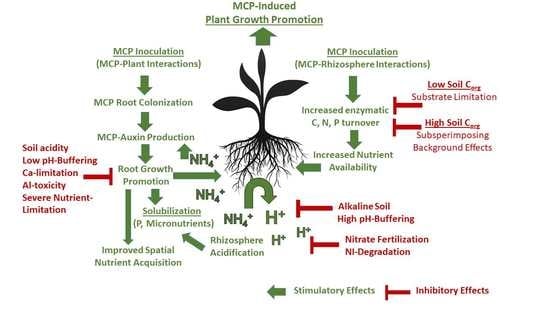
Maize Inoculation with Microbial Consortia: Contrasting Effects on Rhizosphere Activities, Nutrient Acquisition and Early Growth in Different Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Properties
2.2. Fertilization
2.3. Test Plant and Culture Conditions
2.4. MCP Inoculation
2.5. Plant Growth and Nutritional Status
2.6. Expression of Auxin-Responsive Genes in the Root Tissue
2.7. Marker Enzymes as Indicators for C, N and P Cycling in the Rhizosphere
2.8. Experimental Design and Statistical Evaluation
3. Results
3.1. Plant Growth
3.2. Rhizosphere Chemistry
3.3. Plant-Nutritional Status
3.4. MCP Effects on Root Growth and Expression Auf Auxin-Responsive Genes in the Root Tissue
3.5. Phosphate-Solubilizing Potential of the MCP Inoculant
4. Discussion
4.1. Effects of N-Form Supply on MCP Performance on Different Soils
4.2. Limitations of Combined MCP Application with Ammonium Fertilizers
4.3. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bashan, Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol. Adv. 1998, 16, 729–770. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Hartmann, A.; Schmid, M. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O.; Fertilizers, V.S.P. Microbial Consortia: Promising Probiotics as Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 7–12. [Google Scholar] [CrossRef]
- Menzies, N.; Harbison, D.; Dart, P. Soil chemistry-facts and fiction and their influence on the fertilizer decision making process. In Proceedings of the 26th Annual Conference of the Grassland Society of NSW, Bathurst, Australia, 26–28 July 2011; pp. 49–63. [Google Scholar]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N.; Scotti, R. Improving Crop Yield and Nutrient Use Efficiency via Biofertilization - A Global Meta-analysis. Front. Plant Sci. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Nuti, M.; Giovannetti, G. Borderline Products between Bio-fertilizers/Bio-effectors and Plant Protectants: The Role of Microbial Consortia. J. Agric. Sci. Technol. A 2015, 5, 305–315. [Google Scholar] [CrossRef]
- Lopez-Cervantes, J.; Thorpe, D.T. Microbial Composition Comprising Liquid Fertilizer and Processes for Agricultural Use. U.S. Patent 2013/0255338 A1, 3 October 2013. [Google Scholar]
- Liu, H.; White, P.J.; Li, C. Biomass partitioning and rhizosphere responses of maize and faba bean to phosphorus deficiency. Crop Pasture Sci. 2016, 67, 847–856. [Google Scholar] [CrossRef]
- Bradáčová, K.; Kandeler, E.; Berger, N.; Ludewig, U.; Neumann, G. Microbial Consortia Stimulate Early Growth of Maize Depending on N and P Supply. Plant Soil Environm. 2019. submitted. [Google Scholar]
- VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten e.V. Speyer, Germany). Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik Methodenbuch Band I Die Untersuchung von Böden, 4th ed.; VDLUFA Verlag: Darmsatdt, Germany, 1991. [Google Scholar]
- Gericke, S.; Kurmies, B. Die kolorimetrische Phosphorsäure bestimmung mit Ammonium, Vanadat, Molybdat und ihre Anwendung in der Pflanzenanalyse. Z. für Pflanz. Düngung Bodenkd. 1952, 59, 235–247. [Google Scholar]
- Stemmer, M. Multiple-substrate enzyme assays: A useful approach for profiling enzyme activity in soils? Soil Biol. Biochem. 2004, 36, 519–527. [Google Scholar] [CrossRef]
- Chrominski, K.; Tkacz, M. Comparison of outlier detection methods in biomedical data. J. Med. Inform. Technol. 2010, 16, 1–6. [Google Scholar]
- Campbell, R.C. Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States; Southern Cooperative Series Bulletin #394; North Carolina Department of Agriculture and Consumer Services Agronomic Division: Raleigh, NC, USA, 2009; ISBN 1581613946. [Google Scholar]
- Mpanga, I.K.; Gomez-Genao, N.J.; Moradtalab, N.; Wanke, D.; Chrobaczek, V.; Ahmed, A.; Windisch, S.; Geistlinger, J.; Walker, F.; Ludewig, U.; et al. The role of N form supply for PGPM-host plant interactions in maize. J. Plant Nutr. Soil Sci. 2019, in press. [Google Scholar] [CrossRef]
- Park, R.M.; Hasenstein, K.H. Hormone-Induced Gene Expression During Gravicurvature of Brassica Roots. J. Plant Growth Regul. 2015, 1–13. [Google Scholar] [CrossRef]
- Gälweiler, L.; Guan, C.; Müller, A.; Wisman, E.; Mendgen, K.; Yephremov, A.; Palme, K. Regulation of Polar Auxin Transport by AtPIN1 in Arabidopsis Vascular Tissue Regulation of Polar Auxin Transport by AtPIN1 in Arabidopsis Vascular Tissue. Science 1999, 282, 2226–2230. [Google Scholar] [CrossRef]
- Mpanga, I.A.; Nkebiwe, P.M.; Kuhlmann, K.; Cozzolino, V.; Piccolo, A.; Geistlinger, G.; Berger, N.; Ludewig, U.; Neumann, G. The Form of N Supply Determines Plant Growth Promotion by P-Solubilizing Microorganisms in Maize. Microorganisms 2019, 7, 38. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. Root-induced changes in the availability of nutrients in the rhizosphere. In Plant Roots the Hidden Half, 3rd ed.; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Duus, J.; Lekfeldt, S.; Rex, M.; Mercl, F.; Kulhánek, M.; Tlustoš, P.; Magid, J. Effect of bioeffectors and recycled P - fertiliser products on the growth of spring wheat. Chem. Biol. Technol. Agric. 2016, 3, 1–18. [Google Scholar] [CrossRef]
- Thonar, C.; Duus, J.; Lekfeldt, S.; Cozzolino, V.; Kundel, D.; Kulhánek, M.; Mosimann, C.; Neumann, G.; Piccolo, A.; Rex, M.; et al. Potential of three microbial bio - effectors to promote maize growth and nutrient acquisition from alternative phosphorous fertilizers in contrasting soils. Chem. Biol. Technol. Agric. 2017, 4, 1–16. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Dapaah, H.K.; Geistlinger, J.; Ludewig, U. Soil Type-Dependent Interactions of P-Solubilizing Microorganisms with Organic and Inorganic Fertilizers Mediate Plant Growth Promotion in Tomato. Agronomy 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Robinson, D.; Rorison, I.H. Root hairs and plant growth at low nitrogen availabilities. New Phytol. 1987, 107, 681–693. [Google Scholar] [CrossRef]
- Kania, A.; Guldner, M.; Szabo, B.; Kazem, S.; Römheld, V.; Neumann, G.; Morhard, J.; Evers, M.; Terlouw, T. Functional characterization of the stabilized organic turf grass fertilizer ‘Marathon’ . Rasen. Turf. Gazon. 2007, 1, 192–195. [Google Scholar]
- Patil, N.B.; Gajbhiye, M.; Ahiwale, S.S.; Gunjal, A.B.; Kapadnis, B.P. Optimization of Indole 3 acetic acid (IAA) production by Acetobacter diazotrophicus L1 isolated from Sugarcane. Int. J. Environ. Sci. 2011, 2, 295–302. [Google Scholar]
- Bharucha, U.; Patel, K.; Trivedi, U.B. Optimization of Indole Acetic Acid Production by Pseudomonas putida UB1 and its Effect as Plant Growth-Promoting Rhizobacteria on Mustard (Brassica nigra). Agric. Res. 2013, 2, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009. [Google Scholar] [CrossRef]
- Hartmann, A.; Rothballer, M.; Hense, B.A.; Schröder, P. Bacterial quorum sensing compounds are important modulators of microbe-plant interactions. Front. Plant Sci. 2014. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C. Revisiting bacterial volatile-mediated plant growth promotion: Lessons from the past and objectives for the future. Ann. Bot. 2018, 122, 349–358. [Google Scholar] [CrossRef]
- Petrášek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef] [Green Version]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Mu, E.; Raya-gonz, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2015, 209, 1496–1512. [Google Scholar] [CrossRef]
- Vinci, G.; Cozzolino, V.; Mazzei, P.; Monda, H.; Spaccini, R.; Piccolo, A. An alternative to mineral phosphorus fertilizers: The combined effects of Trichoderma harzianum and compost on Zea mays, as revealed by 1 H NMR and GC-MS metabolomics. PLoS ONE 2018. [Google Scholar] [CrossRef]
- Vinci, G.; Cozzolino, V.; Mazzei, P.; Monda, H.; Savy, D.; Drosos, M.; Piccolo, A. Effects of Bacillus amyloliquefaciens and different phosphorus sources on Maize plants as revealed by NMR and GC-MS based metabolomics. Plant Soil 2018, 429, 437–450. [Google Scholar] [CrossRef]
- Bradáčová, K.; Florea, A.S.; Bar-Tal, A.; Minz, D.; Yermiyahu, U.; Shawahna, R.; Kraut-Cohen, J.; Zolti, A.; Erel, R.; Dietel, K.; et al. Microbial Consortia versus Single-Strain Ionculants: An Advantage in PGPM-Assisted Tomato Production? Agronomy 2019. [Google Scholar] [CrossRef]
- Benckiser, G.; Christ, E.; Herbert, T.; Weiske, A.; Blome, J.; Hardt, M. The nitrification inhibitor 3, 4-dimethylpyrazole-phosphat (DMPP)- quantification and effects on soil metabolism. Plant Soil 2013, 1–11. [Google Scholar] [CrossRef]
- Bradáčová, K.; Weber, N.F.; Talab, N.M.; Asim, M.; Imran, M.; Weinmann, M.; Neumann, G. Micronutrients (Zn/Mn), seaweed extracts, and plant growth - promoting bacteria as cold - stress protectants in maize. Chem. Biol. Technol. Agric. 2016, 1–10. [Google Scholar] [CrossRef]
- Moradtalab, N.; Weinmann, M.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Silicon Improves Chilling Tolerance During Early Growth of Maize by Effects on Micronutrient Homeostasis and Hormonal Balances. Front. Plant Sci. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pin, M.A.; Hoekenga, O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Review NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Emanuelsson, J. Root growth and calcium uptake in relation to calcium concentration. Plant Soil 1984, 78, 325–334. [Google Scholar] [CrossRef]
- Njoku, B.O.; Enwezor, W.O.; Onyenakwe, B.I. Calcium deficiency identified as an important factor limiting maize growth in acid ultisols of eastern Nigeria Laboratory incubation. Fertil. Res. 1987, 14, 113–123. [Google Scholar] [CrossRef]
- Jakli-Hauer, M.; Tränkner, M. Critical Leaf Magnesium Thresholds and the Impact of Magnesium on Plant Growth and Photo-Oxidative Defense: A Systematic Review and Meta-Analysis from 70 Years of Research. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Jing, J.; Rui, Y.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Res. 2010, 119, 355–364. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Weinmann, M.; Müller, T. Improving fertilizer-depot exploitation and maize growth by inoculation with plant growth-promoting bacteria: From lab to field. Chem. Biol. Technol. Agric. 2016, 3, 1–16. [Google Scholar] [CrossRef]


| Plant Response | MCP Treatments | Soil 1 | Soil 2 | ||
|---|---|---|---|---|---|
| NO3− | NH4+ | NO3− | NH4+ | ||
| Shoot DW [g] | no MCP | 3.32 ± 0.3 a | 3.96 ± 0.2 a | 2.09 ± 0.4 b | 2.63 ± 0.3 b |
| with MCP | 3.37 ± 0.1 ab | 3.90 ± 0.2 a | 2.28 ± 0.5 b | 3.4 ± 0.3 a | |
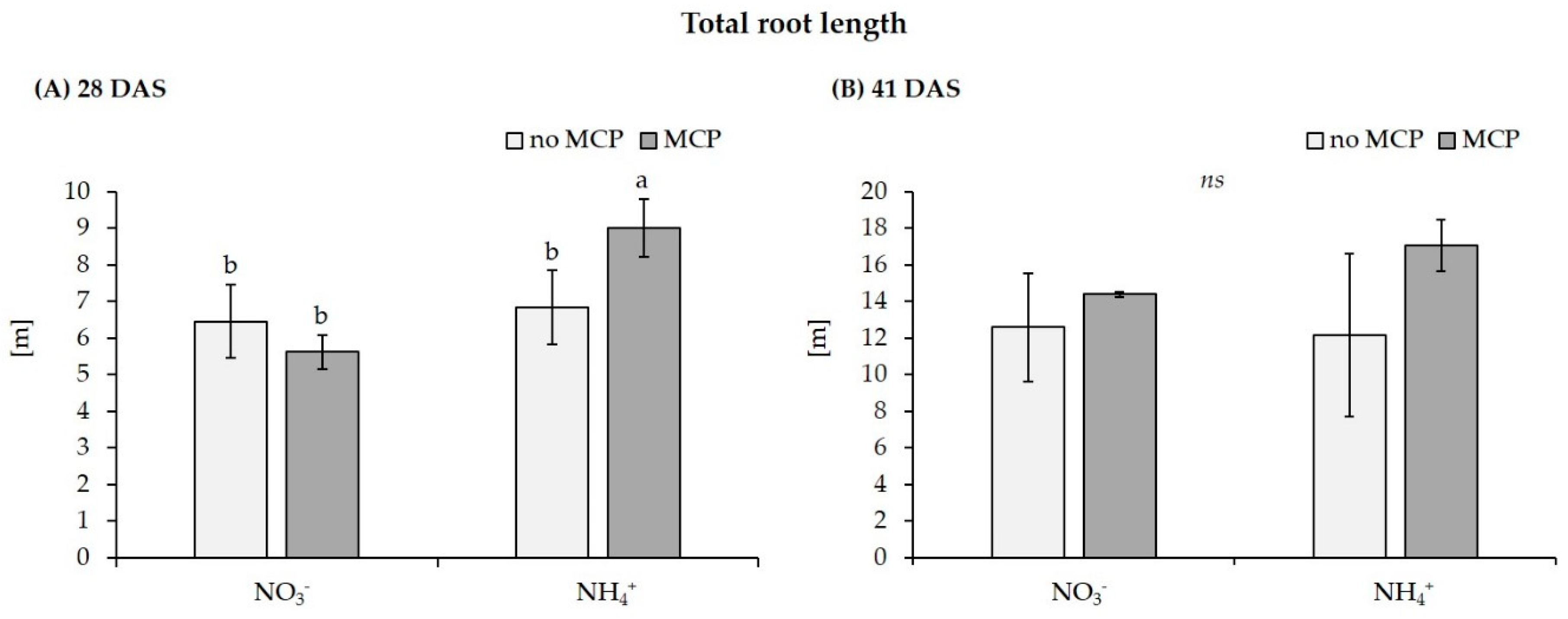
| Total root length [cm] | no MCP | 2718.9 ± 787.0 a | 1048.3 ± 170.2 b | 6454.6 ± 2954.1 b | 6836.5 ± 4455.3 b |
| with MCP | 2325.3 ± 232.9 a | 988.9 ± 448.6 b | 5615.1 ± 132.0 b | 9008.4 ± 1409.8 a | |
| P content [mg plant−1] | no MCP | 6.05 ± 0.8 b | 8.10 ± 0.4 a | 2.89 ± 0.5 b | 3.67 ± 0.4 b |
| with MCP | 6.50 ± 0.4 b | 7.60 ± 0.6 a | 2.95 ± 0.8 b | 4.93 ± 0.4 a | |
| MCP Treatments | Soil 1 | Soil 2 | |||
|---|---|---|---|---|---|
| NO3− | NH4+ | NO3− | NH4+ | ||
| no MCP | 5.11 ± 0.1 a | 4.60 b ± 0.1 | 5.79 ± 0.02 a | 5.35 ± 0.04 b | |
| With MCP | 5.42 ± 0.4 a | 5.11 ± 0.2 a | 5.79 ± 0.01 a | 5.32 ± 0.06 b | |
| Rhizosphere Enzymatic Activities [nmol g−1 soil h−1] | |||||
| Peptidase | no MCP | 42.01 ± 10.9 | 36.34 ± 6.2 | 160.28 ± 12.2 | 140.81 ± 6.6 |
| with MCP | 47.92 ± 0.3 | 49.54 ± 1.6 * | 142.60 ± 6.8 | 144.42 ± 11.9 | |
| Cellulase | no MCP | 8.49 ± 0.9 | 7.67 ± 0.7 | 51.34 ± 5.5 | 52.28 ± 4.2 |
| with MCP | 9.74 ± 1.0 | 8.15 ± 1.1 | 52.10 ± 1.1 | 48.19 ± 6.6 | |
| Acid Phosphatase | no MCP | 111.76 ± 7.1 | 131.78 ± 22.2 | 964.96 ± 128.1 | 891.49 ± 28.0 |
| with MCP | 125.62 ± 4.3 * | 182.89 ± 19.5 * | 948.89 ± 45.2 | 934.45 ± 125.2 | |
| Alkaline Phosphatase | no MCP | 12.45 ± 3.6 | 7.0 ± 0.5 | 122.56 ± 18.8 | 113.96 ± 17.2 |
| with MCP | 13.15 ± 2.3 | 8.98 ± 1.1 * | 113.66 ± 17.3 | 127.16 ± 23.4 | |
| Shoot Mineral Concentration (g kg DM−1) | |||||||
|---|---|---|---|---|---|---|---|
| N Forms | MCP Treatments | N | P | K | Ca | Mg | |
| Soil 1 | NO3− | no MCP | 26.21 a | 1.87 bc | 51.19 a | 3.94 a | 1.99 a |
| with MCP | 25.25 a | 1.82 c | 49.22 a | 3.95 a | 1.99 a | ||
| NH4+ | no MCP | 26.75 a | 2.10 a | 50.38 a | 2.73 b | 1.82 b | |
| with MCP | 27.37 a | 2.01 ab | 49.55 a | 2.75 b | 1.74 b | ||
| Soil 2 | NO3− | no MCP | 37.54 a | 1.38 a | 36.75 b | 4.92 a | 2.71 a |
| with MCP | 35.42 b | 1.24 b | 36.61 b | 4.81 a | 2.65 a | ||
| NH4+ | no MCP | 37.50 a | 1.42 a | 38.60 ab | 4.15 b | 2.33 b | |
| with MCP | 37.73 a | 1.45 a | 39.88 a | 4.00 b | 2.12 c | ||
| Deficiency Threshold [16] | 30.00 | 3.00 | 20.00 | 2.50 | 1.50 | ||
| Shoot Mineral Content (mg Plant−1) | |||||||
|---|---|---|---|---|---|---|---|
| N Form | MCP Treatments | N | P | K | Ca | Mg | |
| Soil 1 | NO3− | no MCP | 88.62 a | 6.05 b | 163.7 b | 12.67 ab | 6.42 a |
| with MCP | 90.96 a | 6.54 b | 177.3 ab | 14.60 a | 7.28 a | ||
| NH4+ | no MCP | 104.7 a | 8.13 a | 195.9 a | 10.57 b | 6.87 a | |
| with MCP | 103.2 a | 7.59 a | 186.9 a | 10.40 b | 6.87 a | ||
| Soil 2 | NO3− | no MCP | 78.10 b | 2.89 b | 76.66 b | 10.23 b | 5.40 a |
| with MCP | 80.64 b | 2.95 b | 83.79 b | 10.98 ab | 5.84 a | ||
| NH4+ | no MCP | 98.67 b | 3.67 b | 101.7 b | 10.93 ab | 5.87 a | |
| with MCP | 128.1 a | 4.93 a | 135.5 a | 13.57 a | 7.21 a | ||
| N Form | MCP Treatments | Nmin Total | Soil NO3−-N | Soil NH4+-N | |
|---|---|---|---|---|---|
| 28 DAS | NO3− | no MCP | 60.45 ± 5.1a | 60.45 ± 5.1 a | 0 ± 0 c |
| with MCP | 45.46 ± 2.8 b | 45.46 ± 2.8 b | 0 ± 0 c | ||
| NH4+ | no MCP | 42.58 ± 4.1 b | 42.12 ± 4.1 b | 0.46 ± 0.05 a | |
| with MCP | 19.34 ± 0.5 c | 19.26 ± 0.4 c | 0.08 ± 0.04 b | ||
| 41 DAS | NO3− | no MCP | 2.73 ± 1.0 b | 2.67 ± 0.9 bc | 0.06 ± 0.02c |
| with MCP | 3.72 ± 4.4 b | 3.69 ± 4.4 b | 0.06 ± 0.04c | ||
| NH4+ | no MCP | 7.09 ± 1.6 a | 6.8 ± 1.4 a | 0.29 ± 0.05b | |
| with MCP | 2.42 ± 0.2 b | 2.06 ± 0.1 bc | 0.39 ± 0.04 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradáčová, K.; Sittinger, M.; Tietz, K.; Neuhäuser, B.; Kandeler, E.; Berger, N.; Ludewig, U.; Neumann, G. Maize Inoculation with Microbial Consortia: Contrasting Effects on Rhizosphere Activities, Nutrient Acquisition and Early Growth in Different Soils. Microorganisms 2019, 7, 329. https://doi.org/10.3390/microorganisms7090329
Bradáčová K, Sittinger M, Tietz K, Neuhäuser B, Kandeler E, Berger N, Ludewig U, Neumann G. Maize Inoculation with Microbial Consortia: Contrasting Effects on Rhizosphere Activities, Nutrient Acquisition and Early Growth in Different Soils. Microorganisms. 2019; 7(9):329. https://doi.org/10.3390/microorganisms7090329
Chicago/Turabian StyleBradáčová, Klára, Maximilian Sittinger, Katharina Tietz, Benjamin Neuhäuser, Ellen Kandeler, Nils Berger, Uwe Ludewig, and Günter Neumann. 2019. "Maize Inoculation with Microbial Consortia: Contrasting Effects on Rhizosphere Activities, Nutrient Acquisition and Early Growth in Different Soils" Microorganisms 7, no. 9: 329. https://doi.org/10.3390/microorganisms7090329