Potentiality of Native Ascomycete Strains in Bioremediation of Highly Polychlorinated Biphenyl Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. PCB Congeners Analysed
2.2. Soil Sampling
2.3. Inoculum Preparation
2.4. Design of Mesocosm Experiments
2.5. PCB Extraction and Determination of PCB Concentration in Soils
2.6. Fungal Concentrations in Soil
2.7. Microbial Extracellular Catabolic Enzymatic Activities
2.8. Statistical Analyses
3. Results
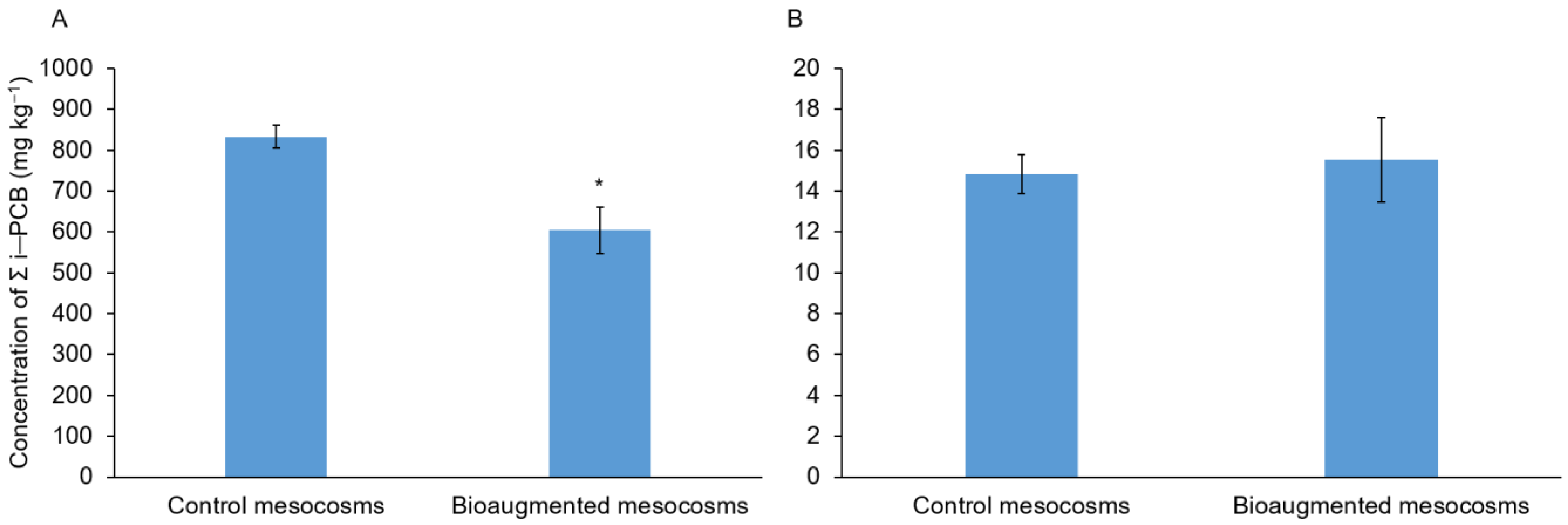
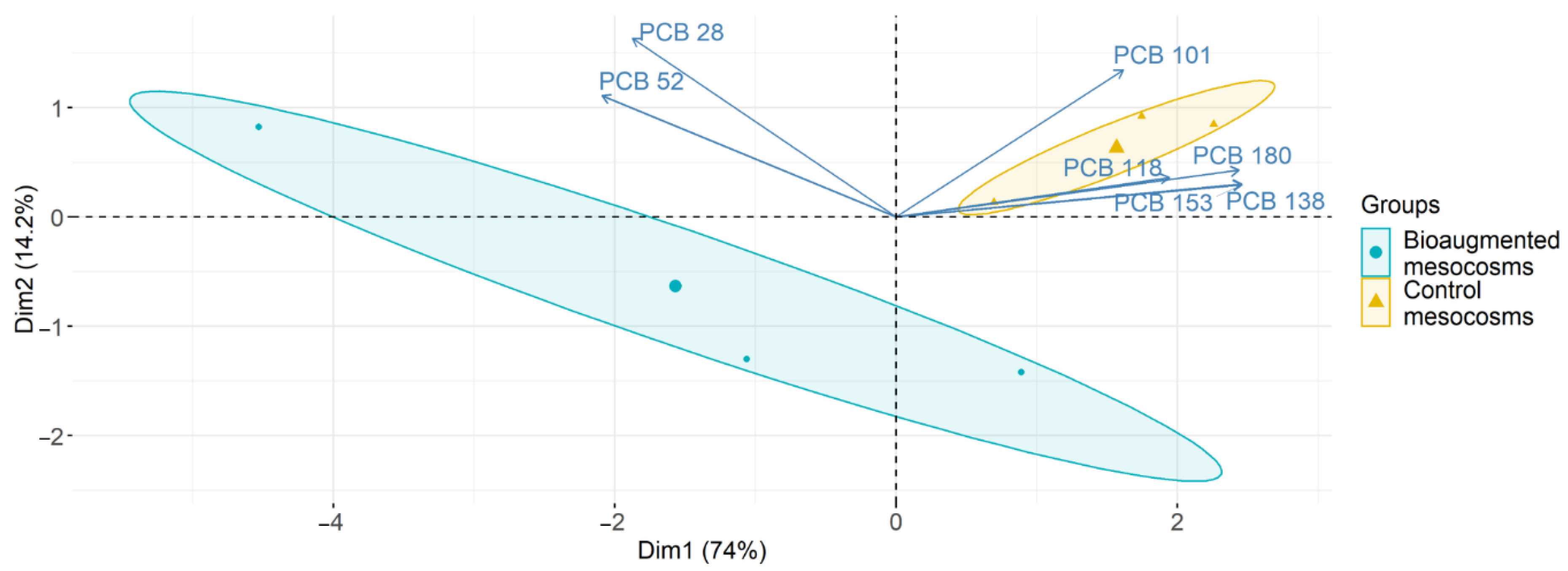
3.1. PCB Degradation in Soil
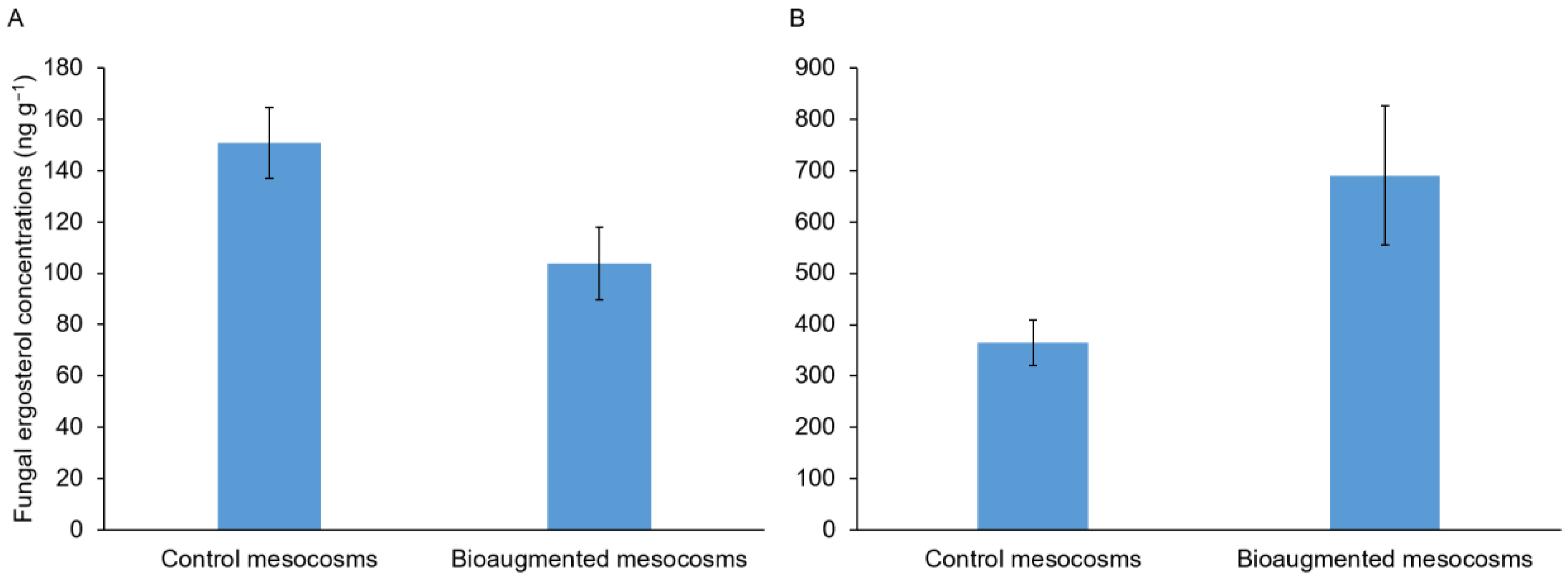
3.2. Soil Fungal Colonization
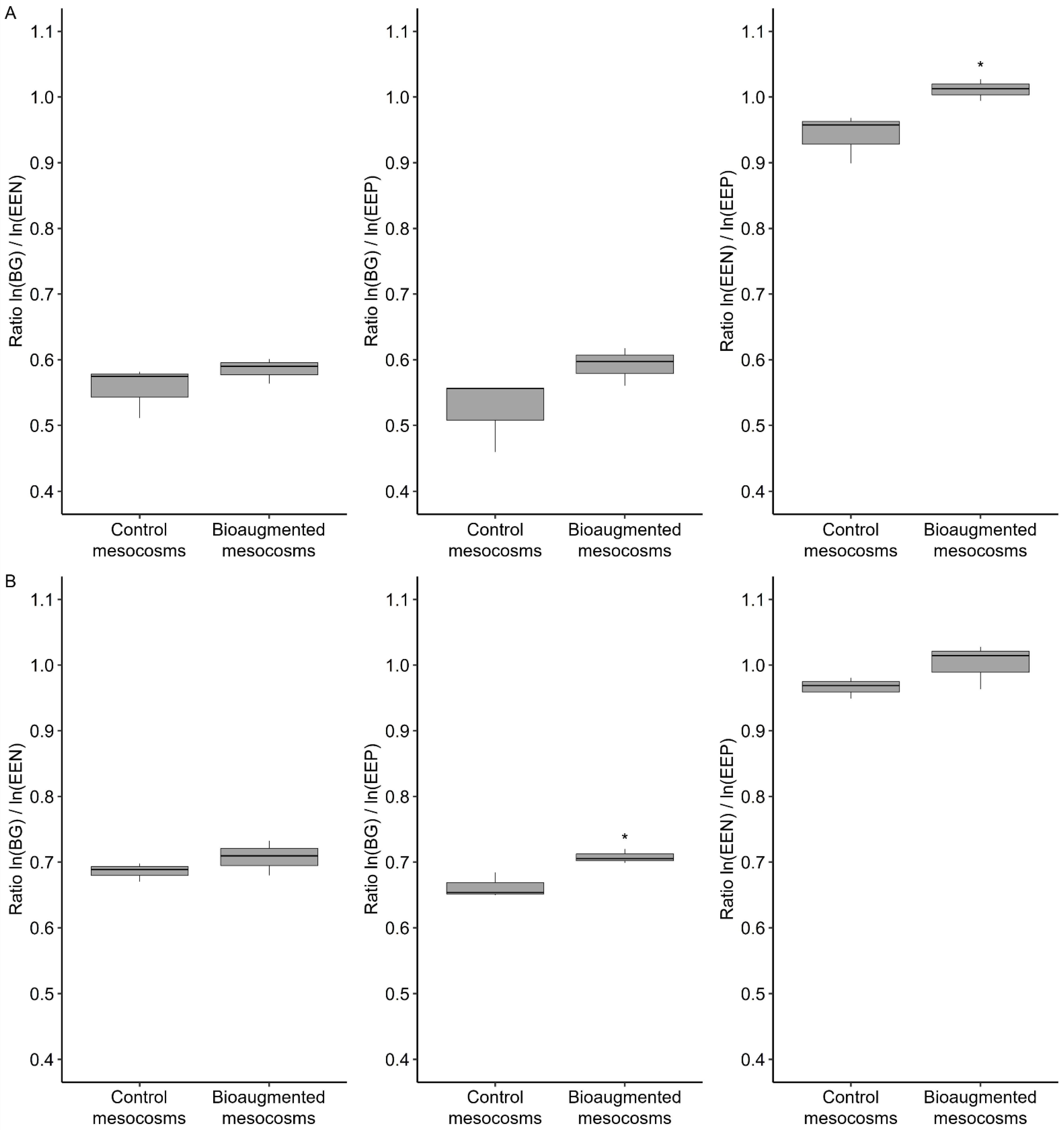
3.3. Soil Hydrolase Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chun, S.C.; Muthu, M.; Hasan, N.; Tasneem, S.; Gopal, J. Mycoremediation of PCBs by Pleurotus Ostreatus: Possibilities and Prospects. Appl. Sci. 2019, 9, 4185. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.K.; Gautam, R.K.; Nanekar, S.V.; Weber, R.; Singh, B.K.; Singh, S.K.; Juwarkar, A.A. Advances and Perspective in Bioremediation of Polychlorinated Biphenyl-Contaminated Soils. Environ. Sci. Pollut. Res. 2018, 25, 16355–16375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Harner, T.; Liu, L.; Zhang, Z.; Ren, N.-Q.; Jia, H.; Ma, J.; Sverko, E. Polychlorinated Biphenyls in Global Air and Surface Soil: Distributions, Air−Soil Exchange, and Fractionation Effect. Environ. Sci. Technol. 2010, 44, 2784–2790. [Google Scholar] [CrossRef] [PubMed]
- Stella, T.; Covino, S.; Čvančarová, M.; Filipová, A.; Petruccioli, M.; D’Annibale, A.; Cajthaml, T. Bioremediation of Long-Term PCB-Contaminated Soil by White-Rot Fungi. J. Hazard. Mater. 2017, 324, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Sage, L.; Périgon, S.; Faure, M.; Gaignaire, C.; Abdelghafour, M.; Mehu, J.; Geremia, R.A.; Mouhamadou, B. Autochthonous Ascomycetes in Depollution of Polychlorinated Biphenyls Contaminated Soil and Sediment. Chemosphere 2014, 110, 62–69. [Google Scholar] [CrossRef]
- Mourier, B.; Desmet, M.; Van Metre, P.C.; Mahler, B.J.; Perrodin, Y.; Roux, G.; Bedell, J.-P.; Lefèvre, I.; Babut, M. Historical Records, Sources, and Spatial Trends of PCBs along the Rhône River (France). Sci. Total Environ. 2014, 476–477, 568–576. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Li, J.; Zhang, G. Polychlorinated Biphenyls in Nepalese Surface Soils: Spatial Distribution, Air-Soil Exchange, and Soil-Air Partitioning. Ecotoxicol. Environ. Saf. 2017, 144, 498–506. [Google Scholar] [CrossRef]
- Géorisques. Available online: https://www.georisques.gouv.fr/risques/sites-et-sols-pollues/donnees#/type=instructions (accessed on 3 March 2021).
- Faroon, O.; Jones, D.; De Rosa, C. Effects of Polychlorinated Biphenyls on the Nervous System. Toxicol. Ind. Health 2000, 16, 305–333. [Google Scholar] [CrossRef] [Green Version]
- Bedard, D.L. A Case Study for Microbial Biodegradation: Anaerobic Bacterial Reductive Dechlorination of Polychlorinated Biphenyls—From Sediment to Defined Medium. Annu. Rev. Microbiol. 2008, 62, 253–270. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Loomis, D.; Grosse, Y.; Ghissassi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. Carcinogenicity of Polychlorinated Biphenyls and Polybrominated Biphenyls. Lancet Oncol. 2013, 14, 287–288. [Google Scholar] [CrossRef] [Green Version]
- Stølevik, S.B.; Nygaard, U.C.; Namork, E.; Haugen, M.; Meltzer, H.M.; Alexander, J.; Knutsen, H.K.; Aaberge, I.; Vainio, K.; van Loveren, H.; et al. Prenatal Exposure to Polychlorinated Biphenyls and Dioxins from the Maternal Diet May Be Associated with Immunosuppressive Effects That Persist into Early Childhood. Food Chem. Toxicol. 2013, 51, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Anitescu, G.; Tavlarides, L.L. Supercritical extraction of contaminants from soils and sediments. J. Supercrit. Fluids 2006, 38, 167–180. [Google Scholar] [CrossRef]
- Sadañoski, M.A.; Benítez, S.F.; Fonseca, M.I.; Velázquez, J.E.; Zapata, P.D.; Levin, L.N.; Villalba, L.L. Mycoremediation of High Concentrations of Polychlorinated Biphenyls with Pleurotus Sajor-Caju LBM 105 as an Effective and Cheap Treatment. J. Environ. Chem. Eng. 2019, 7, 103453. [Google Scholar] [CrossRef]
- Sadañoski, M.A.; Benitez, S.F.; Velázquez, J.E.; Fonseca, M.I.; Zapata, P.D.; Levin, L.N.; Villalba, L.L. Bioprocess Conditions for Treating Mineral Transformer Oils Contaminated with Polychlorinated Biphenyls (PCBs). J. Environ. Chem. Eng. 2020, 8, 104068. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped Potential: Exploiting Fungi in Bioremediation of Hazardous Chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Germain, J.; Raveton, M.; Binet, M.N.; Mouhamadou, B. Screening and Metabolic Potential of Fungal Strains Isolated from Contaminated Soil and Sediment in the Polychlorinated Biphenyl Degradation. Ecotoxicol. Environ. Saf. 2021, 208, 111703. [Google Scholar] [CrossRef]
- Cruz-Izquierdo, R.I.; Paz-González, A.D.; Reyes-Espinosa, F.; Vazquez-Jimenez, L.K.; Salinas-Sandoval, M.; González-Domínguez, M.I.; Rivera, G. Analysis of Phenanthrene Degradation by Ascomycota Fungi Isolated from Contaminated Soil from Reynosa, Mexico. Lett. Appl. Microbiol. 2021. [Google Scholar] [CrossRef]
- Siracusa, G.; Yuan, Q.; Chicca, I.; Bardi, A.; Spennati, F.; Becarelli, S.; Levin, D.B.; Munz, G.; Petroni, G.; Di Gregorio, S. Mycoremediation of Old and Intermediate Landfill Leachates with an Ascomycete Fungal Isolate, Lambertella Sp. Water 2020, 12, 800. [Google Scholar] [CrossRef] [Green Version]
- González-Abradelo, D.; Pérez-Llano, Y.; Peidro-Guzmán, H.; Sánchez-Carbente, M.d.R.; Folch-Mallol, J.L.; Aranda, E.; Vaidyanathan, V.K.; Cabana, H.; Gunde-Cimerman, N.; Batista-García, R.A. First Demonstration That Ascomycetous Halophilic Fungi (Aspergillus Sydowii and Aspergillus Destruens) Are Useful in Xenobiotic Mycoremediation under High Salinity Conditions. Bioresour. Technol. 2019, 279, 287–296. [Google Scholar] [CrossRef]
- Mouhamadou, B.; Faure, M.; Sage, L.; Marçais, J.; Souard, F.; Geremia, R.A. Potential of Autochthonous Fungal Strains Isolated from Contaminated Soils for Degradation of Polychlorinated Biphenyls. Fungal Biol. 2013, 117, 268–274. [Google Scholar] [CrossRef]
- Périgon, S.; Massier, M.; Germain, J.; Binet, M.-N.; Legay, N.; Mouhamadou, B. Metabolic Adaptation of Fungal Strains in Response to Contamination by Polychlorinated Biphenyls. Environ. Sci. Pollut. Res. 2019, 26, 14943–14950. [Google Scholar] [CrossRef]
- Galzy, P.; Slominski, P. Variations Physiologiques de La Levure Au Cours de La Croissance Sur l’acide Lactique Comme Seule Sorce de Carbone. Comptes Rendus Académie Sci. 1957, 245, 2423–2426. [Google Scholar]
- Petrić, I.; Hršak, D.; Fingler, S.; Udiković-Kolić, N.; Bru, D.; Martin-Laurent, F. Insight in the PCB-Degrading Functional Community in Long-Term Contaminated Soil under Bioremediation. J. Soils Sediments 2011, 11, 290–300. [Google Scholar] [CrossRef]
- Viisimaa, M.; Karpenko, O.; Novikov, V.; Trapido, M.; Goi, A. Influence of Biosurfactant on Combined Chemical–Biological Treatment of PCB-Contaminated Soil. Chem. Eng. J. 2013, 220, 352–359. [Google Scholar] [CrossRef]
- AFNOR Groupe. Available online: https://www.boutique.afnor.org/norme/nf-en-16167/sols-biodechets-traites-et-boues-dosage-des-polychlorobiphenyles-pcbs-par-chromatographie-en-phase-gazeuse-spectrometrie-gazeuse/article/906803/fa190960 (accessed on 11 January 2021).
- Gong, P.; Guan, X.; Witter, E. A Rapid Method to Extract Ergosterol from Soil by Physical Disruption. Appl. Soil Ecol. 2001, 17, 285–289. [Google Scholar] [CrossRef]
- Piton, G.; Foulquier, A.; Martínez-García, L.B.; Legay, N.; Hedlund, K.; Martins da Silva, P.; Nascimento, E.; Reis, F.; Sousa, J.P.; De Deyn, G.B.; et al. Disentangling Drivers of Soil Microbial Potential Enzyme Activity across Rain Regimes: An Approach Based on the Functional Trait Framework. Soil Biol. Biochem. 2020, 148, 107881. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: https://pbil.univ-lyon1.fr/CRAN/ (accessed on 3 March 2021).
- Lee, S.-H.; Kim, M.-S.; Kim, J.-G.; Kim, S.-O. Use of Soil Enzymes as Indicators for Contaminated Soil Monitoring and Sustainable Management. Sustainability 2020, 12, 8209. [Google Scholar] [CrossRef]
- Federici, E.; Giubilei, M.; Santi, G.; Zanaroli, G.; Negroni, A.; Fava, F.; Petruccioli, M.; D’Annibale, A. Bioaugmentation of a Historically Contaminated Soil by Polychlorinated Biphenyls with Lentinus Tigrinus. Microb. Cell Factories 2012, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Tigini, V.; Prigione, V.; Di Toro, S.; Fava, F.; Varese, G.C. Isolation and Characterisation of Polychlorinated Biphenyl (PCB) Degrading Fungi from a Historically Contaminated Soil. Microb. Cell Factories 2009, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Vyas, B.R.M.; Šašek, V.; Matucha, M.; Bubner, M. Degradation of 3,3′,4,4′-Tetrachlorobiphenyl by Selected White Rot Fungi. Chemosphere 1994, 28, 1127–1134. [Google Scholar] [CrossRef]
- Dietrich, D.; Hickey, W.J.; Lamar, R. Degradation of 4,4′-Dichlorobiphenyl, 3,3′,4,4′-Tetrachlorobiphenyl, and 2,2′,4,4′,5,5′-Hexachlorobiphenyl by the White Rot Fungus Phanerochaete Chrysosporium. Appl. Environ. Microbiol. 1995, 61, 3904–3909. [Google Scholar] [CrossRef] [Green Version]
- Beaudette, L.A.; Davies, S.; Fedorak, P.M.; Ward, O.P.; Pickard, M.A. Comparison of Gas Chromatography and Mineralization Experiments for Measuring Loss of Selected Polychlorinated Biphenyl Congeners in Cultures of White Rot Fungi. Appl. Environ. Microbiol. 1998, 64, 2020–2025. [Google Scholar] [CrossRef] [Green Version]
- Beaudette, L.A.; Ward, O.P.; Pickard, M.A.; Fedorak, P.M. Low Surfactant Concentration Increases Fungal Mineralization of a Polychlorinated Biphenyl Congener but Has No Effect on Overall Metabolism. Lett. Appl. Microbiol. 2000, 30, 155–160. [Google Scholar] [CrossRef]
- Stella, T.; Covino, S.; Burianová, E.; Filipová, A.; Křesinová, Z.; Voříšková, J.; Větrovský, T.; Baldrian, P.; Cajthaml, T. Chemical and Microbiological Characterization of an Aged PCB-Contaminated Soil. Sci. Total Environ. 2015, 533, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Passatore, L.; Rossetti, S.; Juwarkar, A.A.; Massacci, A. Phytoremediation and Bioremediation of Polychlorinated Biphenyls (PCBs): State of Knowledge and Research Perspectives. J. Hazard. Mater. 2014, 278, 189–202. [Google Scholar] [CrossRef]
- Borja, J.; Taleon, D.M.; Auresenia, J.; Gallardo, S. Polychlorinated Biphenyls and Their Biodegradation. Process Biochem. 2005, 40, 1999–2013. [Google Scholar] [CrossRef]
- Vasilyeva, G.K.; Strijakova, E.R. Bioremediation of Soils and Sediments Contaminated by Polychlorinated Biphenyls. Microbiology 2007, 76, 639–653. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Li, S.; Chen, M.; Chen, H.; Liu, B. Bioleaching Waste Printed Circuit Boards by Acidithiobacillus Ferrooxidans and Its Kinetics Aspect. J. Biotechnol. 2014, 173, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry of Microbial Organic Nutrient Acquisition in Soil and Sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
| Soil | Σ7 Indicator PCBs (mg kg−1) | pH | Total Organic Matter (%) | Composition (%) | ||
|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||
| H | 850 ± 89.00 | 8.21 ± 0.06 | 3.61 ± 0.13 | 20 | 50 | 30 |
| L | 36 ± 1.80 | 7.80 ± 0.50 | 2.55 ± 0.11 | 45 | 35 | 20 |
| PCB Congeners | Control Mesocoms PCBs Residuals (mg kg−1) | Bioaugmented Mesocoms PCBs Residuals (mg kg−1) | p-Value | PCB Depletion (%) |
|---|---|---|---|---|
| 28 | 0.37 ± 0.01 | 0.39 ± 0.08 | 0.8549 | −5.41 |
| 52 | 18.53 ± 0.50 | 24.40 ± 5.81 | 0.4966 | −31.65 |
| 101 | 120.00 ± 4.19 | 72.17 ± 7.87 | 0.02128 * | 39.86 |
| 118 | 52.63 ± 2.23 | 46.97 ± 3.74 | 0.3601 | 10.77 |
| 138 | 234.67 ± 8.85 | 169.33 ± 22.16 | 0.1242 | 27.84 |
| 153 | 235.67 ± 8.28 | 172.33 ± 21.94 | 0.13 | 26.87 |
| 180 | 171.00 ± 7.13 | 118.63 ± 16.06 | 0.1005 | 30.62 |
| PCB Congeners | Control Mesocoms PCBs Residuals (mg kg−1) | Bioaugmented Mesocoms PCBs Residuals (mg kg−1) | p-Value | PCB Depletion (%) |
|---|---|---|---|---|
| 28 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.3868 | −12.5 |
| 52 | 0.24 ± 0.04 | 0.20 ± 0.03 | 0.2406 | 17.61 |
| 101 | 0.960 ± 0.24 | 0.963 ± 0.11 | 0.5827 | −0.26 |
| 118 | 0.410 ± 0.06 | 0.408 ± 0.04 | 0.07827 | 0.61 |
| 138 | 4.99 ± 0.40 | 5.47 ± 0.38 | 0.9593 | −9.74 |
| 153 | 4.31 ± 0.77 | 4.85 ± 0.55 | 0.828 | −12.5 |
| 180 | 4.56 ± 0.41 | 5.63 ± 0.68 | 0.4705 | −23.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germain, J.; Raveton, M.; Binet, M.-N.; Mouhamadou, B. Potentiality of Native Ascomycete Strains in Bioremediation of Highly Polychlorinated Biphenyl Contaminated Soils. Microorganisms 2021, 9, 612. https://doi.org/10.3390/microorganisms9030612
Germain J, Raveton M, Binet M-N, Mouhamadou B. Potentiality of Native Ascomycete Strains in Bioremediation of Highly Polychlorinated Biphenyl Contaminated Soils. Microorganisms. 2021; 9(3):612. https://doi.org/10.3390/microorganisms9030612
Chicago/Turabian StyleGermain, Joaquim, Muriel Raveton, Marie-Noëlle Binet, and Bello Mouhamadou. 2021. "Potentiality of Native Ascomycete Strains in Bioremediation of Highly Polychlorinated Biphenyl Contaminated Soils" Microorganisms 9, no. 3: 612. https://doi.org/10.3390/microorganisms9030612





