Cloning and Expression Analysis of Two Kdm Lysine Demethylases in the Testes of Mature Yaks and Their Sterile Hybrids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
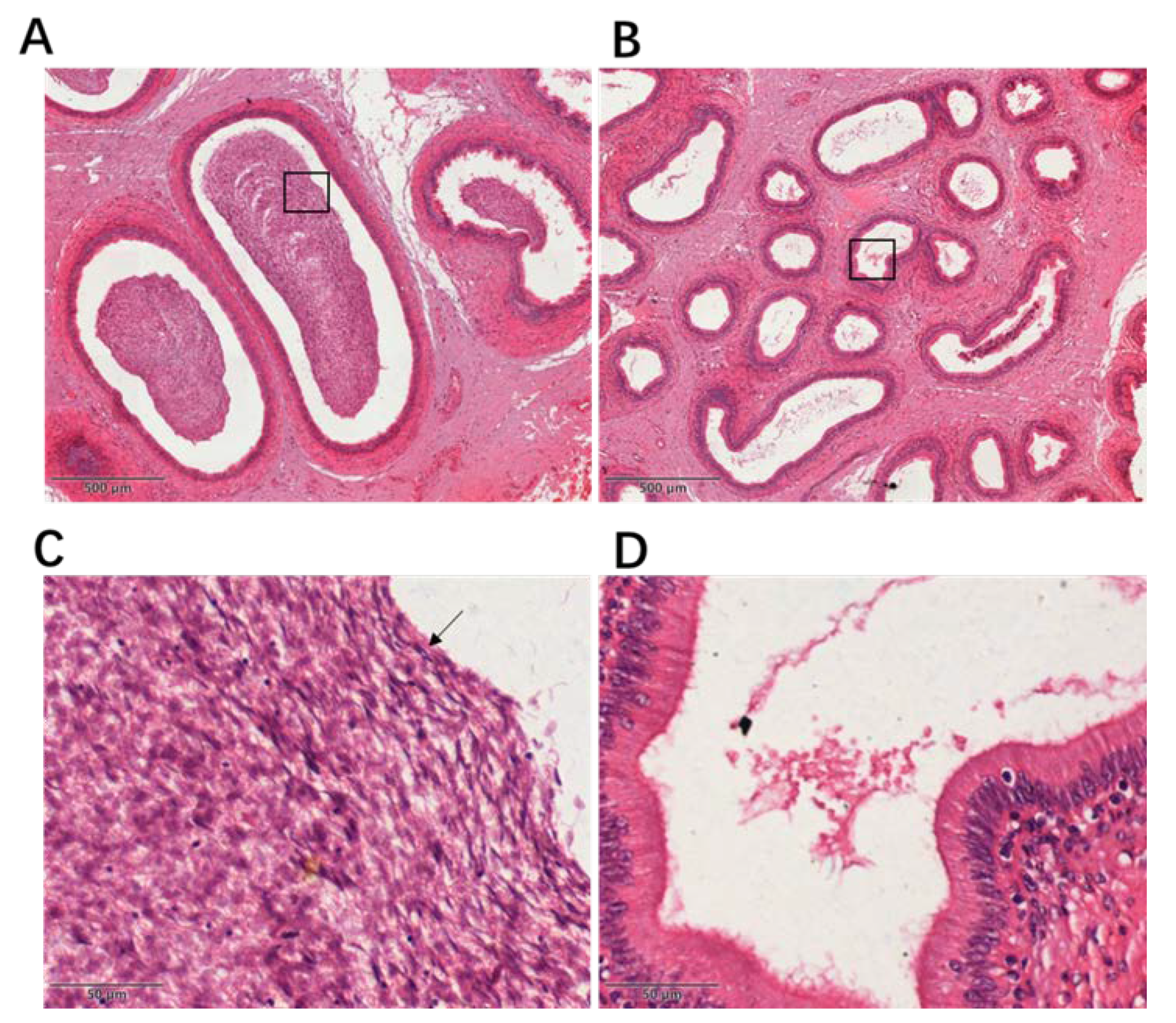
2.2. Histological Comparison of Yak and Cattle–Yak Epididymes
2.3. RNA Extraction and cDNA Synthesis
2.4. Cloning and Sequencing of the KDM1A and KDM4B Genes of Yaks
2.5. Analysis of KDM1A and KDM4B mRNA Expressions by Quantitative Real-time PCR
2.6. Analysis of KDM1A and KDM4B Protein Expression by Western Blotting
2.7. Quantification of H3K36 and H3K27 Trimethylation by ELISA
2.8. Statistical Analysis
3. Results
3.1. H&E-Stained Sections of Epididymes from Yaks and Cattle–Yaks
3.2. Cloning and Sequencing of the Yak KDM1A and KDM4B Genes
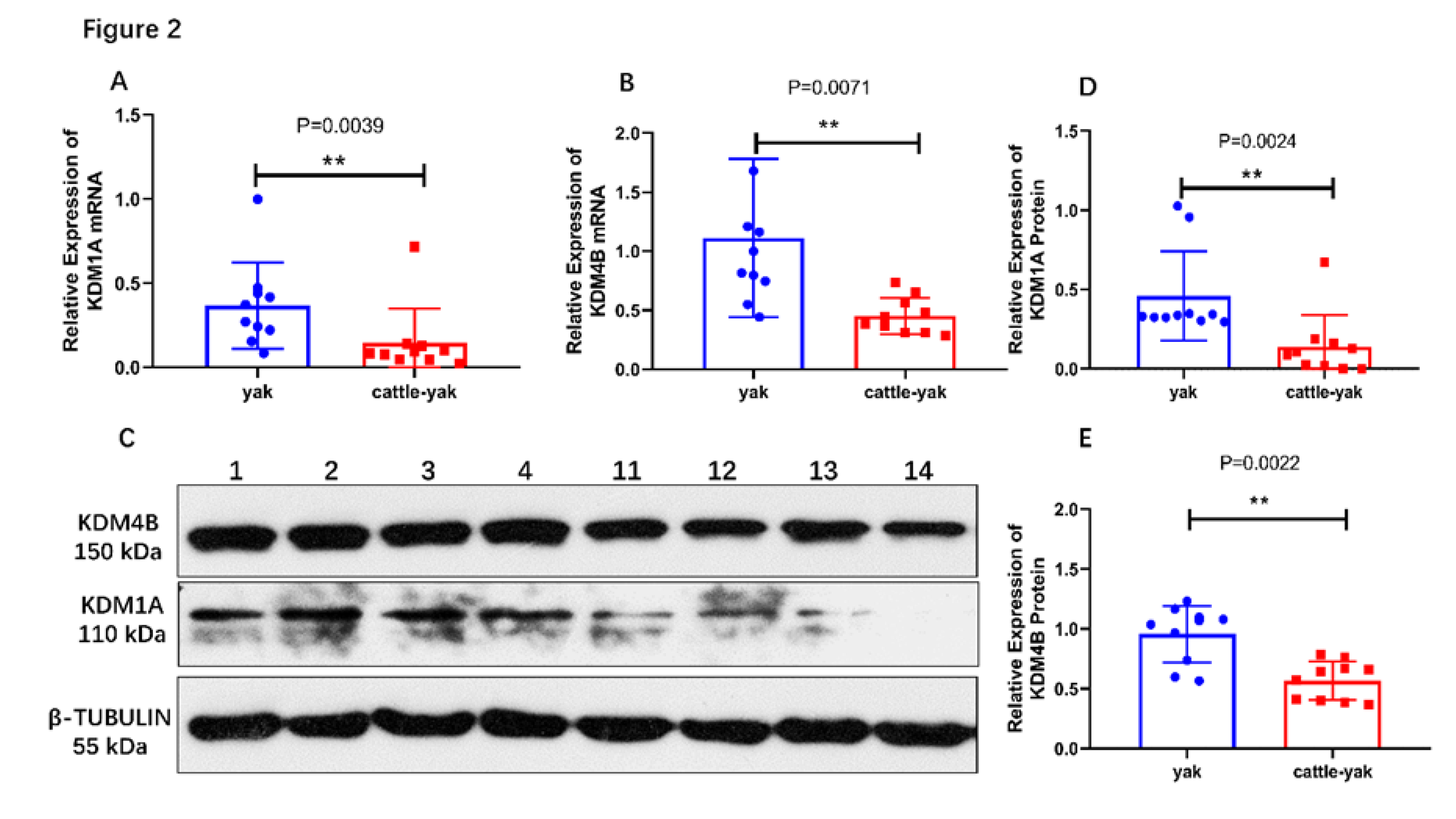
3.3. KDM1A and KDM4B Expressions in the Testes of Yaks and Cattle–Yaks
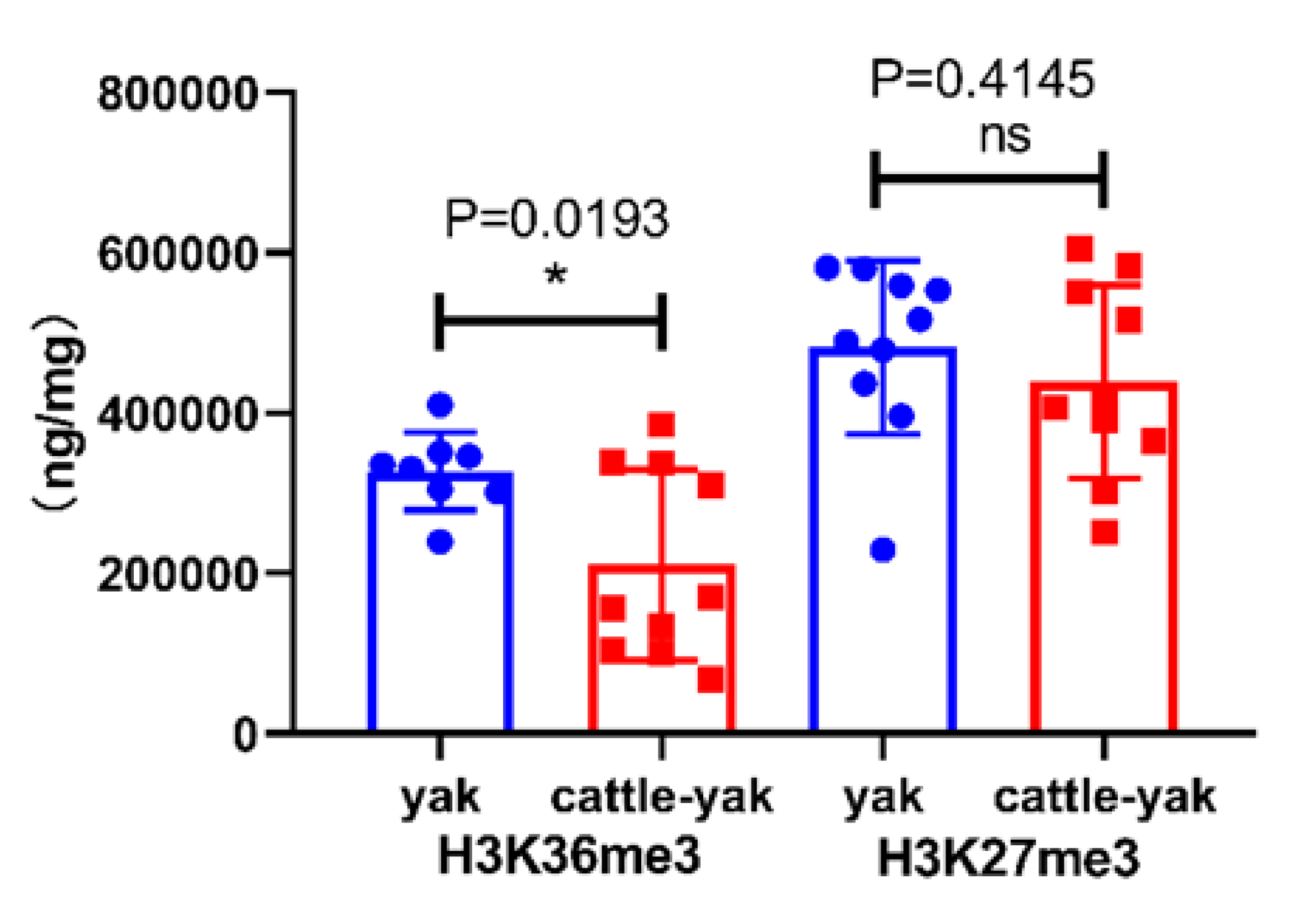
3.4. The Levels of H3K36me3 and H3K27me3 in the Testes of Yaks and Cattle–Yaks
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Jiang, M. Metabolomic profiles in yak mammary gland tissue during the lactation cycle. PLoS ONE 2019, 14, e0219220. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.N.; Liu, W.J.; Wang, C.L.; Huang, L.; Jin, S.Y.; Lin, Y.Q.; Zheng, Y.C. Histological evaluation and Prdm9 expression level in the testis of sterile male cattle-yaks. Livest. Sci. 2014, 160, 208–213. [Google Scholar] [CrossRef]
- Tumennasan, K.; Tuya, T.; Hotta, Y.; Takase, H.; Speed, R.M.; Chandley, A.C. Fertility investigations in the F1 hybrid and backcross progeny of cattle (Bos taurus) and yak (B. grunniens) in Mongolia. Cytogenet. Cell Genet. 1997, 78, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pan, Z.; Zhang, Q.; Xie, Z.; Liu, H.; Li, Q. Differential mRNA expression and promoter methylation status of SYCP3 gene in testes of yaks and cattle-yaks. Reprod. Domest. Anim. Zuchthyg. 2012, 47, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, Q.; Pan, Z.; Li, M.; Luo, H.; Xie, Z. Molecular cloning, gene expression and methylation status analysis of PIWIL1 in cattle-yaks and the parental generation. Anim. Reprod. Sci. 2013, 140, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yu, S.; Cui, Y.; He, J.; Zhang, Q.; Sun, J.; Huang, Y.; Yang, X.; Cao, M.; Liao, B.; et al. Regulation by Hsp27/P53 in testis development and sperm apoptosis of male cattle (cattle-yak and yak). J. Cell. Physiol. 2018, 234, 650–660. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Luo, H.; Weng, Q.; Wang, S.; Pan, Z.; Xie, Z.; Wu, W.; Liu, H.; Li, Q. Differential DNA methylation of the meiosis-specific gene FKBP6 in testes of yak and cattle-yak hybrids. Reprod. Domest. Anim. Zuchthyg. 2016, 51, 1030–1038. [Google Scholar] [CrossRef]
- Yan, P.; Xiang, L.; Guo, X.; Bao, P.J.; Jin, S.; Wu, X.Y. The low expression of Dmrt7 is associated with spermatogenic arrest in cattle-yak. Mol. Biol. Rep. 2014, 41, 7255–7263. [Google Scholar] [CrossRef]
- Li, B.; Wu, W.; Luo, H.; Liu, Z.; Liu, H.; Li, Q.; Pan, Z. Molecular characterization and epigenetic regulation of Mei1 in cattle and cattle-yak. Gene 2015, 573, 50–56. [Google Scholar] [CrossRef]
- Dong, L.Y.; Li, Q.F.; Qu, X.G.; Li, Y.X.; Li, X.F.; Hu, H.T.; Xie, Z. [Expression levels of Cdc2 and Cdc25A mRNA in cattle, yak, and cattle-yak testis]. Yi Chuan Hered. 2009, 31, 495–499. [Google Scholar] [CrossRef]
- Cui, X.; Jing, X.; Wu, X.; Yan, M.; Li, Q.; Shen, Y.; Wang, Z. DNA methylation in spermatogenesis and male infertility. Exp. Ther. Med. 2016, 12, 1973–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.; Qin, J.; Wan, Y.; Zhang, Y.; Hu, Y.; Zhang, C.; Zeng, W. Histone lysine methylation exhibits a distinct distribution during spermatogenesis in pigs. Theriogenology 2015, 84, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Rajender, S.; Avery, K.; Agarwal, A. Epigenetics, spermatogenesis and male infertility. Mutat. Res. 2011, 727, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Luense, L.J.; Wang, X.; Schon, S.B.; Weller, A.H.; Lin Shiao, E.; Bryant, J.M.; Bartolomei, M.S.; Coutifaris, C.; Garcia, B.A.; Berger, S.L. Comprehensive analysis of histone post-translational modifications in mouse and human male germ cells. Epigenetics Chromatin 2016, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Garcia, B.A. Comprehensive Catalog of Currently Documented Histone Modifications. Cold Spring Harb. Perspect Biol. 2015, 7, a025064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachner, M.; Jenuwein, T. The many faces of histone lysine methylation. Curr. Opin. Cell Biol. 2002, 14, 286–298. [Google Scholar] [CrossRef]
- Fuks, F. DNA methylation and histone modifications: Teaming up to silence genes. Curr. Opin. Genet. Dev. 2005, 15, 490–495. [Google Scholar] [CrossRef]
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Anim. Int. J. Anim. Biosci. 2018, 12, s27–s35. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.L.; Petkova, P.; Walker, M.; Flachs, P.; Mihola, O.; Trachtulec, Z.; Petkov, P.M.; Paigen, K. Multimer Formation Explains Allelic Suppression of PRDM9 Recombination Hotspots. PLoS Genet. 2015, 11, e1005512. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, M.; Nozaki, M.; Takeda, N.; Shinkai, Y. Functional dynamics of H3K9 methylation during meiotic prophase progression. EMBO J. 2007, 26, 3346–3359. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, S.; Liao, L.; Chen, X.; Meistrich, M.; Xu, J. Jmjd1a demethylase-regulated histone modification is essential for cAMP-response element modulator-regulated gene expression and spermatogenesis. J. Biol. Chem. 2010, 285, 2758–2770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Li, Q.; Pan, Z.; Qu, X.; Zhang, C.; Xie, Z. Comparative analysis on mRNA expression level and methylation status of DAZL gene between cattle-yaks and their parents. Anim. Reprod. Sci. 2011, 126, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.W.; Wu, Y.; Luo, Z.; Guan, J.; Wang, L.; Luo, X.; Zuo, F. Comparison of Y-chromosome-linked TSPY, TSPY2, and PRAMEY genes in Taurus cattle, yaks, and interspecific hybrid bulls. J. Dairy Sci. 2019, 102, 6263–6275. [Google Scholar] [CrossRef] [PubMed]
- Fellous, A.; Earley, R.L.; Silvestre, F. The Kdm/Kmt gene families in the self-fertilizing mangrove rivulus fish, Kryptolebias marmoratus, suggest involvement of histone methylation machinery in development and reproduction. Gene 2019, 687, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Mukai, M.; Hira, S.; Nakamura, K.; Nakamura, S.; Kimura, H.; Sato, M.; Kobayashi, S. H3K36 Trimethylation-Mediated Epigenetic Regulation is Activated by Bam and Promotes Germ Cell Differentiation During Early Oogenesis in Drosophila. Biol. Open 2015, 4, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Rajput, S.; Kumar, S.; De, S.; Chakravarty, A.K.; Kumar, R.; Datta, T.K. Differential histone modification status of spermatozoa in relation to fertility of buffalo bulls. J. Cell. Biochem. 2015, 116, 743–753. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sun, L.; Mipam, T.D.; Zhao, F.; Liu, W.; Zhao, W.; Wu, S.; Xu, C.; Yu, S.; Cai, X. Comparative testis proteome of cattleyak from different developmental stages. Anim. Int. J. Anim. Biosci. 2017, 11, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, J.; Li, Q.; Li, X.; Liu, Z.; Song, D.; Xie, Z. Cloning and characterization of the gene encoding the bovine BOULE protein. Mol. Genet. Genom. 2009, 281, 67–75. [Google Scholar] [CrossRef]
- Presnyak, V.; Alhusaini, N.; Chen, Y.H.; Martin, S.; Morris, N.; Kline, N.; Olson, S.; Weinberg, D.; Baker, K.E.; Graveley, B.R.; et al. Codon optimality is a major determinant of mRNA stability. Cell 2015, 160, 1111–1124. [Google Scholar] [CrossRef] [Green Version]
- Kudla, G.; Murray, A.W.; Tollervey, D.; Plotkin, J.B. Coding-sequence determinants of gene expression in Escherichia coli. Science 2009, 324, 255–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komar, A.A. The Yin and Yang of codon usage. Hum. Mol. Genet. 2016, 25, R77–R85. [Google Scholar] [CrossRef] [PubMed]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, E.; Wissmann, M.; Yin, N.; Muller, J.M.; Schneider, R.; Peters, A.H.; Gunther, T.; Buettner, R.; Schule, R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 2005, 437, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Scoumanne, A.; Chen, X. The lysine-specific demethylase 1 is required for cell proliferation in both p53-dependent and -independent manners. J. Biol. Chem. 2007, 282, 15471–15475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whyte, W.A.; Bilodeau, S.; Orlando, D.A.; Hoke, H.A.; Frampton, G.M.; Foster, C.T.; Cowley, S.M.; Young, R.A. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature 2012, 482, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Su, S.T.; Ying, H.Y.; Chiu, Y.K.; Lin, F.R.; Chen, M.Y.; Lin, K.I. Involvement of histone demethylase LSD1 in Blimp-1-mediated gene repression during plasma cell differentiation. Mol. Cell. Biol. 2009, 29, 1421–1431. [Google Scholar] [CrossRef] [Green Version]
- Lambrot, R.; Lafleur, C.; Kimmins, S. The histone demethylase KDM1A is essential for the maintenance and differentiation of spermatogonial stem cells and progenitors. J. Off. Publ. FASEB 2015, 29, 4402–4416. [Google Scholar] [CrossRef]
- Saleque, S.; Kim, J.; Rooke, H.M.; Orkin, S.H. Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Mol. Cell 2007, 27, 562–572. [Google Scholar] [CrossRef]
- Choi, J.; Jang, H.; Kim, H.; Kim, S.T.; Cho, E.J.; Youn, H.D. Histone demethylase LSD1 is required to induce skeletal muscle differentiation by regulating myogenic factors. Biochem. Biophys. Res. Commun. 2010, 401, 327–332. [Google Scholar] [CrossRef]
- Myrick, D.A.; Christopher, M.A.; Scott, A.M.; Simon, A.K.; Donlin-Asp, P.G.; Kelly, W.G.; Katz, D.J. KDM1A/LSD1 regulates the differentiation and maintenance of spermatogonia in mice. PLoS ONE 2017, 12, e0177473. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, S.M.; Helin, K. Molecular mechanisms and potential functions of histone demethylases. Nat. Rev. Mol. Cell Biol. 2012, 13, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Labbe, R.M.; Holowatyj, A.; Yang, Z.Q. Histone lysine demethylase (KDM) subfamily 4: Structures, functions and therapeutic potential. Am. J. Transl. Res. 2013, 6, 1–15. [Google Scholar] [PubMed]
- Wilson, C.; Krieg, A.J. KDM4B: A Nail for Every Hammer? Genes 2019, 10, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillringhaus, L.; Yue, W.W.; Rose, N.R.; Ng, S.S.; Gileadi, C.; Loenarz, C.; Bello, S.H.; Bray, J.E.; Schofield, C.J.; Oppermann, U. Structural and evolutionary basis for the dual substrate selectivity of human KDM4 histone demethylase family. J. Biol. Chem. 2011, 286, 41616–41625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, L.C.; McDonald, D.W.; Hendzel, M.J. Kdm4b histone demethylase is a DNA damage response protein and confers a survival advantage following gamma-irradiation. J. Biol. Chem. 2013, 288, 21376–21388. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Antony, J.; Meng, F.; MacLean, P.; Rhind, R.; Laible, G.; Oback, B. KDM4B-mediated reduction of H3K9me3 and H3K36me3 levels improves somatic cell reprogramming into pluripotency. Sci. Rep. 2017, 7, 7514. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Fan, Z.; Yu, B.; Chang, J.; Al Hezaimi, K.; Zhou, X.; Park, N.H.; Wang, C.Y. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell 2012, 11, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Cooper, S.; Brockdorff, N. The interplay of histone modifications—writers that read. EMBO Rep. 2015, 16, 1467–1481. [Google Scholar] [CrossRef]
- Powers, N.R.; Parvanov, E.D.; Baker, C.L.; Walker, M.; Petkov, P.M.; Paigen, K. The Meiotic Recombination Activator PRDM9 Trimethylates Both H3K36 and H3K4 at Recombination Hotspots in Vivo. PLoS Genet. 2016, 12, e1006146. [Google Scholar] [CrossRef]
- Pfister, S.X.; Ahrabi, S.; Zalmas, L.P.; Sarkar, S.; Aymard, F.; Bachrati, C.Z.; Helleday, T.; Legube, G.; La Thangue, N.B.; Porter, A.C.; et al. SETD2-dependent histone H3K36 trimethylation is required for homologous recombination repair and genome stability. Cell Rep. 2014, 7, 2006–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, J.; Mak, W.; Zvetkova, I.; Appanah, R.; Nesterova, T.B.; Webster, Z.; Peters, A.H.; Jenuwein, T.; Otte, A.P.; Brockdorff, N. Establishment of histone H3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev. Cell 2003, 4, 481–495. [Google Scholar] [CrossRef] [Green Version]
- Bosselut, R. Pleiotropic Functions of H3K27Me3 Demethylases in Immune Cell Differentiation. Trends Immunol. 2016, 37, 102–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| GenBank Accession No. | Gene Name | Primer Sequence (5′–3′) | Annealing Temperature (°C) | Product Size (bp) | Application |
|---|---|---|---|---|---|
| XM_005203319.2 | KDM1A | F: CCATGGAAACGGGAATCGCAG R: ACGTCTGTCTCACATGCTCG | 59 | 2342 | CDS clone |
| XM_005203319.2 | KDM1A | F: GCGGACTGTGTGAAGAGAG R: CTCGTCTTCTGAGAGGTTGG | 60 | 497 | CDS clone |
| MK411808 | KDM1A | F: TGTGCTTGTCCACCGAGT R: CCTGGCTTCCAGAAGTGTG | 60 | 193 | Real-time PCR |
| XM_014482676.1 | KDM4B | F: TGACAAGGAACCGTGAAGTGT R: ATTCACAGCAAGCAAACGCCAG | 59 | 3467 | CDS clone |
| MH510242 | KDM4B | F: GTGGCCTACATCGAGTCC R: GTGCAGTACTTCTCGCTG | 60 | 233 | Real-time PCR |
| NM 001034034 | GAPDH | F: CGACTTCAACAGCGACACTCA R: GGTCCAGGGACCTTACTCCTT | 60 | 169 | Real-time PCR |
| NR 036642 | 18S RNA | F: CTGAGAAACGGCTACCACATC R: CAGACTTGCCCTCCAATGG | 59 | 168 | Real-time PCR |
| Species | Variant | Nucleotide Position | |||||
|---|---|---|---|---|---|---|---|
| 21 | 36 | 520-579 | 1431 | 1524 | 1860 | ||
| Cattle | 1 | G | G | G-A | G | A | C |
| Cattle | 2 | G | G | - | G | A | C |
| Yak | 1 | A | T | G-A | A | G | G |
| Yak | 2 | A | T | - | A | G | G |
| Species | Variant | Amino Acid Position |
|---|---|---|
| 174–193 | ||
| Cattle | 1 | GlyGlnAlaGlyGlyLeuGlnAspAspSerSerGlyGlyTyrGlyAspGlyGlnAlaSer |
| Cattle | 2 | — |
| Yak | 1 | GlyGlnAlaGlyGlyLeuGlnAspAspSerSerGlyGlyTyrGlyAspGlyGlnAlaSer |
| Yak | 2 | — |
| Species | Nucleotide Position | ||||||||||||
| 147 | 278 | 372 | 390 | 399 | 507 | 778 | 836 | 846 | 1101 | 1221 | 1295 | 1668 | |
| Cattle | T | T | G | A | T | C | C | G | T | T | A | T | C |
| Yak | C | C | A | G | C | T | T | A | C | C | T | C | T |
| Species | Nucleotide Position | ||||||||||||
| 1686 | 1827 | 2057 | 2078 | 2084 | 2169 | 2220 | 2262 | 2292 | 2399 | 2576 | 2706 | 2769 | |
| Cattle | A | T | A | T | T | T | T | G | C | A | A | T | T |
| Yak | G | C | G | C | C | C | C | A | T | G | G | C | C |
| Species | Nucleotide Position | ||||||||||||
| 2797–2871 | 2979 | 3162 | |||||||||||
| Cattle | GTGAGTGCCCGTCTGCCCCACAGTCTGTTCCCCGGCCCCG CTGTCCTGCTGTGTTCTCATCCCCTCCACCTGCAG | C | G | ||||||||||
| Yak | — | T | A | ||||||||||
| Species | Amino Acid Position | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 93 | 260 | 279 | 432 | 686 | 693 | 695 | 800 | 859 | 933–957 | |
| Cattle | Met | Arg | Gly | Val | Asn | Phe | Val | Asn | Asp | Val–Gln |
| Yak | Thr | Trp | Asp | Ala | Ser | Ser | Ala | Ser | Gly | — |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Huang, L.; Jin, S.; Zheng, Y. Cloning and Expression Analysis of Two Kdm Lysine Demethylases in the Testes of Mature Yaks and Their Sterile Hybrids. Animals 2020, 10, 521. https://doi.org/10.3390/ani10030521
Shen Z, Huang L, Jin S, Zheng Y. Cloning and Expression Analysis of Two Kdm Lysine Demethylases in the Testes of Mature Yaks and Their Sterile Hybrids. Animals. 2020; 10(3):521. https://doi.org/10.3390/ani10030521
Chicago/Turabian StyleShen, Zhenhua, Lin Huang, Suyu Jin, and Yucai Zheng. 2020. "Cloning and Expression Analysis of Two Kdm Lysine Demethylases in the Testes of Mature Yaks and Their Sterile Hybrids" Animals 10, no. 3: 521. https://doi.org/10.3390/ani10030521




