Maternal Gut Microbiome Decelerates Fetal Endochondral Bone Formation by Inducing Inflammatory Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Skeletal Preparation
2.3. Micro-CT Analysis
2.4. Primary Cell Culture
2.5. Cell Proliferation
2.6. RNA Preparation and RNA Sequence
2.7. Real-Time PCR
2.8. Statistical Analysis
3. Results
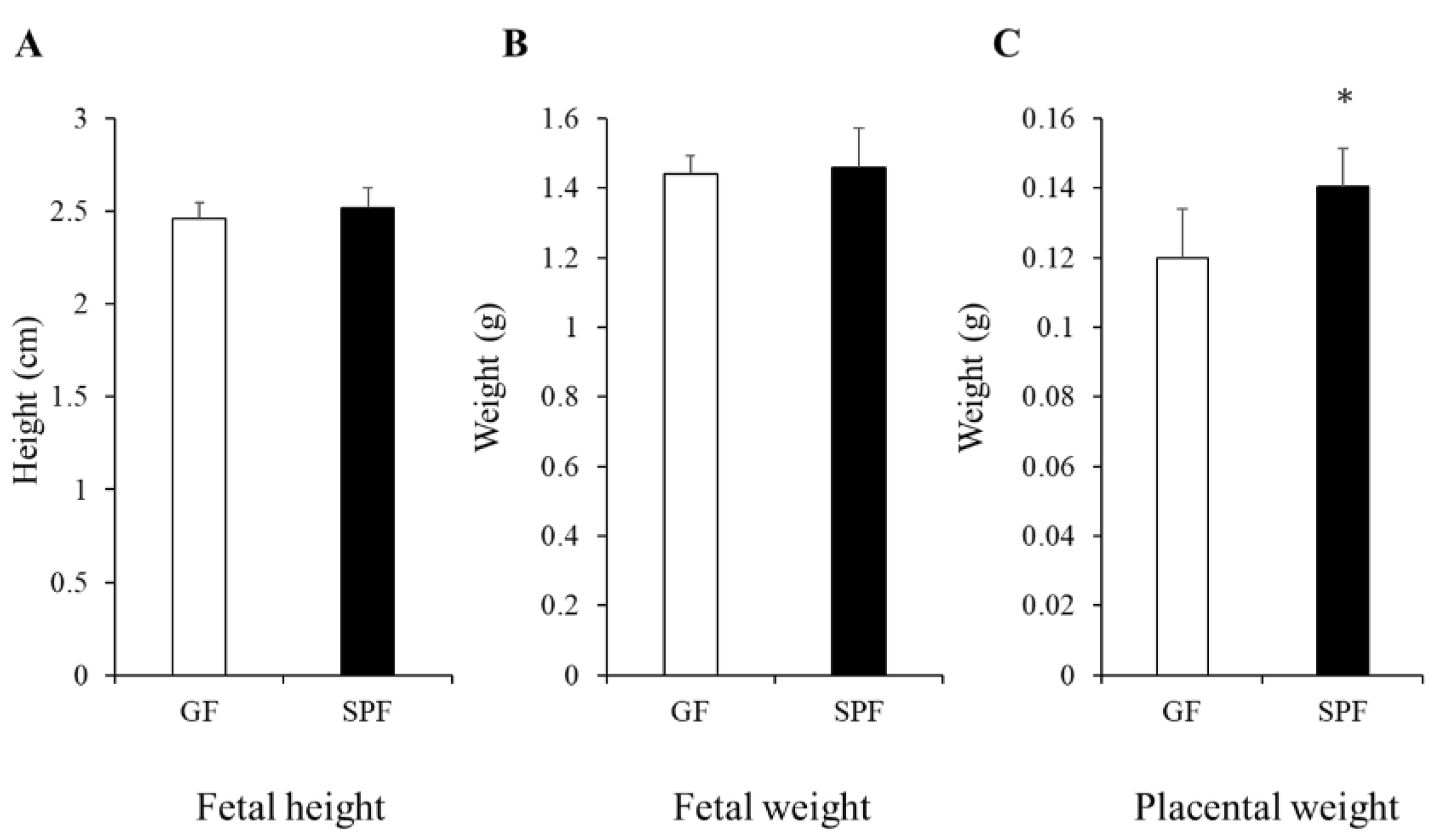
3.1. Comparison of Fetal and Placental Size from GF and SPF Embryos
3.2. Less Mineralization in Fetal SPF Bone
3.3. Decelerated Chondrocyte Proliferation and Accumulation of Extracellular Matrices in SPF Cartilage
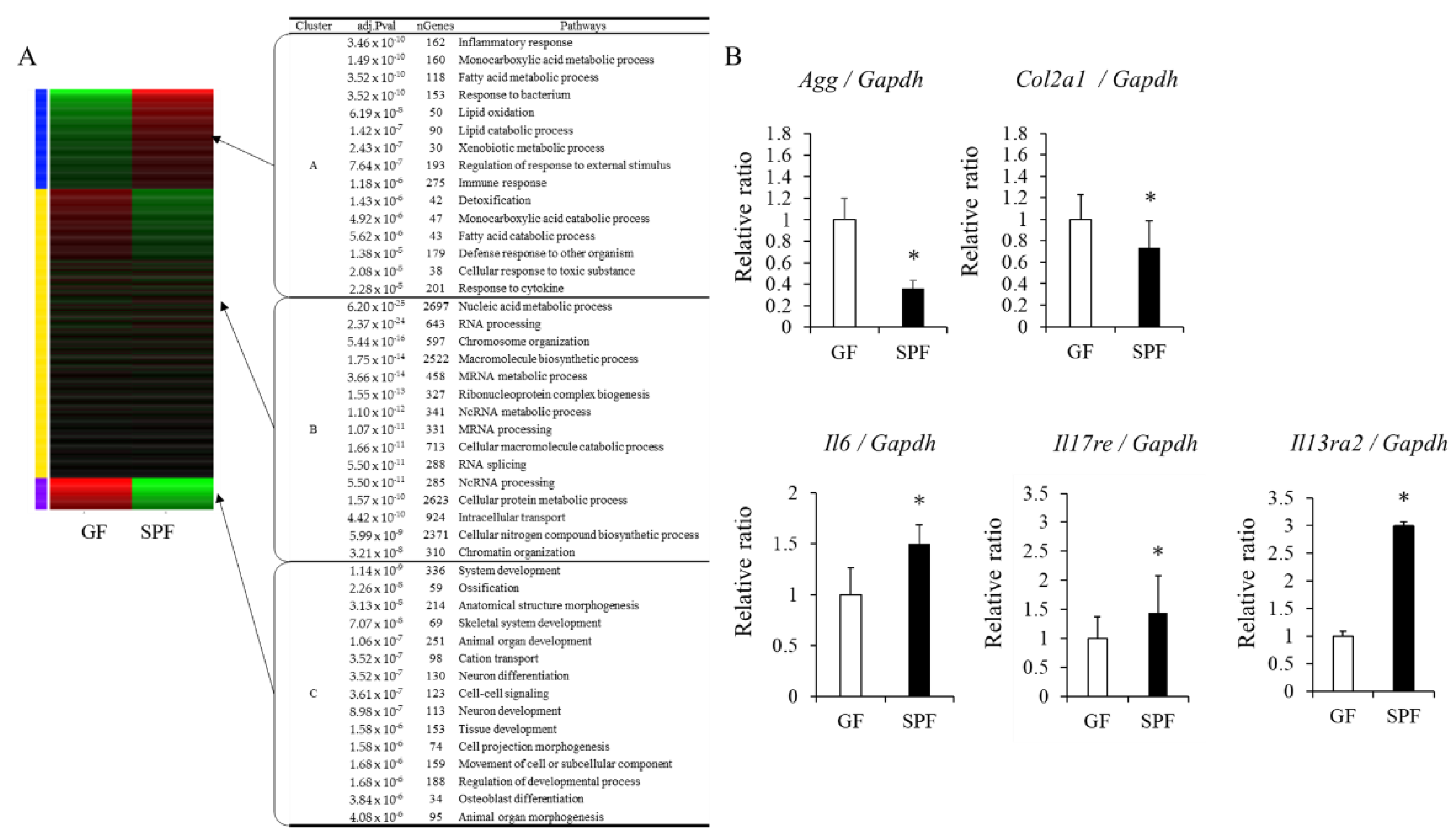
3.4. Gene-Expression Analysis in GF and SPF Fetal Chondrocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baquero, F.; Nombela, C. The Microbiome as a Human Organ. Clin. Microbiol. Infect. 2012, 18 (Suppl. 4), 2–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, R.W.; Arhire, L.; Covasa, M. Gut Microbiota: From Microorganisms to Metabolic Organ Influencing Obesity. Obesity 2018, 26, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peirce, J.M.; Alviña, K. The Role of Inflammation and the Gut Microbiome in Depression and Anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Lee, S.K.; Zhang, D.; Frenette, P.S. The Gut Microbiome Regulates Psychological-Stress-Induced Inflammation. Immunity 2020, 53, 417–428.e4. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The Gut-Bone Axis: How Bacterial Metabolites Bridge the Distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef] [Green Version]
- Riccio, P.; Rossano, R. The Human Gut Microbiota Is Neither an Organ nor a Commensal. FEBS Lett. 2020, 594, 3262–3271. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Edwards, S.M.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The Maternal Gut Microbiome During Pregnancy. MCN Am. J. Matern./Child Nursing 2017, 42, 310–317. [Google Scholar] [CrossRef]
- Berry, A.S.F.; Pierdon, M.K.; Misic, A.M.; Sullivan, M.C.; O’Brien, K.; Chen, Y.; Murray, S.J.; Ramharack, L.A.; Baldassano, R.N.; Parsons, T.D.; et al. Remodeling of the Maternal Gut Microbiome during Pregnancy Is Shaped by Parity. Microbiome 2021, 9, 146. [Google Scholar] [CrossRef]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the Gut Microbiota Composition during Pregnancy in Patients with Gestational Diabetes Mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef] [PubMed]
- Pronovost, G.N.; Hsiao, E.Y. Perinatal Interactions between the Microbiome, Immunity, and Neurodevelopment. Immunity 2019, 50, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Franchi, L.; Kambe, N.; Meng, G.; Strober, W.; Núñez, G. Critical Role for Mast Cells in Interleukin-1β-Driven Skin Inflammation Associated with an Activating Mutation in the nlrp3 Protein. Immunity 2012, 37, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Ruiz, A.; Mosley, M.; George, A.J.; Mussaji, L.F.; Fullerton, E.F.; Ruszkowski, E.M.; Jacobs, A.J.; Gewirtz, A.T.; Chassaing, B.; Forger, N.G. The Microbiota Influences Cell Death and Microglial Colonization in the Perinatal Mouse Brain. Brain Behav. Immun. 2018, 67, 218–229. [Google Scholar] [CrossRef]
- Erkkola, M.; Nwaru, B.I.; Kaila, M.; Kronberg-Kippilä, C.; Ilonen, J.; Simell, O.; Veijola, R.; Knip, M.; Virtanen, S.M. Risk of Asthma and Allergic Outcomes in the Offspring in Relation to Maternal Food Consumption during Pregnancy: A Finnish Birth Cohort Study. Pediatr. Allergy Immunol. 2012, 23, 186–194. [Google Scholar] [CrossRef]
- Baïz, N.; Just, J.; Chastang, J.; Forhan, A.; de Lauzon-Guillain, B.; Magnier, A.-M.; Annesi-Maesano, I.; EDEN Mother-Child Cohort Study Group. Maternal Diet before and during Pregnancy and Risk of Asthma and Allergic Rhinitis in Children. Allergy Asthma Clin. Immunol. 2019, 15, 40. [Google Scholar] [CrossRef] [Green Version]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D.; et al. Maternal Gut Microbiota in Pregnancy Influences Offspring Metabolic Phenotype in Mice. Science 2020, 367, eaaw8429. [Google Scholar] [CrossRef]
- Sjögren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The Gut Microbiota Regulates Bone Mass in Mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut Microbiota Induce IGF-1 and Promote Bone Formation and Growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef] [Green Version]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-Chain Fatty Acids Regulate Systemic Bone Mass and Protect from Pathological Bone Loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Uchida, Y.; Irie, K.; Fukuhara, D.; Kataoka, K.; Hattori, T.; Ono, M.; Ekuni, D.; Kubota, S.; Morita, M. Commensal Microbiota Enhance Both Osteoclast and Osteoblast Activities. Molecules 2018, 23, 1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooney, O.D.; Nagareddy, P.R.; Murphy, A.J.; Lee, M.K.S. Healthy Gut, Healthy Bones: Targeting the Gut Microbiome to Promote Bone Health. Front. Endocrinol. 2020, 11, 620466. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yu, X.-J.; Yu, L.-L.; Tian, F.-W.; Zhao, J.-X.; Zhang, H.; Chen, W.; Zhai, Q.-X. The Influence of Gut Microbiome on Bone Health and Related Dietary Strategies against Bone Dysfunctions. Food Res. Int. 2021, 144, 110331. [Google Scholar] [CrossRef]
- Hattori, T.; Müller, C.; Gebhard, S.; Bauer, E.; Pausch, F.; Schlund, B.; Bösl, M.R.; Hess, A.; Surmann-Schmitt, C.; von der Mark, H.; et al. SOX9 Is a Major Negative Regulator of Cartilage Vascularization, Bone Marrow Formation and Endochondral Ossification. Development 2010, 137, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Kuwahara, M.; Uchida, Y.; Koudo, S.; Hayashi, D.; Shimomura, Y.; Takagaki, A.; Nishida, T.; Maruyama, Y.; Ikegame, M.; et al. Circadian Production of Melatonin in Cartilage Modifies Rhythmic Gene Expression. J. Endocrinol. 2019, 241, 161–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An Integrated Web Application for Differential Expression and Pathway Analysis of RNA-Seq Data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [Green Version]
- Fukuhara, D.; Irie, K.; Uchida, Y.; Kataoka, K.; Akiyama, K.; Ekuni, D.; Tomofuji, T.; Morita, M. Impact of Commensal Flora on Periodontal Immune Response to Lipopolysaccharide. J. Periodontol. 2018, 89, 1213–1220. [Google Scholar] [CrossRef]
- Berthelot, J.-M.; Sellam, J.; Maugars, Y.; Berenbaum, F. Cartilage-Gut-Microbiome Axis: A New Paradigm for Novel Therapeutic Opportunities in Osteoarthritis. RMD Open 2019, 5, e001037. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Horst, R.T.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1897. [Google Scholar] [CrossRef] [Green Version]
- Buford, T.W. (Dis)Trust Your Gut: The Gut Microbiome in Age-Related Inflammation, Health, and Disease. Microbiome 2017, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The Role of the Gut Microbiome in Systemic Inflammatory Disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Hu, J.; Seki, A.; Eisele, C.; Nair, N.; Huang, R.; Tarassishin, L.; Jharap, B.; Cote-Daigneault, J.; Mao, Q.; et al. Infants Born to Mothers with IBD Present with Altered Gut Microbiome That Transfers Abnormalities of the Adaptive Immune System to Germ-Free Mice. Gut 2020, 69, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, M.; Yamaoka, K.; Sonomoto, K.; Fukuyo, S.; Oshita, K.; Okada, Y.; Tanaka, Y. IL-17 Inhibits Chondrogenic Differentiation of Human Mesenchymal Stem Cells. PLoS ONE 2013, 8, e79463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmele, E.P.; Pasricha, T.S.; Ramalingam, T.R.; Thompson, R.W.; Gieseck, R.L., 3rd; Knilans, K.J.; Hegen, M.; Farmer, M.; Jin, F.; Kleinman, A.; et al. Anti-IL-13Rα2 Therapy Promotes Recovery in a Murine Model of Inflammatory Bowel Disease. Mucosal Immunol. 2019, 12, 1174–1186. [Google Scholar] [CrossRef] [Green Version]
- Olaniyi, K.S.; Moodley, J.; Mahabeer, Y.; Mackraj, I. Placental Microbial Colonization and Its Association with Pre-Eclampsia. Front. Cell. Infect. Microbiol. 2020, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Sterpu, I.; Fransson, E.; Hugerth, L.W.; Du, J.; Pereira, M.; Cheng, L.; Radu, S.A.; Calderón-Pérez, L.; Zha, Y.; Angelidou, P.; et al. No Evidence for a Placental Microbiome in Human Pregnancies at Term. Am. J. Obstet. Gynecol. 2021, 224, e296.e1–e296.e23. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi-Tabata, A.; Takeuchi, N.; Uchida, Y.; Ekuni, D.; Morita, M. Association between Maternal Periodontal Status and Ultrasonographic Measurement of Fetal Growth: A Longitudinal Study. Sci. Rep. 2020, 10, 1402. [Google Scholar] [CrossRef]
- Brett, K.E.; Ferraro, Z.M.; Yockell-Lelievre, J.; Gruslin, A.; Adamo, K.B. Maternal-Fetal Nutrient Transport in Pregnancy Pathologies: The Role of the Placenta. Int. J. Mol. Sci. 2014, 15, 16153–16185. [Google Scholar] [CrossRef] [Green Version]


| Gene | F/R | Primer Sequences (5′–3′) |
|---|---|---|
| Agg | F | gttcctgcacagcttcacaa |
| R | aaacagcccagtgaccattc | |
| Col2a1 | F | gaactgcaacacattgtggg |
| R | attgatggtgaggtgtgcaa | |
| IL6 | F | agttgccttcttgggactga |
| R | cagaattgccattgcacaac | |
| IL17re | F | cagtaacagtgacgctagac |
| R | acccactagagcggtgagag | |
| IL13ra2 | F | gcaaaggaggacaaagaggtc |
| R | gatttagtgtgctgaaagctctactc | |
| Gapdh | F | tgtgatgggtgtgaaccacgagaa |
| R | gagcccttccacaatgccaaagtt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida-Fukuhara, Y.; Hattori, T.; Fu, S.; Kondo, S.; Kuwahara, M.; Fukuhara, D.; Islam, M.M.; Kataoka, K.; Ekuni, D.; Kubota, S.; et al. Maternal Gut Microbiome Decelerates Fetal Endochondral Bone Formation by Inducing Inflammatory Reaction. Microorganisms 2022, 10, 1000. https://doi.org/10.3390/microorganisms10051000
Uchida-Fukuhara Y, Hattori T, Fu S, Kondo S, Kuwahara M, Fukuhara D, Islam MM, Kataoka K, Ekuni D, Kubota S, et al. Maternal Gut Microbiome Decelerates Fetal Endochondral Bone Formation by Inducing Inflammatory Reaction. Microorganisms. 2022; 10(5):1000. https://doi.org/10.3390/microorganisms10051000
Chicago/Turabian StyleUchida-Fukuhara, Yoko, Takako Hattori, Shanqi Fu, Sei Kondo, Miho Kuwahara, Daiki Fukuhara, Md Monirul Islam, Kota Kataoka, Daisuke Ekuni, Satoshi Kubota, and et al. 2022. "Maternal Gut Microbiome Decelerates Fetal Endochondral Bone Formation by Inducing Inflammatory Reaction" Microorganisms 10, no. 5: 1000. https://doi.org/10.3390/microorganisms10051000
APA StyleUchida-Fukuhara, Y., Hattori, T., Fu, S., Kondo, S., Kuwahara, M., Fukuhara, D., Islam, M. M., Kataoka, K., Ekuni, D., Kubota, S., Morita, M., Iikegame, M., & Okamura, H. (2022). Maternal Gut Microbiome Decelerates Fetal Endochondral Bone Formation by Inducing Inflammatory Reaction. Microorganisms, 10(5), 1000. https://doi.org/10.3390/microorganisms10051000








