Unveiling Antibacterial Potential and Physiological Characteristics of Thermophilic Bacteria Isolated from a Hot Spring in Iran
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reference Strains
2.2. Sampling of Mahallat Hot Spring
2.3. Isolation of Thermophilic Bacteria
2.4. Screening Thermophilic Bacterial Isolates for Potential Antibacterial Activity
2.5. Phenotypic Characterizations
2.6. Assessment of Extracellular Enzyme Production by Selected Strains
2.7. Molecular Identification of Promising Isolates by 16S rRNA Gene Amplification and Sequence Analysis
2.8. Growth Kinetics of Antibacterial-Producing Strains
2.9. Physiological Characterizations
2.10. Heat Stability of Cell-Free Supernatant
3. Results
3.1. Isolation and Screening of Thermophilic Bacteria with Antibacterial Activity
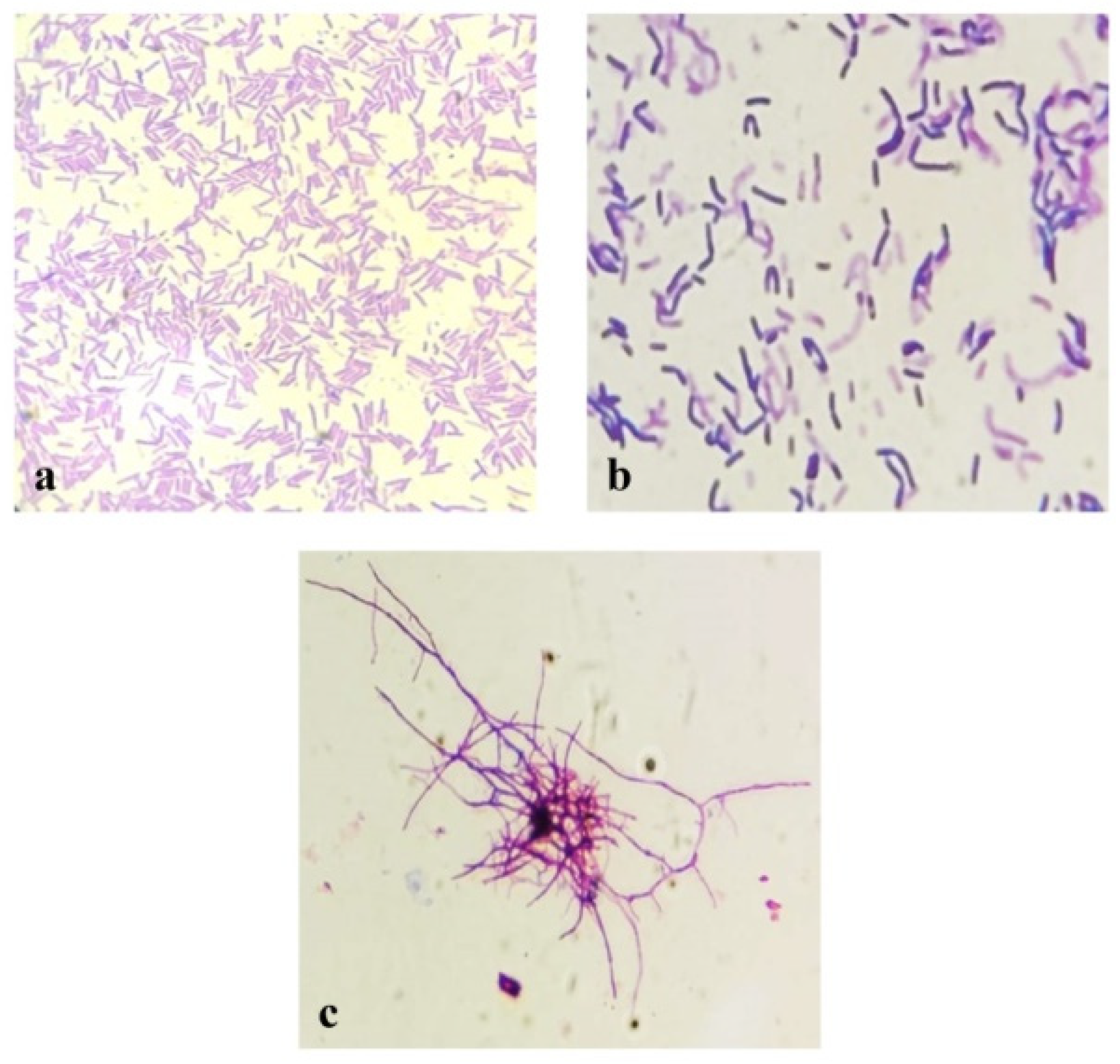
3.2. Phenotypic Characterizations of Selected Thermophilic Bacterial Isolates
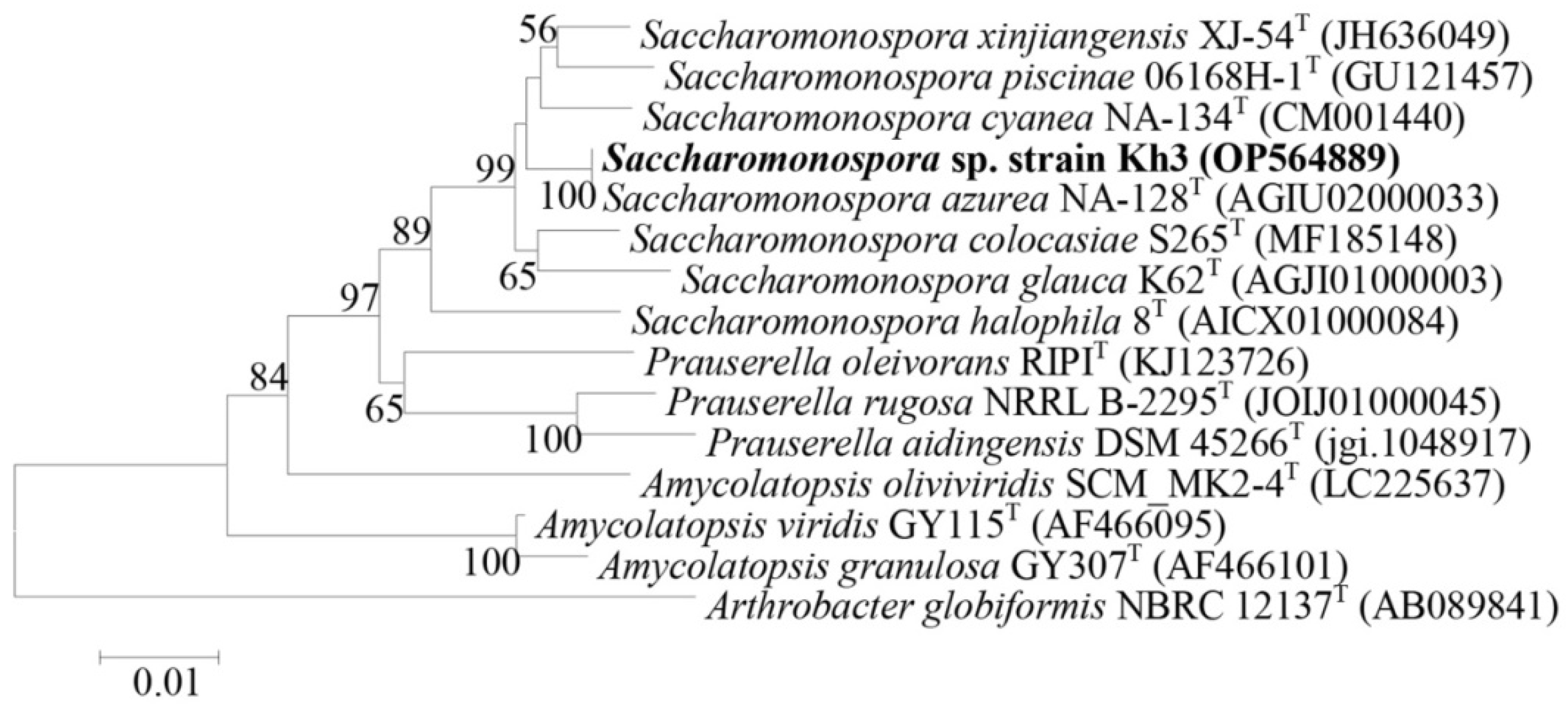
3.3. Molecular Identification and Phylogenetic Analysis of Selected Thermophilic Bacterial Isolates
3.4. Growth Kinetics of Selected Thermophilic Strains and Their Antibacterial Activity
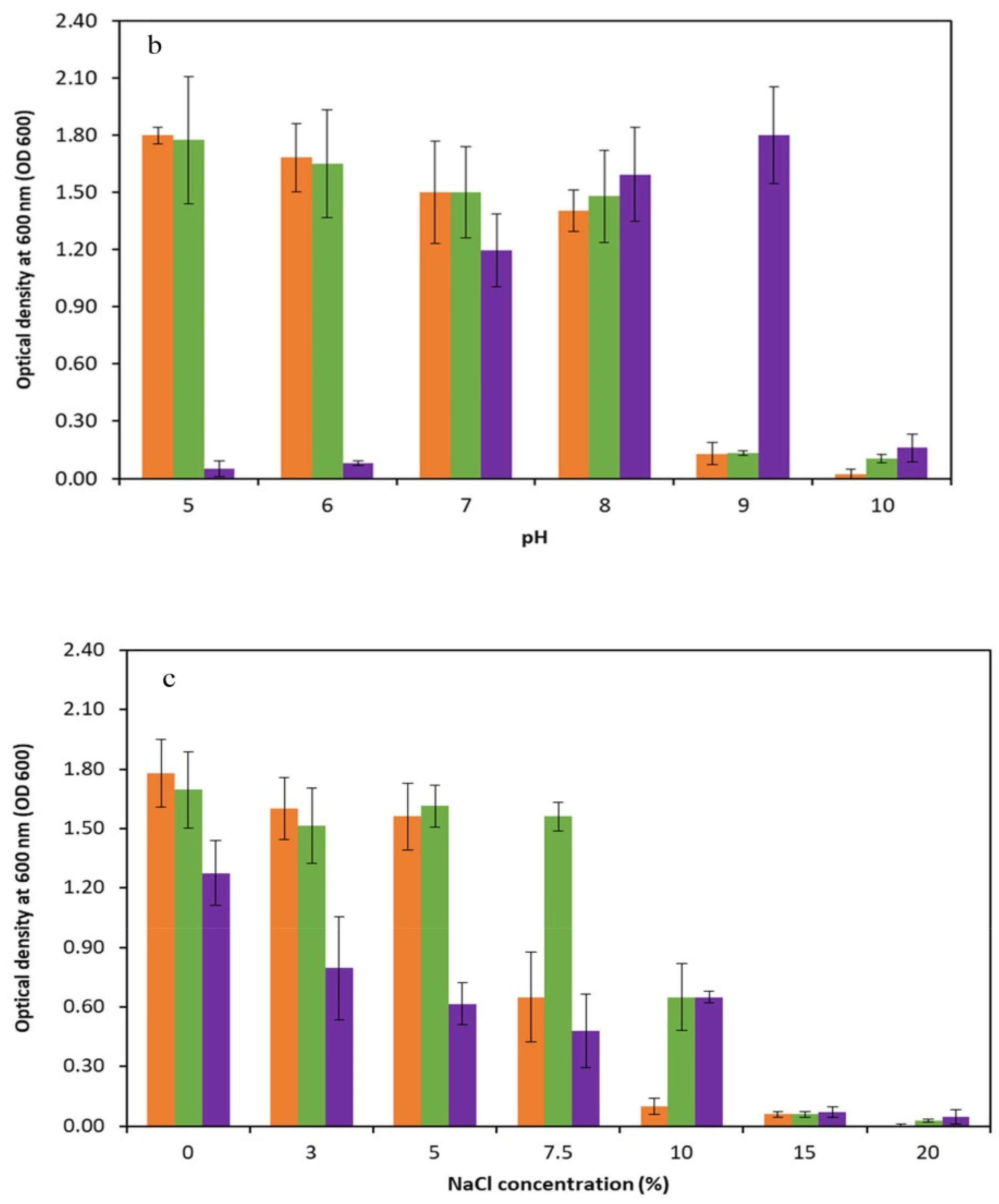
3.5. Physiological Characterizations of Selected Thermophilic Strains
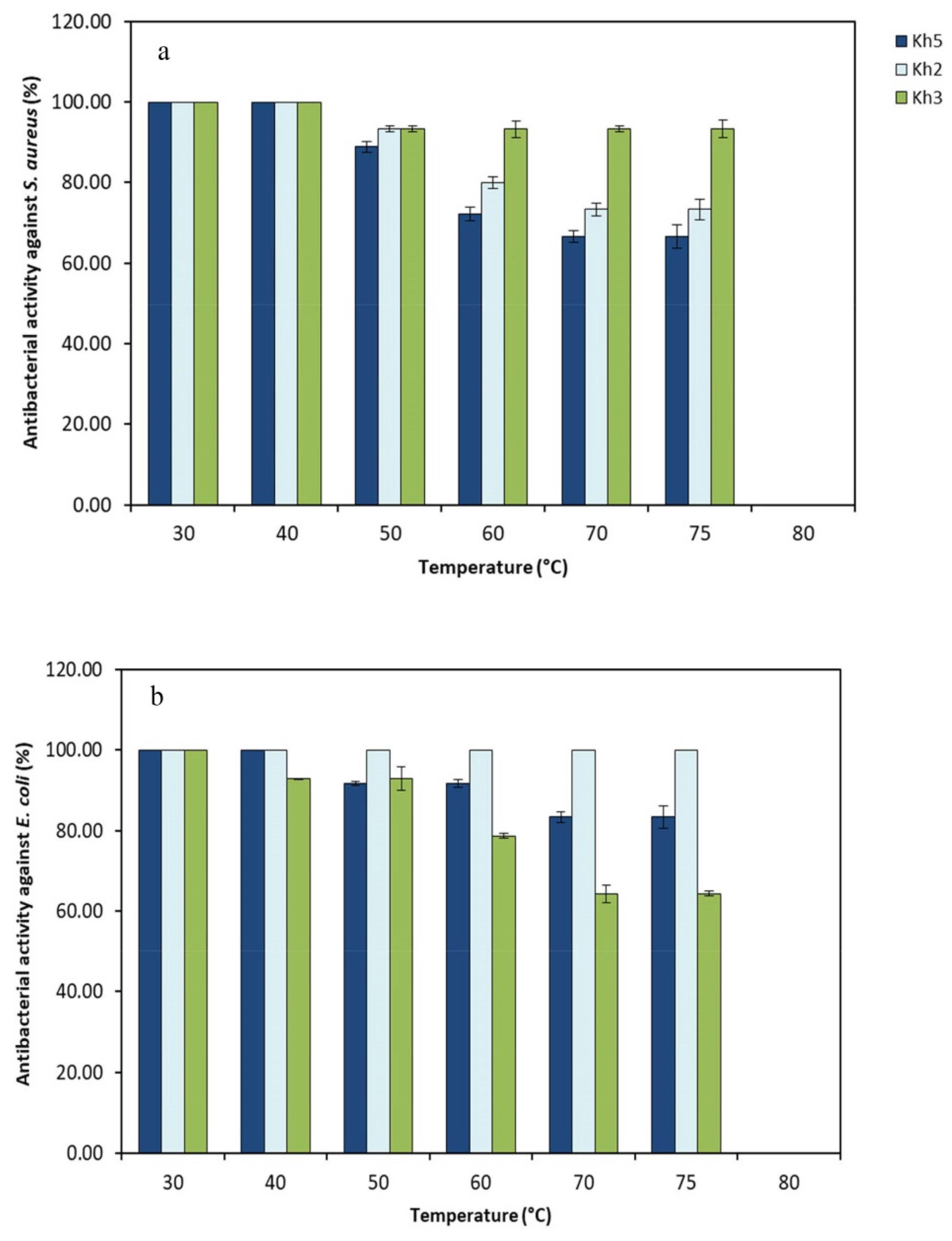
3.6. Heat Stability of the Cell-Free Supernatant of Selected Thermophilic Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, N.; Singh, S.P.; Zhang, C. Microorganisms under extreme environments and their applications. Curr. Res. Microb. Sci. 2022, 3, 100141. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, G.B.; Balachandran, L. Sources of antibiotics: Hot springs. Biochem. Pharmacol. 2017, 134, 35–41. [Google Scholar] [CrossRef]
- King, S.A.; Behnke, S.; Slack, K.; Krabbenhoft, D.P.; Nordstrom, D.K.; Burr, M.D.; Striegl, R.G. Mercury in water and biomass of microbial communities in hot springs of Yellowstone National Park, USA. Appl. Geochem. 2006, 21, 1868–1879. [Google Scholar] [CrossRef]
- Fandi, K.; Al-Muaikel, N.; Al-Momani, F. Antimicrobial activities of some thermophiles isolated from Jordan hot springs. Int. J. Chem. Environ. Biol. Sci. 2014, 2, 57–60. [Google Scholar]
- Wang, X.-W.; Tan, X.; Dang, C.-C.; Lu, Y.; Xie, G.-J.; Liu, B.-F. Thermophilic microorganisms involved in the nitrogen cycle in thermal environments: Advances and prospects. Sci. Total Environ. 2023, 896, 165259. [Google Scholar] [CrossRef]
- Bajpai, P. (Ed.) Developments and Applications of Enzymes from Thermophilic Microorganisms; Academic Press: Cambridge, MA, USA, 2023; pp. 17–27. [Google Scholar]
- Urbieta, M.S.; Donati, E.R.; Chan, K.-G.; Shahar, S.; Sin, L.L.; Goh, K.M. Thermophiles in the genomic era: Biodiversity, science, and applications. Biotechnol. Adv. 2015, 33, 633–647. [Google Scholar] [CrossRef]
- Alrumman, S.; Mostafa, Y.; Al-Qahtani, S.T.; Sahlabji, T.; Taha, T. Antimicrobial activity and GC-MS analysis of bioactive constituents of Thermophilic bacteria isolated from Saudi hot springs. Arab. J. Sci. Eng. 2019, 44, 75–85. [Google Scholar] [CrossRef]
- Esikova, T.; Temirov, Y.V.; Sokolov, S.; Alakhov, Y.B. Secondary antimicrobial metabolites produced by thermophilic Bacillus spp. strains VK2 and VK21. Appl. Biochem. Microbiol. 2002, 38, 226–231. [Google Scholar] [CrossRef]
- Arya, M.; Joshi, G.K.; Gupta, A.K.; Kumar, A.; Raturi, A. Isolation and characterization of thermophilic bacterial strains from Soldhar (Tapovan) hot spring in Central Himalayan Region, India. Ann. Microbiol. 2015, 65, 1457–1464. [Google Scholar] [CrossRef]
- Saikia, R.; Gogoi, D.; Mazumder, S.; Yadav, A.; Sarma, R.; Bora, T.; Gogoi, B. Brevibacillus laterosporus strain BPM3, a potential biocontrol agent isolated from a natural hot water spring of Assam, India. Microbiol. Res. 2011, 166, 216–225. [Google Scholar] [CrossRef]
- Ghrairi, T.; Braiek, O.B.; Hani, K. Detection and characterization of a bacteriocin, putadicin T01, produced by Pseudomonas putida isolated from hot spring water. Apmis 2015, 123, 260–268. [Google Scholar] [CrossRef]
- Mahajan, G.B. Antibiotics: Current Innovations and Future Trends; Caister Academic Press: Norfolk, UK, 2015; pp. 206–212. [Google Scholar]
- Zarqa, M.A.; Al-Qaryouti, M. Production of antimicrobial agents from thermophilic Yersinia sp. 1 and Aeromonas hydrophila isolated from hot spring in Jordan Valley. Biotechnology 2006, 5, 252–256. [Google Scholar]
- Biondi, N.; Martina, M.R.; Centini, M.; Anselmi, C.; Tredici, M.R. Hot Springs Cyanobacteria Endowed with Biological Activities for Cosmetic Applications: Evaluation of On-Site Collected Communities and Isolated Strains. Cosmetics 2023, 10, 81. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Duraipandiyan, V.; Valan Arasu, M.; Salem-Bekhit, M.M. Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles 2016, 20, 79–90. [Google Scholar] [CrossRef]
- Hussein, E.I.; Jacob, J.H.; Shakhatreh, M.A.K.; Al-Razaq, M.A.A.; Juhmani, A.-S.F.; Cornelison, C.T. Detection of antibiotic-producing Actinobacteria in the sediment and water of Ma’in thermal springs (Jordan). Germs 2018, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-H.; Tiang, Y.-Q.; Zhang, Y.-F.; Zhao, L.-X.; Jiang, C.-L. Streptomyces thermogriseus, a new species of the genus Streptomyces from soil, lake and hot-spring. Int. J. Syst. Evol. Microbiol. 1998, 48, 1089–1093. [Google Scholar] [CrossRef]
- Duan, Y.-Y.; Ming, H.; Dong, L.; Yin, Y.-R.; Zhang, Y.; Zhou, E.-M.; Liu, L.; Nie, G.-X.; Li, W.-J. Streptomyces calidiresistens sp. nov., isolated from a hot spring sediment. Antonie Van Leeuwenhoek 2014, 106, 189–196. [Google Scholar] [PubMed]
- Valverde, A.; Tuffin, M.; Cowan, D.A. Biogeography of bacterial communities in hot springs: A focus on the actinobacteria. Extremophiles 2012, 16, 669–679. [Google Scholar] [CrossRef]
- Yim, C.-Y.; Le, T.C.; Lee, T.G.; Yang, I.; Choi, H.; Lee, J.; Kang, K.-Y.; Lee, J.S.; Lim, K.-M.; Yee, S.-T. Saccharomonopyrones A–C, new α-pyrones from a marine sediment-derived bacterium Saccharomonospora sp. CNQ-490. Mar. Drugs 2017, 15, 239. [Google Scholar] [CrossRef]
- Zahra, A.; Zoheir, H.; Reza, M.M. Anti-bacterial potential of new Streptomyces sp. nov isolated from hot-springs North of Iran. Adv. Environ. Biol. 2014, 8, 2008–2012. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.; Falkow, S.; Rosenberg, E.; Stackebrandt, E.; Schleifer, K.-H. The Prokaryotes: A Handbook on the Biology of Bacteria; Springer: Berlin/Heidelberg, Germany, 2006; Volume 1. [Google Scholar]
- Logan, N.A.; De Vos, P. Genus I. bacillus. Bergeys Man. Syst. Bacteriol. 2009, 3, 21–128. [Google Scholar]
- Tille, P.M. Bailey & Scott’s Diagnostic Microbiology; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Peng, X.; Yu, K.Q.; Deng, G.H.; Jiang, Y.X.; Wang, Y.; Zhang, G.X.; Zhou, H.W. Comparison of direct boiling method with commercial kits for extracting fecal microbiome DNA by Illumina sequencing of 16S rRNA tags. J. Microbiol. Methods 2013, 95, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Harirchi, S.; Etemadifar, Z.; Mahboubi, A.; Yazdian, F.; Taherzadeh, M.J. The effect of calcium/magnesium ratio on the biomass production of a novel thermoalkaliphilic Aeribacillus pallidus strain with highly heat-resistant spores. Curr. Microbiol. 2020, 77, 2565–2574. [Google Scholar] [PubMed]
- Tokatli, A.; Idil, O.; Veyisoglu, A.; Saygin, H.; Guven, K.; Cetin, D.; Sahin, N. Streptomyces boluensis sp. nov., isolated from lake sediment. Arch. Microbiol. 2020, 202, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updates 2023, 68, 100954. [Google Scholar]
- Heidari, F.; Hauer, T.; Zima, J.R.H.; Riahi, H. New simple trichal cyanobacterial taxa isolated from radioactive thermal springs. Fottea 2018, 18, 137–149. [Google Scholar] [CrossRef]
- Beitollahi, M.; Ghiassi-Nejad, M.; Esmaeli, A.; Dunker, R. Radiological studies in the hot spring region of Mahallat, Central Iran. Radiat. Prot. Dosim. 2007, 123, 505–508. [Google Scholar] [CrossRef]
- Aissaoui, N.; Mahjoubi, M.; Nas, F.; Mghirbi, O.; Arab, M.; Souissi, Y.; Hoceini, A.; Masmoudi, A.S.; Mosbah, A.; Cherif, A.; et al. Antibacterial Potential of 2,4-Di-tert-Butylphenol and Calixarene-Based Prodrugs from Thermophilic Bacillus licheniformis Isolated in Algerian Hot Spring. Geomicrobiol. J. 2019, 36, 53–62. [Google Scholar] [CrossRef]
- Dworkin, M. The Prokaryotes: Vol. 1: Symbiotic Associations, Biotechnology, Applied Microbiology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Kushner, D.J. The Biology of Halophilic Bacteria; CRC Press: Boca Raton, FL, USA, 2020; pp. 87–103. [Google Scholar]
- Vos, P.; Garrity, G.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.-H.; Whitman, W.B. Bergey’s Manual of Systematic Bacteriology: Volume 3: The Firmicutes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 3. [Google Scholar]
- Al-Fadhli, A.A.; Threadgill, M.D.; Mohammed, F.; Sibley, P.; Al-Ariqi, W.; Parveen, I. Macrolides from rare actinomycetes: Structures and bioactivities. Int. J. Antimicrob. Agents 2022, 59, 106523. [Google Scholar] [CrossRef] [PubMed]
- Boudjelal, F.; Zitouni, A.; Mathieu, F.; Lebrihi, A.; Sabaou, N. Taxonomy and antimicrobial activities of two novel halophilic Saccharomonospora strains isolated in Algerian Sahara soils. Ann. Microbiol. 2011, 61, 299–305. [Google Scholar] [CrossRef]
- Kovács, M.; Seffer, D.; Pénzes-Hűvös, Á.; Juhász, Á.; Kerepesi, I.; Csepregi, K.; Kovács-Valasek, A.; Fekete, C. Structural and functional comparison of Saccharomonospora azurea strains in terms of primycin producing ability. World J. Microbiol. Biotechnol. 2020, 36, 160. [Google Scholar] [CrossRef] [PubMed]
- Feiszt, P.; Mestyán, G.; Kerényi, M.; Dobay, O.; Szabó, J.; Dombrádi, Z.; Urbán, E.; Emődy, L. Re-evaluation of in vitro activity of primycin against prevalent multiresistant bacteria. Int. J. Med. Microbiol. 2014, 304, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Takeda, I. Antibiotic AB-65, a new antibiotic from Saccharomonospora viride. J. Antibiot. 1975, 28, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, S.; Liu, X.; Zhang, C.; Xu, J.; Chen, Y. Bacillus halotolerans strain LYSX1-induced systemic resistance against the root-knot nematode Meloidogyne javanica in tomato. Ann. Microbiol. 2019, 69, 1227–1233. [Google Scholar] [CrossRef]
- Etemadzadeh, S.S.; Emtiazi, G. In vitro identification of antimicrobial hemolytic lipopeptide from halotolerant Bacillus by Zymogram, FTIR, and GC mass analysis. Iran. J. Basic Med. Sci. 2021, 24, 666. [Google Scholar]



| Parameter | Amount | Unit |
|---|---|---|
| Electrical conductivity (EC) | 1.8 | dS/m |
| Total Dissolved Solids (TDSs) | 1770 | mg/L |
| Total Suspended Solids (TSSs) | ≤50 | mg/L |
| [SO42−] | 960 | mg/L |
| [HCO3−] | 244 | mg/L |
| [CO32−] | 0.0 | mg/L |
| [Cl−] | 49.7 | mg/L |
| [NO3−] | 0.14 | mg/L |
| [Ca2+] | 360 | mg/L |
| [Na+] | 87.4 | mg/L |
| [Mg2+] | 57.6 | mg/L |
| [K+] | 5.8 | mg/L |
| Total Hardness (TH) | 1140 | P.P.M (France Degree) |
| pH | 6.95 | - |
| Isolate Designation | Isolation Source | Zone of Inhibition (mm) | |
|---|---|---|---|
| S. aureus | E. coli | ||
| Kh5 | Hot spring sludge | 18 | 12 |
| Kh2 | Soil | 15 | 8 |
| Kh3 | Hot spring sludge | 12 | 10 |
| Characteristics | Kh2 | Kh5 | Kh3 |
|---|---|---|---|
| Colony morphology | Smooth, circular with undulate margin, wrinkled, opaque, and slightly raised | Rough, circular with undulate margin, opaque, and flat | Rough, circular with undulate margin, opaque, raised, and adhered to the surface of agar |
| Colony color | Creamy | Creamy | White-powdery surface with creamy color for substrate mycelia |
| Pigment | − | − | + |
| Pigment color | − | − | Non-diffusible green |
| Colony size (mm) | 2 mm | 2 mm | 1 mm |
| Cell morphology | Rod-shape occurring singly and palisade form | Rod-shape occurring singly, in pairs, and sometimes palisade form | Filamentous, Branches of mycelia fragmented into rod or coccoid-shaped forms |
| Cell size (µm) | ND | ND | <1 µm in diameter |
| Gram staining | + | + | + |
| Reaction with KOH 3% | − | − | − |
| Motility | + | + | − |
| Spore formation | + | + | + |
| Spore morphology | Spherical | Spherical | Spherical |
| Spore position in the cell | Central to subterminal | Central to subterminal | Released from cells |
| Aerobic growth | + | + | + |
| Anaerobic growth | + | + | − |
| Growth in O/F medium | ND | ND | ± |
| Acid produced from the following: | |||
| D-Arabinose | ND | ND | − |
| D-Xylose | ND | ND | − |
| D-Galactose | ND | ND | − |
| D-Glucose | + | + | + |
| D-Mannose | + | + | − |
| Cellobiose | ND | ND | − |
| Sucrose | − | − | + |
| Glycerol | ND | ND | ND |
| Methyl Red/Voges Proskauer (MR/VP) reaction | ± | ± | ± |
| Gas formation from carbohydrates | ND | ND | − |
| Indole formation | + | + | + |
| H2S formation | − | − | − |
| Nitrate reduction | + | + | + |
| Catalase activity | + | + | + |
| Oxidase activity | − | − | − |
| Amylase activity | − | − | + |
| Gelatinase activity | − | − | + |
| Lecithinase activity | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiee, Z.; Jalili Tabaii, M.; Moradi, M.; Harirchi, S. Unveiling Antibacterial Potential and Physiological Characteristics of Thermophilic Bacteria Isolated from a Hot Spring in Iran. Microorganisms 2024, 12, 834. https://doi.org/10.3390/microorganisms12040834
Rafiee Z, Jalili Tabaii M, Moradi M, Harirchi S. Unveiling Antibacterial Potential and Physiological Characteristics of Thermophilic Bacteria Isolated from a Hot Spring in Iran. Microorganisms. 2024; 12(4):834. https://doi.org/10.3390/microorganisms12040834
Chicago/Turabian StyleRafiee, Zeinab, Maryam Jalili Tabaii, Maryam Moradi, and Sharareh Harirchi. 2024. "Unveiling Antibacterial Potential and Physiological Characteristics of Thermophilic Bacteria Isolated from a Hot Spring in Iran" Microorganisms 12, no. 4: 834. https://doi.org/10.3390/microorganisms12040834






