neutrophil extracellular traps formation by bacteria causing endometritis in the mare. J. Reprod. Immunol. 2014, 106, 41–49. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Foster, D.N. Seminal DNase Frees Spermatozoa Entangled in Neutrophil Extracellular Traps. Boil. Reprod. 2005, 73, 1174–1181. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Lovaas, B.J.; Bird, S.L.; Lamb, G.C.; Rendahl, A.K.; Taube, P.C.; Foster, U.N. Species-specific interaction of seminal plasma on sperm–neutrophil binding. Anim. Reprod. Sci. 2009, 114, 331–344. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Amaral, A.; Łukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Constituents of neutrophil extracellular traps induce in vitro collagen formation in mare endometrium. Theriogenology 2018, 113, 8–18. [Google Scholar] [CrossRef]
- Amaral, A.; Fernandes, C.; Łukasik, K.; Szóstek-Mioduchowska, A.; Baclawska, A.; Rebordão, M.R.; Aguiar-Silva, J.; Pinto-Bravo, P.; Skarzynski, D.J.; Ferreira-Dias, G. Elastase inhibition affects collagen transcription and prostaglandin secretion in mare endometrium during the estrous cycle. Reprod. Domest. Anim. 2018, 53, 66–69. [Google Scholar] [CrossRef] [PubMed]
- A Wynn, T. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandooren, J.; Steen, P.E.V.D.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Boil. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model. Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semo, A.P.; Cabrera, S.; Maldonado, M.; Selman, M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.P.; Montezano, A.C.; Alves-Lopes, R.; Rios, F.J.; Touyz, R.M. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Boil. 2015, 44, 147–156. [Google Scholar] [CrossRef]
- Wang, B.-L.; Tu, Y.; Fu, J.-F.; Zhong, Y.-X.; Fu, G.; Tian, X.-X.; Wang, L.-H.; Gong, L.; Ren, Q. Unbalanced MMP/TIMP-1 expression during the development of experimental pulmonary fibrosis with acute paraquat poisoning. Mol. Med. Rep. 2011, 4, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Szóstek-Mioduchowska, A.; Baclawska, A.; Okuda, K.; Skarzynski, D.J. Effect of proinflammatory cytokines on endometrial collagen and metallopeptidase expression during the course of equine endometrosis. Cytokine 2019, 123, 154767. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.; Słowińska, M.; Pacewicz, J.; Skarzynski, D.J.; Okuda, K. Matrix metallopeptidase expression and modulation by transforming growth factor-β1 in equine endometrosis. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, A.S.; Kühbandner, I.; Gehrig, S.; Rickert-Zacharias, V.; Twigg, M.; Wege, S.; Taggart, C.C.; Herth, F.; Schultz, C.; Mall, M.A. Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis. Eur. Respir. J. 2018, 51, 1701910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, A.D.; Kliment, C.R.; Metz, H.E.; Kim, K.-H.; Kargl, J.; Agostini, B.A.; Crum, L.T.; Oczypok, E.A.; Oury, T.A.; Houghton, A.M. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J. Leukoc. Boil. 2015, 98, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Takemasa, A.; Ishii, Y.; Fukuda, T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur. Respir. J. 2012, 40, 1475–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aikawa, N.; Ishizaka, A.; Hirasawa, H.; Shimazaki, S.; Yamamoto, Y.; Sugimoto, H.; Shinozaki, M.; Taenaka, N.; Endo, S.; Ikeda, T.; et al. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm. Pharmacol. Ther. 2011, 24, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Kido, T.; Muramatsu, K.; Yatera, K.; Asakawa, T.; Otsubo, H.; Kubo, T.; Fujino, Y.; Matsuda, S.; Mayumi, T.; Mukae, H. Efficacy of early sivelestat administration on acute lung injury and acute respiratory distress syndrome. Respirology 2016, 22, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.P.R.; Serrão, P.M.; Monteiro, S.; Pessa, P.; Silva, J.R.; Dias, G.M.L.F. Caspase-3-mediated apoptosis and cell proliferation in the equine endometrium during the oestrous cycle. Reprod. Fertil. Dev. 2007, 19, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Rebordão, M.R.; Amaral, A.; Łukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Impairment of the antifibrotic prostaglandin E2 pathway may influence neutrophil extracellular traps–induced fibrosis in the mare endometrium. Domest. Anim. Endocrinol. 2019, 67, 1–10. [Google Scholar] [CrossRef]
- Kenney, R.M.; Doig, P.A. Equine endometrial biopsy. Current Therapy in Theriogenology 2: Diagnosis, Treatment, and Prevention of Reproductive Diseases in Small and Large Animals, 2nd ed.; Morrow, D.A., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1986; pp. 723–729. [Google Scholar]
- Szóstek-Mioduchowska, A.; Łukasik, K.; Skarzynski, D.J.; Okuda, K. Effect of transforming growth factor -β1 on α-smooth muscle actin and collagen expression in equine endometrial fibroblasts. Theriogenology 2019, 124, 9–17. [Google Scholar] [CrossRef]
- Nash, D.M.; Lane, E.; Herath, S.; Sheldon, I.M. ORIGINAL ARTICLE: Endometrial Explant Culture for Characterizing Equine Endometritis. Am. J. Reprod. Immunol. 2008, 59, 105–117. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.; Łukasik, K.; Galvão, A.; Ferreira-Dias, G.M.; Skarzynski, D.J. Impairment of the Interleukin System in Equine Endometrium During the Course of Endometrosis. Boil. Reprod. 2013, 89, 79. [Google Scholar] [CrossRef]
- Voynow, J.A.; Fischer, B.M.; Zheng, S. Proteases and cystic fibrosis. Int. J. Biochem. Cell Boil. 2008, 40, 1238–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misumi, T.; Tanaka, T.; Mikawa, K.; Nishina, K.; Morikawa, O.; Obara, H. Effects of sivelestat, a new elastase inhibitor, on IL-8 and MCP-1 production from stimulated human alveolar epithelial type II cells. J. Anesth. 2006, 20, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, W.R.; Fischer, L.; Roth, K.; Jüllig, A.K.; Stuckenschneider, J.E.; Schwartz, P.; Weimer, M.; Orlowska-Volk, M.; Hanjalic-Beck, A.; Kranz, I.; et al. Critical evaluation of human endometrial explants as an ex vivo model system: A molecular approach. Mol. Hum. Reprod. 2010, 17, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Huggett, J.F.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 2004, 37, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Boil. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Ladner-Keay, C.; Yang, J.; Turner, R.J.; Edwards, R.A. Visible fluorescent detection of proteins in polyacrylamide gels without staining. Anal. Biochem. 2004, 326, 13–20. [Google Scholar] [CrossRef]
- Gilda, J.E.; Gomes, A.V. Stain-Free total protein staining is a superior loading control to β-actin for Western blots. Anal. Biochem. 2013, 440, 186–188. [Google Scholar] [CrossRef] [Green Version]
- Posch, A.; Kohn, J.; Oh, K.; Hammond, M.; Liu, N. V3 Stain-free Workflow for a Practical, Convenient, and Reliable Total Protein Loading Control in Western Blotting. J. Vis. Exp. 2013, 50948. [Google Scholar] [CrossRef] [Green Version]
- Comajoan, P.; Gubern, C.; Huguet, G.; Serena, J.; Kádár, E.; Castellanos, M. Evaluation of common housekeeping proteins under ischemic conditions and/or rt-PA treatment in bEnd.3 cells. J. Proteom. 2018, 184, 10–15. [Google Scholar] [CrossRef]
- Manuel, J.A.; Gawronska-Kozak, B. Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Boil. 2006, 25, 505–514. [Google Scholar] [CrossRef]
- Raykin, J.; Snider, E.; Bheri, S.; Mulvihill, J.J.; Ethier, C.R. A modified gelatin zymography technique incorporating total protein normalization. Anal. Biochem. 2017, 521, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, M.; Iwasaki, Y.; Suzuki, S. A Protective Effect of Sivelestat From Ischemia/Reperfusion Injury in a Porcine Hepatectomy Model. Int. Surg. 2019, 103, 191–198. [Google Scholar] [CrossRef]
- Yuan, Q.; Jiang, Y.-W.; Fang, Q.-H. Improving effect of Sivelestat on lipopolysaccharide-induced lung injury in rats. APMIS 2014, 122, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Hilscher, M.B.; Sehrawat, T.; Arab, J.P.; Zeng, Z.; Gao, J.; Liu, M.; Kostallari, E.; Gao, Y.; Simonetto, U.A.; Yaqoob, U.; et al. Mechanical Stretch Increases Expression of CXCL1 in Liver Sinusoidal Endothelial Cells to Recruit Neutrophils, Generate Sinusoidal Microthombi, and Promote Portal Hypertension. Gastroenterology 2019, 157, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Kang, C.M.; Rhee, C.K.; Yoon, H.K.; Kim, Y.K.; Moon, H.S.; Park, S.H. Effects of elastase inhibitor on the epithelial cell apoptosis in bleomycin-induced pulmonary fibrosis. Exp. Lung Res. 2009, 35, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Rosales-Mayor, E.; Dale, G.E.; Dembowsky, K.; Torres, A. The Role of Neutrophil Elastase Inhibitors in Lung Diseases. Chest 2017, 152, 249–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Schwarz, R.I. Collagen I and the fibroblast: High protein expression requires a new paradigm of post-transcriptional, feedback regulation. Biochem. Biophys. Rep. 2015, 3, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Nissinen, L.; Kähäri, V. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2571–2580. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell Dev. Boil. 2001, 17, 463–516. [Google Scholar] [CrossRef] [Green Version]
- Nothnick, W.B. Regulation of uterine matrix metalloproteinase-9 and the role of microRNAs. Semin. Reprod. Med. 2008, 26, 494–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iida, J.; McCarthy, J.B. Expression of collagenase-1 (MMP-1) promotes melanoma growth through the generation of active transforming growth factor-? Melanoma Res. 2007, 17, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [PubMed]
- Kobayashi, T.; Kim, H.; Liu, X.; Sugiura, H.; Kohyama, T.; Fang, Q.; Wen, F.-Q.; Abe, S.; Wang, X.; Atkinson, J.J.; et al. Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L1006–L1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Angelo, M.; Billings, P.C.; Pacifici, M.; Leboy, P.S.; Kirsch, T. Authentic Matrix Vesicles Contain Active Metalloproteases (MMP). J. Boil. Chem. 2001, 276, 11347–11353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overall, C.M. Molecular Determinants of Metalloproteinase Substrate Specificity: Matrix Metalloproteinase Substrate Binding Domains, Modules, and Exosites. Mol. Biotechnol. 2002, 22, 51–86. [Google Scholar] [CrossRef]
- Dayer, C.; Stamenkovic, I. Recruitment of Matrix Metalloproteinase-9 (MMP-9) to the Fibroblast Cell Surface by Lysyl Hydroxylase 3 (LH3) Triggers Transforming Growth Factor-β (TGF-β) Activation and Fibroblast Differentiation. J. Boil. Chem. 2015, 290, 13763–13778. [Google Scholar] [CrossRef] [Green Version]
- Hattori, N.; Mochizuki, S.; Kishi, K.; Nakajima, T.; Takaishi, H.; D’Armiento, J.; Okada, Y. MMP-13 Plays a Role in Keratinocyte Migration, Angiogenesis, and Contraction in Mouse Skin Wound Healing. Am. J. Pathol. 2009, 175, 533–546. [Google Scholar] [CrossRef] [Green Version]
- Bracher, V.; Neuschaefer, A.; Allen, W.R. The Effect of Intra-Uterine Kerosene Infusion on the Endometrium of mares. J. Reprod. Fert. Suppl. 1991, 44, 706–707. [Google Scholar]
- Podico, G.; Canisso, I.F.; Roady, P.J.; Austin, S.M.; Carossino, M.; Balasuriya, U.; Ellerbrock, R.E.; Lima, F.S.; Ferreira-Dias, G.; Douglas, R.H. Uterine responses and equine chorionic gonadotropin concentrations after two intrauterine infusions with kerosene post early fetal loss in mares. Theriogenology 2020, 147, 202–210. [Google Scholar] [CrossRef]

| Gene (Accession Number) | Sequence 5′-3′ | Amplicon |
|---|---|---|
| COL1A2 (XM_001492939.3) | Forward: CAAGGGCATTAGGGGACACA | 196 |
| Reverse: ACCCACACTTCCATCGCTTC | ||
| MMP2 (XM_001493281.2) | Forward: TCCCACTTTGATGACGACGA | 115 |
| Reverse: TTGCCGTTGAAGAGGAAAGG | ||
| MMP9 (NM_001111302.1) | Forward: GCGGTAAGGTGCTGCTGTTC | 177 |
| Reverse: GAAGCGGTCCTGGGAGAAGT | ||
| RPL32 (XM_001492042.6) | Forward: AGCCATCTACTCGGCGTCA | 144 |
| Reverse: GTCAATGCCTCTGGGTTTCC |
| Estrous Cycle Phase | FP | MLP | ||||||
|---|---|---|---|---|---|---|---|---|
| Time of Treatment | 24 h | 48 h | 24 h | 48 h | ||||
| Treatment | Control | TGFβ1 (10 ng/mL) | Control | TGFβ1 (10 ng/mL) | Control | TGFβ1 (10 ng/mL) | Control | TGFβ1 (10 ng/mL) |
| COL1A2 transcription (fold increase) | 0.66 ± 0.06 a | 0.97 ± 0.04 b | 1.02 ± 0.86 a | 1.82 ± 0.25 b | 1.00 ± 0.24 a | 2.75 ± 0.47 b | 1.00 ± 0.24 a | 3.86 ± 0.48 b |
| COL1 protein (fold increase) | 1.34 ± 0.05 a | 1.93 ± 0.12 b | 1.37 ± 0.05 a | 1.33 ± 0.05 a | 0.71 ± 0.54 a | 1.06 ± 0.01 b | 0.58 ± 0.02 a | 0.87 ± 0.004 b |
| Time of Incubation | LDH Activity (%) |
|---|---|
| 1 h | 94.3 ± 0.9 a |
| 24 h | 92.6 ± 0.5 a |
| 48 h | 89.0 ± 0.6 b |
| Time of Treatment | 24 h | 48 h | ||
|---|---|---|---|---|
| Treatment | Control | OXT (10−7 M) | Control | OXT (10−7 M) |
| PGF2α secretion (ng/mg) | 7.3 ± 0.8 a | 16.0 ± 1.3 b | 7.6 ± 0.9 a | 14.0 ± 3.2 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, A.; Fernandes, C.; Rebordão, M.R.; Szóstek-Mioduchowska, A.; Lukasik, K.; Gawronska-Kozak, B.; Telo da Gama, L.; Skarzynski, D.J.; Ferreira-Dias, G. The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium. Animals 2020, 10, 863. https://doi.org/10.3390/ani10050863
Amaral A, Fernandes C, Rebordão MR, Szóstek-Mioduchowska A, Lukasik K, Gawronska-Kozak B, Telo da Gama L, Skarzynski DJ, Ferreira-Dias G. The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium. Animals. 2020; 10(5):863. https://doi.org/10.3390/ani10050863
Chicago/Turabian StyleAmaral, Ana, Carina Fernandes, Maria Rosa Rebordão, Anna Szóstek-Mioduchowska, Karolina Lukasik, Barbara Gawronska-Kozak, Luís Telo da Gama, Dariusz J. Skarzynski, and Graça Ferreira-Dias. 2020. "The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium" Animals 10, no. 5: 863. https://doi.org/10.3390/ani10050863






