Changes in Oral Microbial Ecology of C57BL/6 Mice at Different Ages Associated with Sampling Methodology
Abstract
:1. Introduction
2. Materials and Methods
3. Results
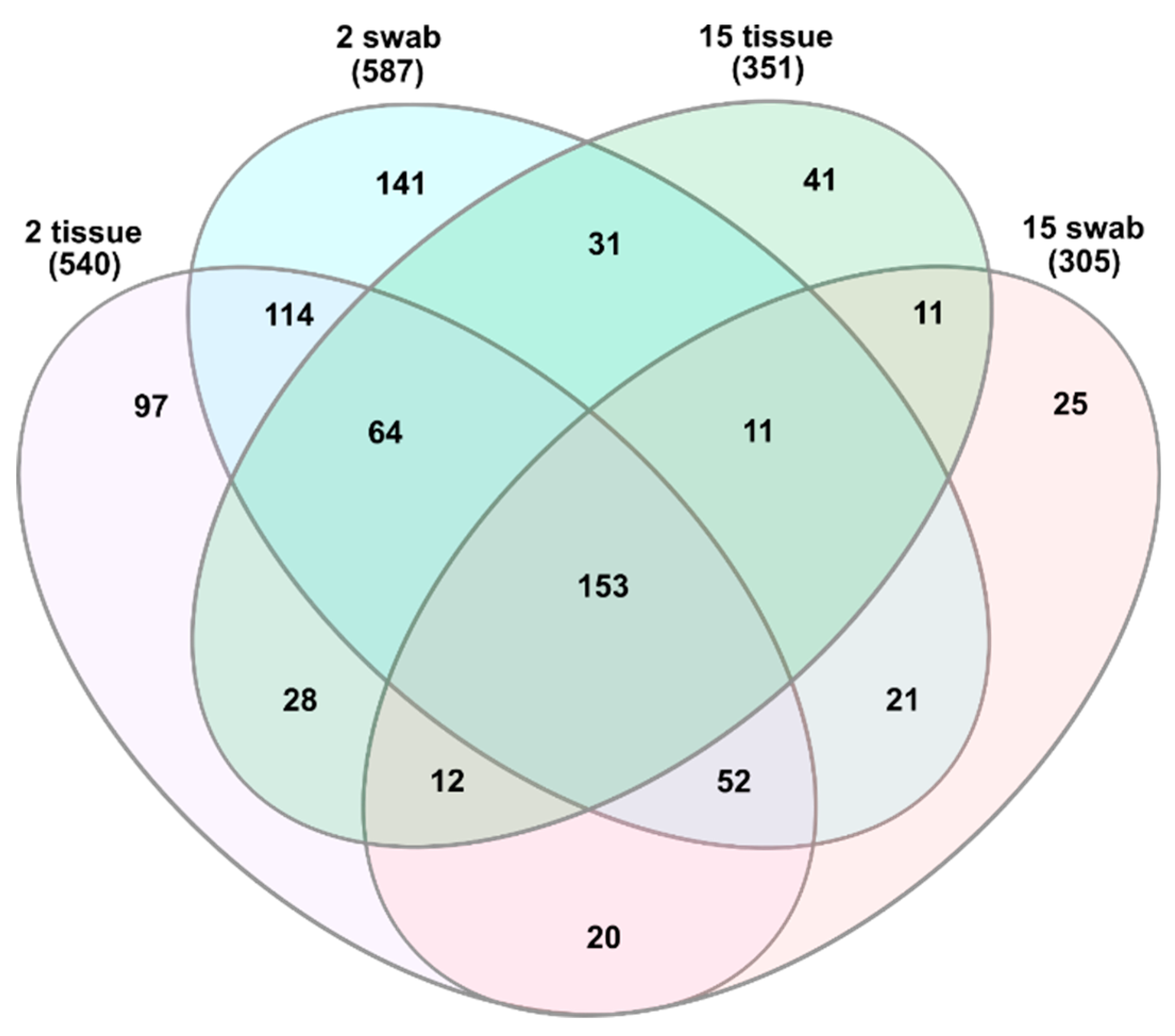
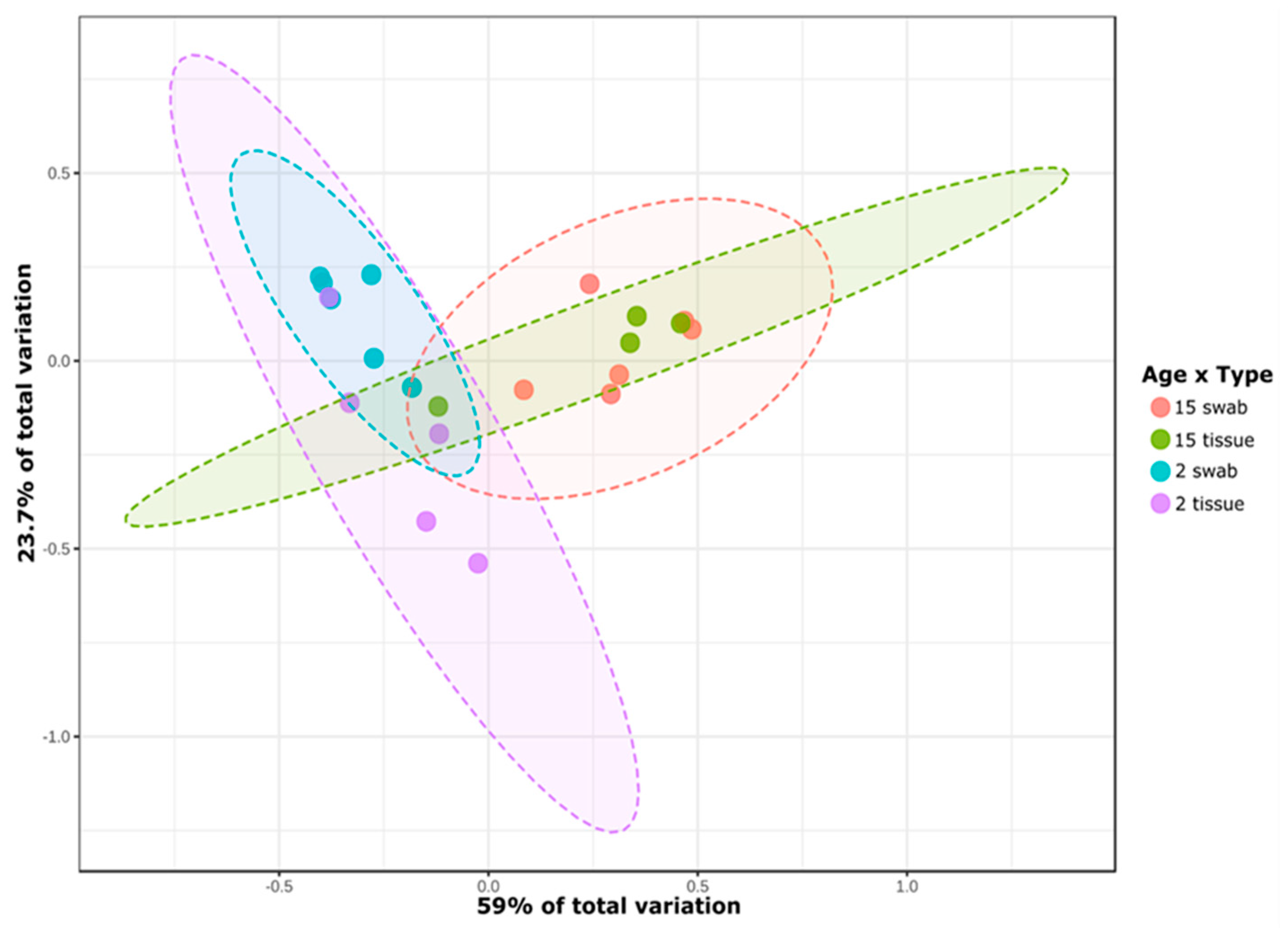
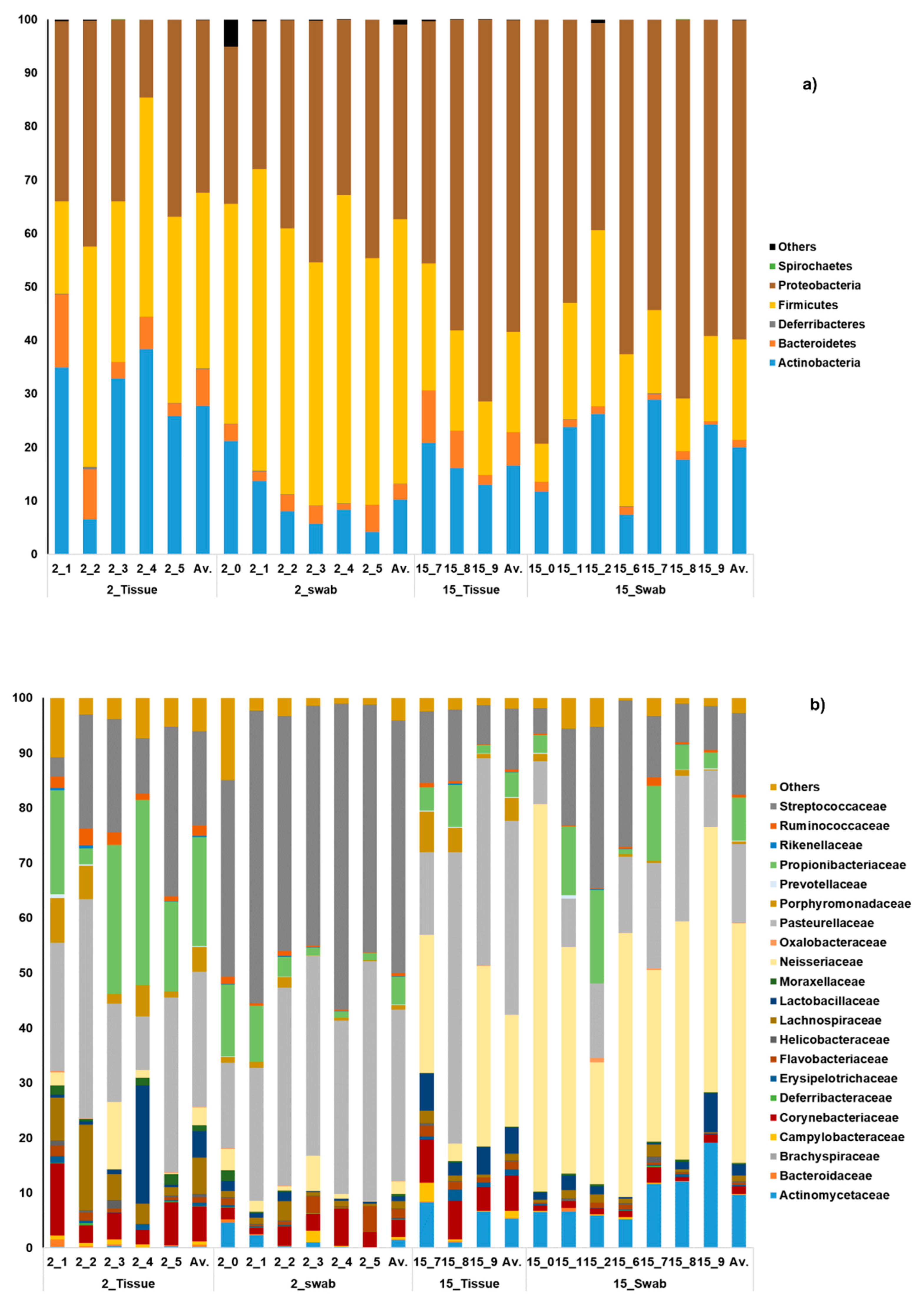
3.1. General Microbial Composition Analysis from the Oral Bacterial Community at Different Ages Using Cotton Swab and Tissue Biopsies
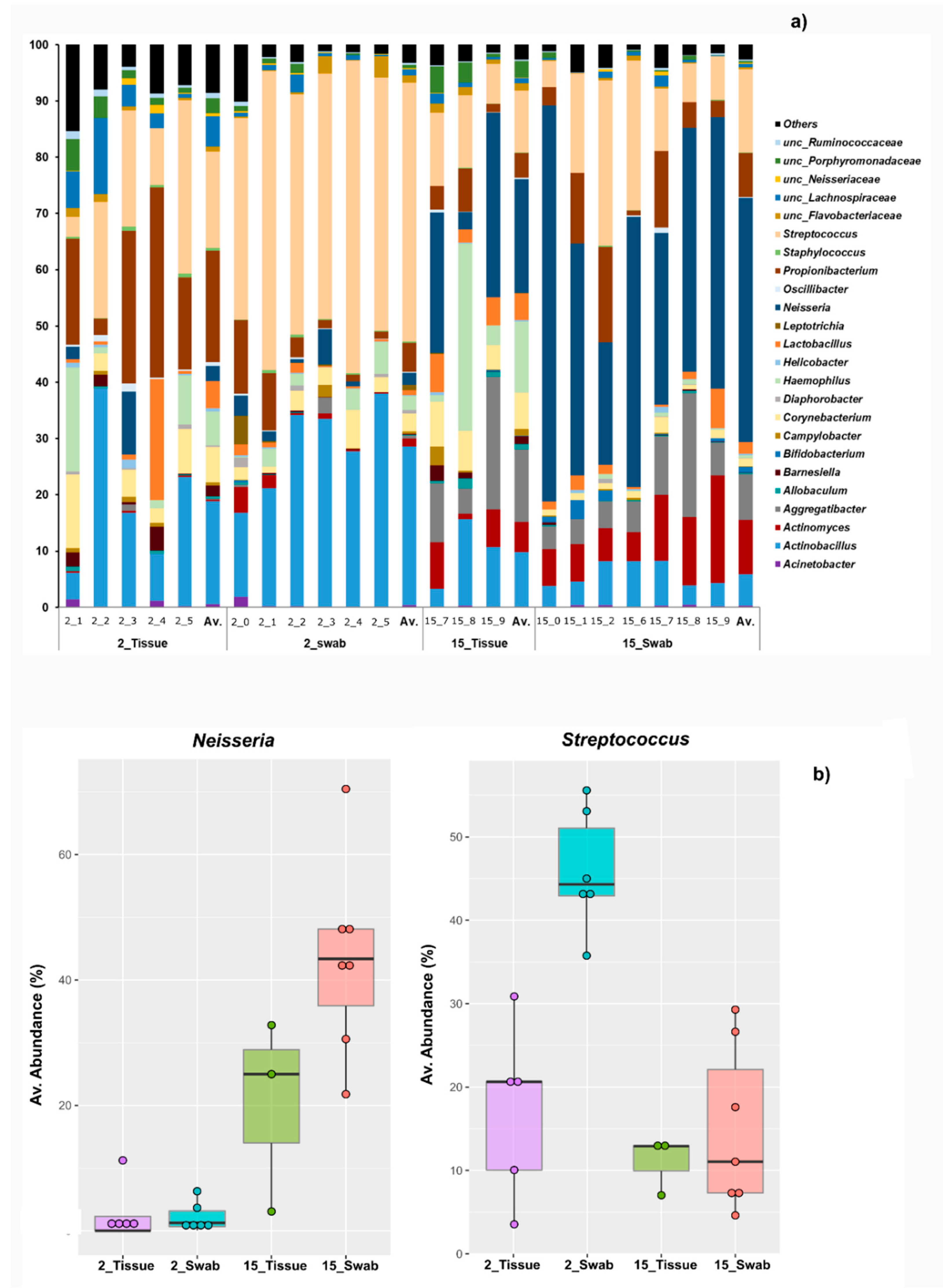
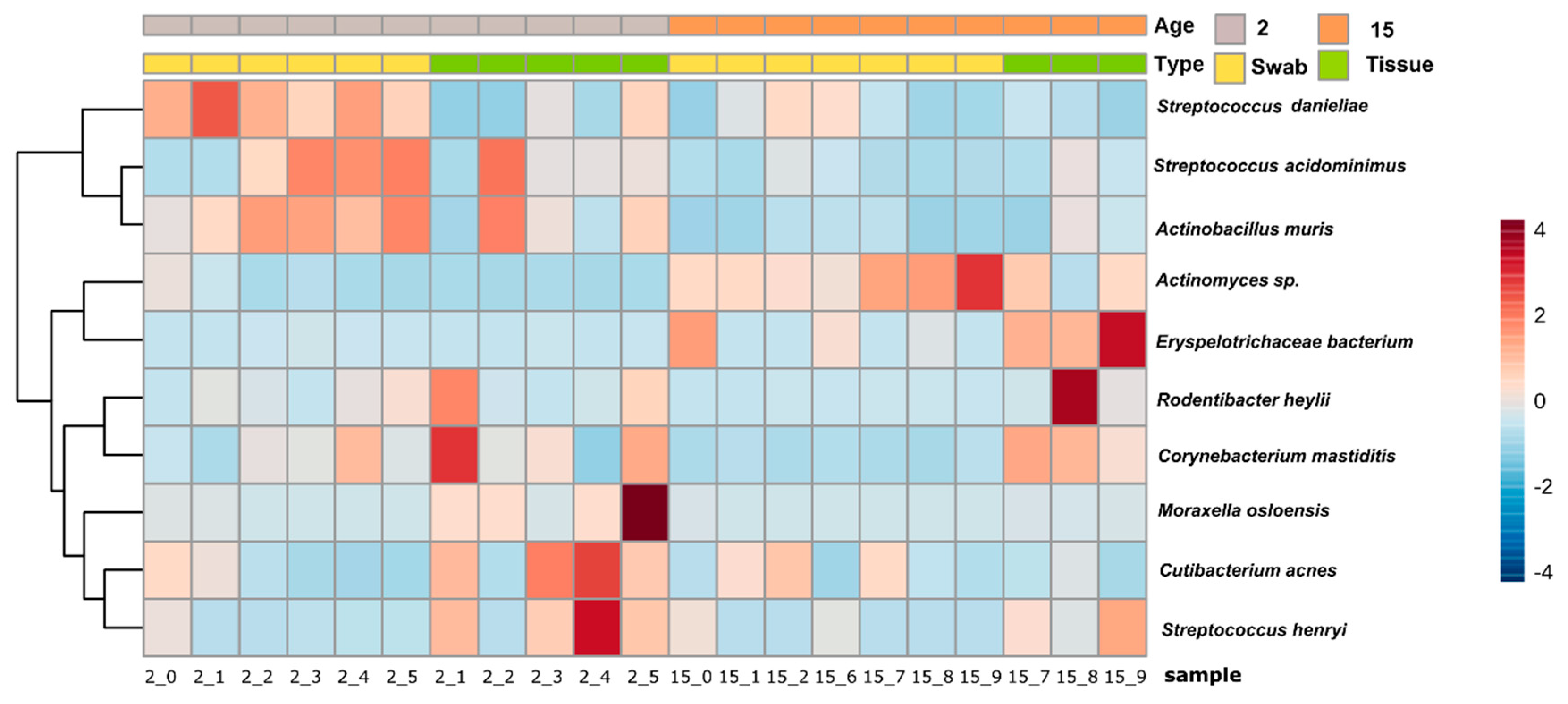
3.2. Taxonomical Bacterial Variation Related to Sampling Approaches and Aging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Vos, W.M.; de Vos, E.A. Role of the intestinal microbiome in health and disease: From correlation to causation. Nutr. Rev. 2012, 70, S45–S56. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Human Microbiome Project Consortium; Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [Green Version]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, R.J. Composition and development of oral bacterial communities. Periodontol. 2000 2014, 64, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Teles, F.; Teles, R.; Figueiredo, L.C.; Faveri, M. The subgingival periodontal microbiota of the aging mouth. Periodontol. 2000 2016, 72, 30–53. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, A.; Morley, J.E. The clinical significance of gastrointestinal changes with aging. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 651–660. [Google Scholar] [CrossRef]
- Xu, F.; Laguna, L.; Sarkar, A. Aging-related changes in quantity and quality of saliva: Where do we stand in our understanding? J. Texture Stud. 2019, 50, 27–35. [Google Scholar] [CrossRef]
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef]
- McInnes, P.; Cutting, M. Manual of Procedures for Human Microbiome Project, Version 12.0 (dbGaP ID: phd003190). Available online: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/document.cgi?study_id=phs000228.v3.p1&phd=3190 (accessed on 5 March 2019).
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Westwater, C.; Xiao, E.; Corrêa, J.D.; Xiao, W.M.; Graves, D.T. Establishment of oral bacterial communities in germ-free mice and the influence of recipient age. Mol. Oral Microbiol. 2018, 33, 38–46. [Google Scholar] [CrossRef]
- Xiao, E.; Mattos, M.; Vieira, G.H.A.; Chen, S.; Corrêa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe 2017, 22, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Palacios, A.; Aladyshkina, N.; Ezeji, J.C.; Erkkila, H.L.; Conger, M.; Ward, J.; Webster, J.; Cominelli, F. ‘Cyclical Bias’ in Microbiome Research Revealed by A Portable Germ-Free Housing System Using Nested Isolation. Sci. Rep. 2018, 8, 3801. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Kaewtapee, C.; Burbach, K.; Tomforde, G.; Hartinger, T.; Camarinha-Silva, A.; Heinritz, S.; Seifert, J.; Wiltafsky, M.; Mosenthin, R.; Rosenfelder-Kuon, P. Effect of Bacillus subtilis and Bacillus licheniformis supplementation in diets with low- and high-protein content on ileal crude protein and amino acid digestibility and intestinal microbiota composition of growing pigs. J. Anim. Sci. Biotechnol. 2017, 8, 37. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.-H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial, 1st ed.; PRIMER-E Ltd.: Devon, UK, 2015. [Google Scholar]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- Hughes, B.D. The influence of factors other than pollution on the value of Shannon’s diversity index for benthic macro-invertebrates in streams. Water Res. 1978, 12, 359–364. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Kostic, A.D.; Howitt, M.R.; Garrett, W.S. Exploring host–microbiota interactions in animal models and humans. Genes Dev. 2013, 27, 701–718. [Google Scholar] [CrossRef]
- Biagi, E.; Candela, M.; Fairweather-Tait, S.; Franceschi, C.; Brigidi, P. Ageing of the human metaorganism: The microbial counterpart. Age 2012, 34, 247–267. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Jilka, R.L. The Relevance of Mouse Models for Investigating Age-Related Bone Loss in Humans. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1209–1217. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontol. 2000 2016, 70, 80–92. [Google Scholar] [CrossRef]
- Wopereis, H.; Oozeer, R.; Knipping, K.; Belzer, C.; Knol, J. The first thousand days—Intestinal microbiology of early life: Establishing a symbiosis. Pediatr. Allergy Immunol. 2014, 25, 428–438. [Google Scholar] [CrossRef]
- Xu, J.; Galley, J.D.; Bailey, M.T.; Thomas-Ahner, J.M.; Clinton, S.K.; Olivo-Marston, S.E. The Impact of Dietary Energy Intake Early in Life on the Colonic Microbiota of Adult Mice. Sci. Rep. 2016, 6, 19083. [Google Scholar] [CrossRef]
- Bik, E.M.; Long, C.D.; Armitage, G.C.; Loomer, P.; Emerson, J.; Mongodin, E.F.; Nelson, K.E.; Gill, S.R.; Fraser-Liggett, C.M.; Relman, D.A. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010, 4, 962–974. [Google Scholar] [CrossRef]
| Phyla | Genera | Cotton Swab | Tissue Biopsy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 Months | 15 Months | 2 Months | 15 Months | p-Value | ||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| Actinobacteria | Propionibacterium | 5.1 * | −0.3–34.2 | 7.8 | 1.9–13.7 | 19.8 * | 5.2–34.3 | 4.4 | 1.9–13.7 | 0.05 |
| Firmicutes | Streptococcus | 45.9 * | 38.3–53.5 | 14.8 | 5.6–23.9 | 17.1 * | 4–30 | 10.9 | 5.6–23.9 | 0.00 |
| Firmicutes | unc_Ruminococcaceae | 0.3 * | 0.4–1.4 | 0.2 | 0.1–0.3 | 0.9 * | 0–0–6 | 0.2 | 0.1–0.4 | 0.02 |
| Proteobacteria | Neisseria | 2.1 | −0.3–4–6 | 43.3 * | 29.1–57.5 | 2.7 | −3.3–8.7 | 20.2 * | 29.1–57.5 | 0.03 |
| Bacteroidetes | unc_Porphyromonadaceae | 0.6 | 0.03–1.3 | 0.3 * | −0.03–0.7 | 2.6 | 0.1–5.2 | 2.9 * | −0.03–0.7 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Arriaga, A.; Baumann, A.; Witte, O.W.; Frahm, C.; Bergheim, I.; Camarinha-Silva, A. Changes in Oral Microbial Ecology of C57BL/6 Mice at Different Ages Associated with Sampling Methodology. Microorganisms 2019, 7, 283. https://doi.org/10.3390/microorganisms7090283
Hernández-Arriaga A, Baumann A, Witte OW, Frahm C, Bergheim I, Camarinha-Silva A. Changes in Oral Microbial Ecology of C57BL/6 Mice at Different Ages Associated with Sampling Methodology. Microorganisms. 2019; 7(9):283. https://doi.org/10.3390/microorganisms7090283
Chicago/Turabian StyleHernández-Arriaga, Angélica, Anja Baumann, Otto W. Witte, Christiane Frahm, Ina Bergheim, and Amélia Camarinha-Silva. 2019. "Changes in Oral Microbial Ecology of C57BL/6 Mice at Different Ages Associated with Sampling Methodology" Microorganisms 7, no. 9: 283. https://doi.org/10.3390/microorganisms7090283





