Cornelian Cherry Pulp Has Beneficial Impact on Dyslipidemia and Reduced Bone Quality in Zucker Diabetic Fatty Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Animals
2.3. Biochemistry
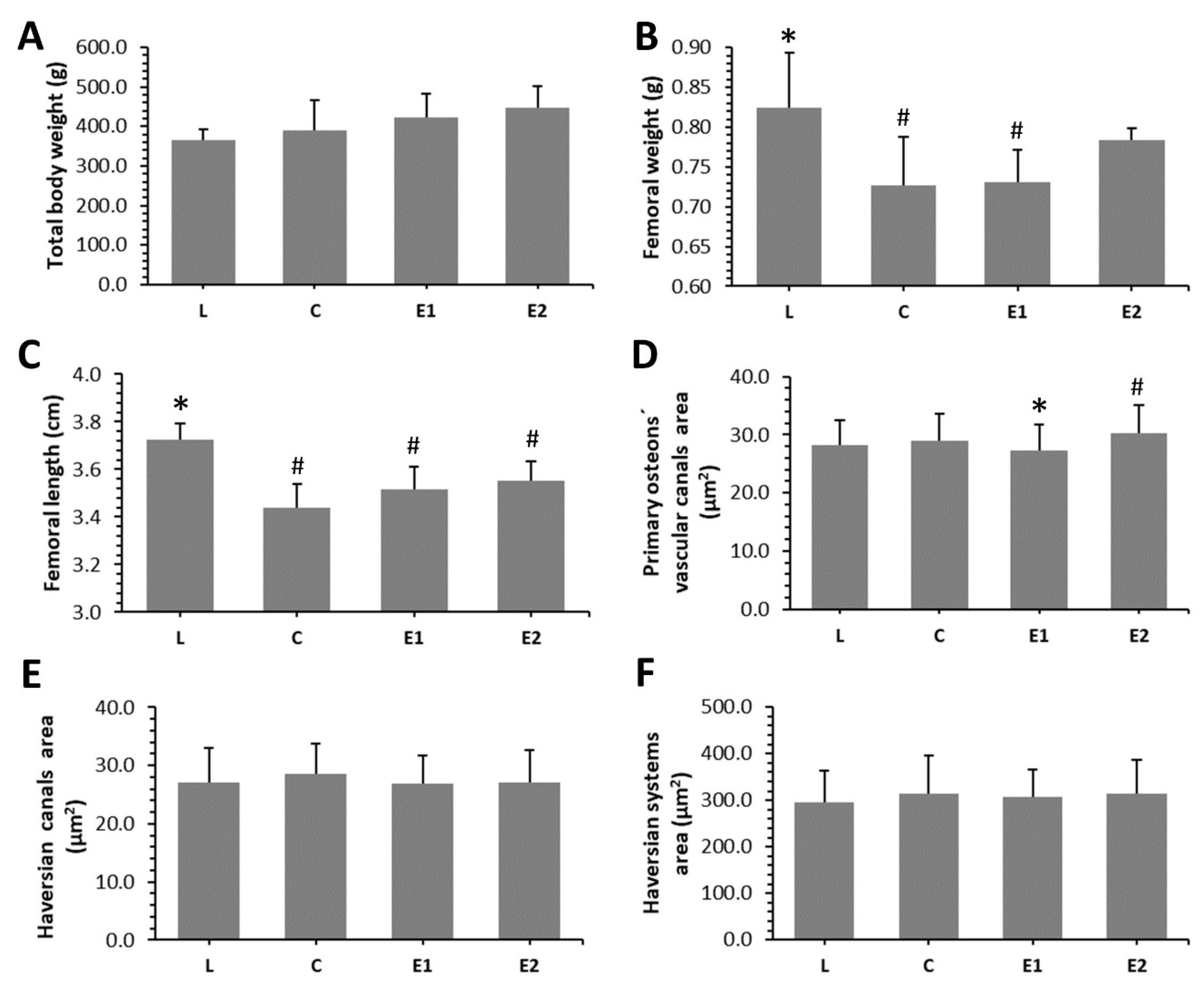
2.4. Macroscopic Measurements
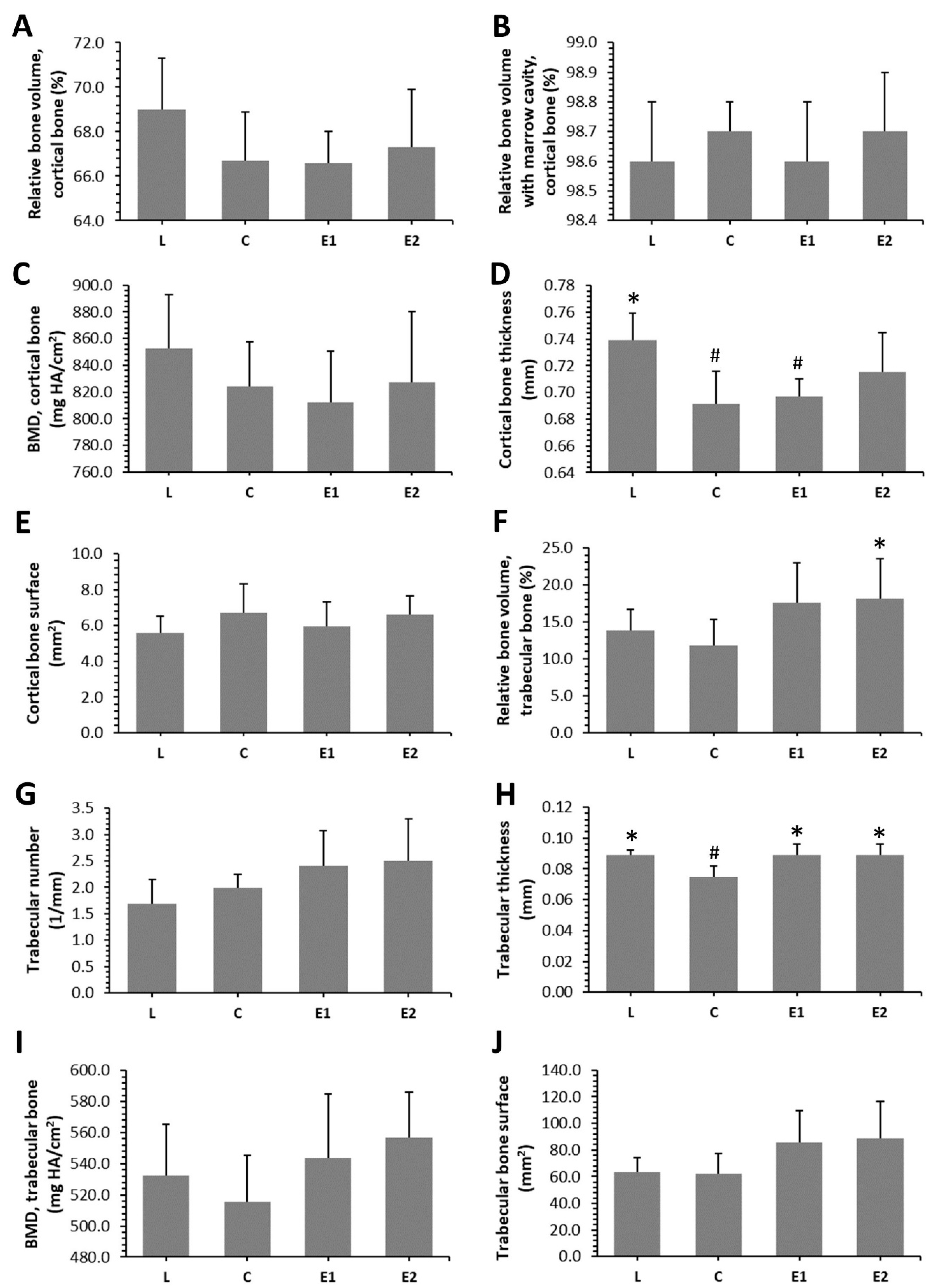
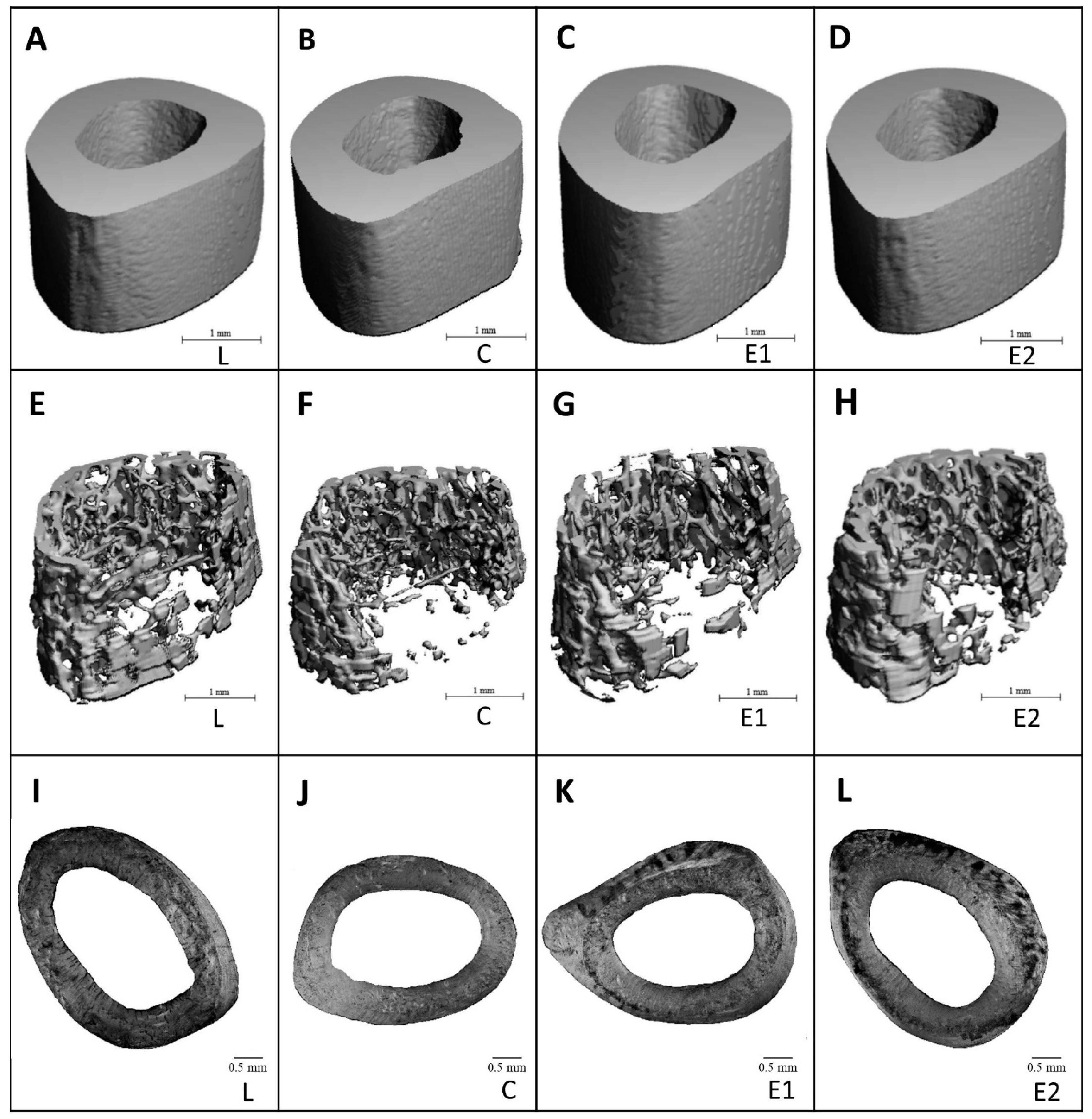
2.5. Microcomputed Tomography
2.6. Histomorphometry
2.7. Data Analysis
3. Results
3.1. Biochemistry
3.2. Macroscopic Measurements
3.3. Microcomputed Tomography
3.4. Histomorphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 Diabetes Mellitus: A Review of Current Trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, C.D.; Jain, A. Diabetes Mellitus: A Review. Int. J. Pure Appl. Biosci. 2015, 3, 224–230. [Google Scholar]
- Sundararaghavan, V.; Mazur, M.M.; Evans, B.; Liu, J.; Ebraheim, N.A. Diabetes and bone health: Latest evidence and clinical implications. Ther. Adv. Musculoskelet. Dis. 2017, 9, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, M.; Larijani, B.; Keshtkar, A.A.; Nasli-Esfahani, E.; Alatab, S.; Mohajeri-Tehrani, M.R. Mechanisms involved in altered bone metabolism in diabetes: A narrative review. J. Diabetes Metab. Disord. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Gaddini, G.W.; Turner, R.T.; Grant, K.A.; Iwaniec, U.T. Alcohol: A Simple Nutrient with Complex Actions on Bone in the Adult Skeleton. Alcohol. Clin. Exp. Res. 2016, 40, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological agents and natural compounds: Available treatments for osteoporosis. J. Physiol. Pharmacol. 2020, 71. [Google Scholar] [CrossRef]
- Costantini, S.; Conte, C. Bone health in diabetes and prediabetes. World J. Diabetes 2019, 10, 421–445. [Google Scholar] [CrossRef]
- Hamilton, E.J.; Rakic, V.; Davis, W.A.; Paul Chubb, S.A.; Kamber, N.; Prince, R.L.; Davis, T.M.E. A five-year prospective study of bone mineral density in men and women with diabetes: The Fremantle Diabetes Study. Acta Diabetol. 2012, 49, 153–158. [Google Scholar] [CrossRef]
- Starup-Linde, J.; Vestergaard, P. Biochemical bone turnover markers in diabetes mellitus—A systematic review. Bone 2016, 82, 69–78. [Google Scholar] [CrossRef]
- Sharifi, F.; Ahmadi Moghaddam, N.; Mousavi-Nasab, N. The Relationship Between Type 2 Diabetes mellitus And Bone Density In Postmenopausal Women. Int. J. Endocrinol. Metab. IJEM 2006, 4, 117–122. [Google Scholar]
- Dumic-Cule, I.; Ivanac, G.; Lucijanic, T.; Katicic, D. Type 2 diabetes and osteoporosis: Current knowledge. Endocr. Oncol. Metab. 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Thakur, A.K.; Dash, S. Estimation of bone mineral density among type 2 diabetes mellitus patients in western Odisha. Int. J. Res. Med. Sci. 2018, 6, 459–464. [Google Scholar] [CrossRef][Green Version]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef]
- Marin, C.; Luyten, F.P.; Van der Schueren, B.; Kerckhofs, G.; Vandamme, K. The Impact of Type 2 Diabetes on Bone Fracture Healing. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Linda Kao, W.H.; Kammerer, C.M.; Schneider, J.L.; Bauer, R.L.; Mitchell, B.D. Type 2 diabetes is associated with increased bone mineral density in Mexican-American women. Arch. Med. Res. 2003, 34, 399–406. [Google Scholar] [CrossRef]
- Janle, E.M.; Kissinger, P.T.; Pesek, J.F. Short Interval Monitoring of Glucose in Zucker Diabetic Fatty (ZDF) Rats. Curr. Sep. 1995, 14, 58–63. [Google Scholar]
- Capcarova, M.; Kalafova, A.; Schwarzova, M.; Soltesova Prnova, M.; Svik, K.; Schneidgenova, M.; Slovak, L.; Bovdisova, I.; Toman, R.; Lory, V.; et al. The high-energy diet affecting development of diabetes symptoms in Zucker diabetic fatty rats. Biologia 2018, 73, 659–671. [Google Scholar] [CrossRef]
- Zeitoun, D.; Caliaperoumal, G.; Bensidhoum, M.; Constans, J.M.; Anagnostou, F.; Bousson, V. Microcomputed tomography of the femur of diabetic rats: Alterations of trabecular and cortical bone microarchitecture and vasculature-a feasibility study. Eur. Radiol. Exp. 2019, 3, 17. [Google Scholar] [CrossRef]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Asgary, S.; Rafieian-Kopaei, M.; Shamsi, F.; Najafi, S.; Sahebkar, A. Biochemical and histopathological study of the anti-hyperglycemic and anti-hyperlipidemic effects of cornelian cherry (Cornus mas L.) in alloxan-induced diabetic rats. J. Complement. Integr. Med. 2014, 11, 63–69. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Fitsiou, E.; Spyridopoulou, K.; Vasileiadis, S.; Iliopoulos, C.; Galanis, A.; Vekiari, S.; Pappa, A.; Chlichlia, K. Evaluation of Antioxidant and Antiproliferative Properties of Cornus mas L. Fruit Juice. Antioxidants 2019, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Narimani-Rad, M.; Lotfi, A.; Mesgari Abbasi, M.; Abdollahi, B. The Effect of Cornelian Cherry (Cornus mas L.) Extract on Serum Ghrelin and Corticosterone Levels in Rat Model. J. Pharm. Biomed. Sci. 2013, 3, 7–9. [Google Scholar]
- Soltani, R.; Gorji, A.; Asgary, S.; Sarrafzadegan, N.; Siavash, M. Evaluation of the Effects of Cornus mas L. Fruit Extract on Glycemic Control and Insulin Level in Type 2 Diabetic Adult Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Evid. Based Complement. Altern. Med. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, M.E.; Melzig, M.F. Cornus mas and Cornus Officinalis—Analogies and Differences of Two Medicinal Plants Traditionally Used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef]
- Lotfi, A.; Aghdam Shahryar, H.; Rasoolian, H. Effects of cornelian cherry (Cornus mas L.) fruit on plasma lipids, cortisol, T3 and T4 levels in hamsters. J. Anim. Plant Sci. 2014, 24, 459–462. [Google Scholar]
- Capcarova, M.; Kalafova, A.; Schwarzova, M.; Schneidgenova, M.; Švík, K.; Prnova, M.; Slovák, L.; Kovacik, A.; Lóry, V.; Zórad, S.; et al. Cornelian cherry fruit improves glycaemia and manifestations of diabetes in obese Zucker diabetic fatty rats. Res. Vet. Sci. 2019, 126. [Google Scholar] [CrossRef]
- Omelka, R.; Martiniakova, M.; Svik, K.; Slovak, L.; Payer, J.; Oppenbergerova, I.; Kovacova, V.; Babikova, M.; Soltesova-Prnova, M. The effects of eggshell calcium (Biomin H®) and its combinations with alfacalcidol (1α-hydroxyvitamin D3) and menaquinone-7 (vitamin K2) on ovariectomy-induced bone loss in a rat model of osteoporosis. J. Anim. Physiol. Anim. Nutr. 2020. [Google Scholar] [CrossRef]
- Martiniaková, M.; Boboňová, I.; Omelka, R.; Grosskopf, B.; Stawarz, R.; Toman, R. Structural changes in femoral bone tissue of rats after subchronic peroral exposure to selenium. Acta Vet. Scand. 2013, 55, 8. [Google Scholar] [CrossRef]
- Martiniaková, M.; Chovancová, H.; Omelka, R.; Grosskopf, B.; Toman, R. Effects of a single intraperitoneal administration of cadmium on femoral bone structure in male rats. Acta Vet. Scand. 2011, 53, 49. [Google Scholar] [CrossRef]
- Enlow, D.H.; Brown, S.O. A comparative histological study of fossil and recent bone tissues. Part I. Tex. J. Sci. 1956, 8, 405–412. [Google Scholar]
- de Ricqles, A.J.; Meunier, F.J.; Castanet, J.; Francillon-Vieillot, H. Comparative microstructure of bone. In Bone Matrix and Bone Specific Products; CRC Press, Inc.: Boca Raton, FL, USA, 1991; Volume 3, pp. 1–78. [Google Scholar]
- Pang, Y.; Hu, J.; Liu, G.; Lu, S. Comparative medical characteristics of ZDF-T2DM rats during the course of development to late stage disease. Anim. Models Exp. Med. 2018, 1, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Mirbadalzadeh, R.; Shirdel, Z. Antihyperglycemic and Antihyperlipidemic effects of Cornus mas extract in diabetic rats compared with glibenclamide. Elixir Hormo Signal 2012, 47, 8969–8972. [Google Scholar]
- Dayar, E.; Cebova, M.; Lietava, J.; Panghyova, E.; Pechanova, O. Beneficial Effects of Cornelian Cherries on Lipid Profile and NO/ROS Balance in Obese Zucker Rats: Comparison with CoQ10. Molecules 2020, 25, 1922. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, R.A.; Morrison, M.C.; Sheedfar, F.; Mulder, P.; Schreurs, M.; Hommelberg, P.P.H.; Hofker, M.H.; Schalkwijk, C.; Kleemann, R.; Tietge, U.J.F.; et al. Effects of Anthocyanin and Flavanol Compounds on Lipid Metabolism and Adipose Tissue Associated Systemic Inflammation in Diet-Induced Obesity. Available online: https://www.hindawi.com/journals/mi/2016/2042107/ (accessed on 5 November 2020).
- Shimizu, S.; Matsushita, H.; Morii, Y.; Ohyama, Y.; Morita, N.; Tachibana, R.; Watanabe, K.; Wakatsuki, A. Effect of anthocyanin-rich bilberry extract on bone metabolism in ovariectomized rats. Biomed. Rep. 2018, 8, 198–204. [Google Scholar] [CrossRef]
- Damiano, S.; Lombari, P.; Salvi, E.; Papale, M.; Giordano, A.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Rapisarda, P.; Capasso, G.; et al. A red orange and lemon by-products extract rich in anthocyanins inhibits the progression of diabetic nephropathy. J. Cell. Physiol. 2019, 234, 23268–23278. [Google Scholar] [CrossRef]
- Burr, D.B. Targeted and nontargeted remodeling. Bone 2002, 30, 2–4. [Google Scholar] [CrossRef]
- Bell, K.L.; Loveridge, N.; Reeve, J.; Thomas, C.D.; Feik, S.A.; Clement, J.G. Super-osteons (remodeling clusters) in the cortex of the femoral shaft: Influence of age and gender. Anat. Rec. 2001, 264, 378–386. [Google Scholar] [CrossRef]
- Bruce Martin, R. Fatigue Damage, Remodeling, and the Minimization of Skeletal Weight. J. Theor. Biol. 2003, 220, 271–276. [Google Scholar] [CrossRef]
- Stoclet, J.-C.; Chataigneau, T.; Ndiaye, M.; Oak, M.-H.; El Bedoui, J.; Chataigneau, M.; Schini-Kerth, V.B. Vascular protection by dietary polyphenols. Eur. J. Pharmacol. 2004, 500, 299–313. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Asgary, S.; Adelnia, A.; Setorki, M.; Khazaei, M.; Kazemi, S.; Shamsi, F. The effects of cornelian cherry on atherosclerosis and atherogenic factors in hypercholesterolemic rabbits. J. Med. Plants Res. 2011, 5, 7. [Google Scholar]
- Gasser, J.; Kneissel, M. Bone Physiology and Biology. In Bone Toxicology; Springer: Cham, Switzerland, 2017; pp. 27–94. ISBN 978-3-319-56190-5. [Google Scholar]
- Conte, C.; Bouillon, R.; Napoli, N. Chapter 40—Diabetes and bone. In Principles of Bone Biology, 4th ed.; Bilezikian, J.P., Martin, T.J., Clemens, T.L., Rosen, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 941–969. ISBN 978-0-12-814841-9. [Google Scholar]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef] [PubMed]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health—A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- González, I.; Morales, M.A.; Rojas, A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res. Int. 2020, 129, 108843. [Google Scholar] [CrossRef]
- Đudarić, L.; Fužinac-Smojver, A.; Muhvić, D.; Giacometti, J. The role of polyphenols on bone metabolism in osteoporosis. Food Res. Int. 2015, 77, 290–298. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omelka, R.; Blahova, J.; Kovacova, V.; Babikova, M.; Mondockova, V.; Kalafova, A.; Capcarova, M.; Martiniakova, M. Cornelian Cherry Pulp Has Beneficial Impact on Dyslipidemia and Reduced Bone Quality in Zucker Diabetic Fatty Rats. Animals 2020, 10, 2435. https://doi.org/10.3390/ani10122435
Omelka R, Blahova J, Kovacova V, Babikova M, Mondockova V, Kalafova A, Capcarova M, Martiniakova M. Cornelian Cherry Pulp Has Beneficial Impact on Dyslipidemia and Reduced Bone Quality in Zucker Diabetic Fatty Rats. Animals. 2020; 10(12):2435. https://doi.org/10.3390/ani10122435
Chicago/Turabian StyleOmelka, Radoslav, Jana Blahova, Veronika Kovacova, Martina Babikova, Vladimira Mondockova, Anna Kalafova, Marcela Capcarova, and Monika Martiniakova. 2020. "Cornelian Cherry Pulp Has Beneficial Impact on Dyslipidemia and Reduced Bone Quality in Zucker Diabetic Fatty Rats" Animals 10, no. 12: 2435. https://doi.org/10.3390/ani10122435
APA StyleOmelka, R., Blahova, J., Kovacova, V., Babikova, M., Mondockova, V., Kalafova, A., Capcarova, M., & Martiniakova, M. (2020). Cornelian Cherry Pulp Has Beneficial Impact on Dyslipidemia and Reduced Bone Quality in Zucker Diabetic Fatty Rats. Animals, 10(12), 2435. https://doi.org/10.3390/ani10122435




