Opportunistic Mapping of Strongyloides stercoralis and Hookworm in Dogs in Remote Australian Communities
Abstract
:1. Introduction
2. Results
2.1. Dog DNA Origin
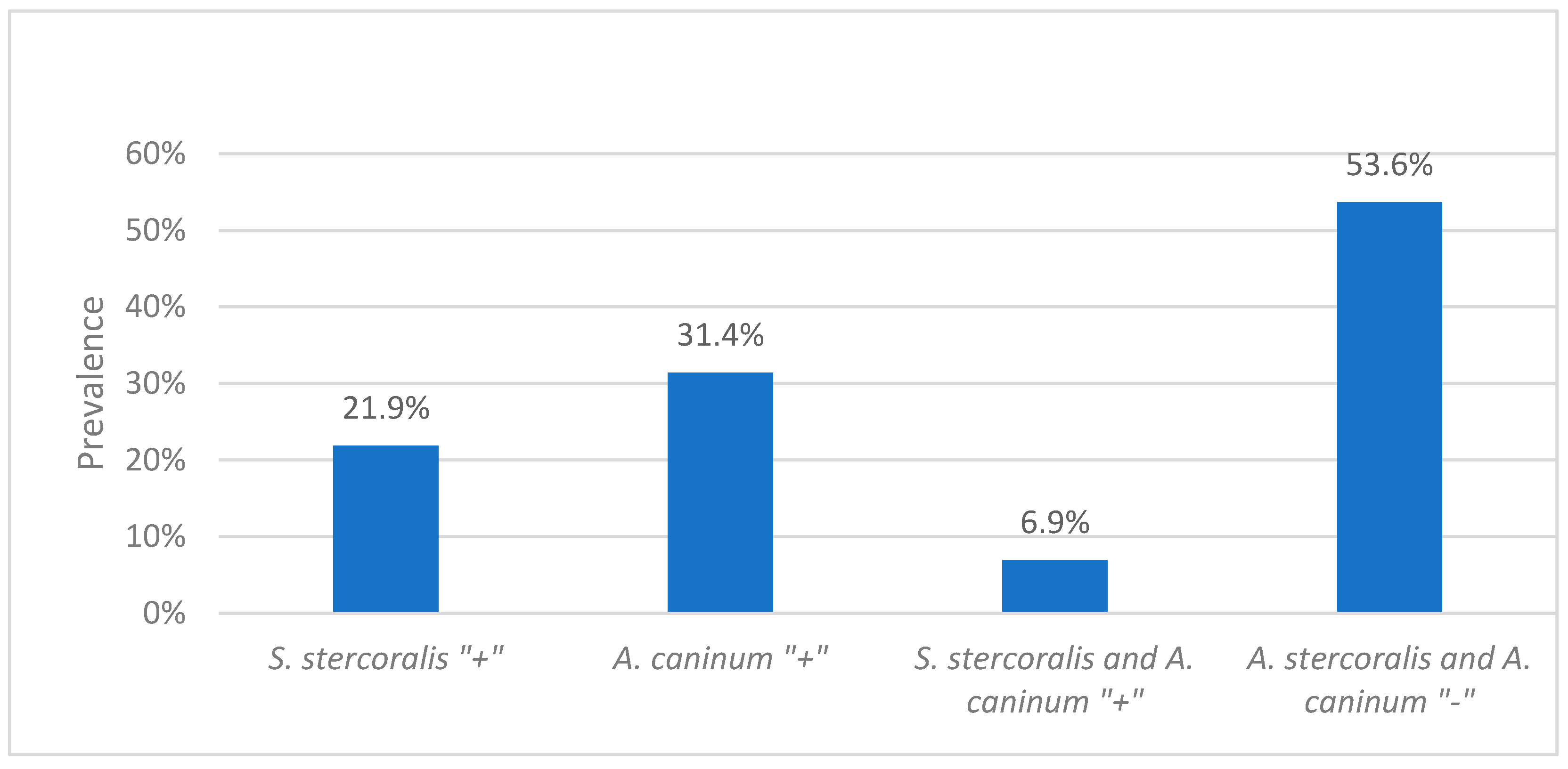
2.2. Prevalence of Strongyloides stercoralis and Hookworms
2.3. Association of Hookworms with Strongyloidiasis
3. Discussion
4. Materials and Methods
4.1. Ethical Considerations
4.2. Study Area and Population
4.3. Specimen Collection and DNA Extraction
4.4. Real-Time PCR Assays
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.M.; Loukas, A.; Diemert, D.; Hotez, P.J. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef]
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef]
- Jex, A.R.; Lim, Y.A.; Bethony, J.M.; Hotez, P.J.; Young, N.D.; Gasser, R.B. Soil-transmitted helminths of humans in Southeast Asia—Towards integrated control. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 74, pp. 231–265. [Google Scholar]
- Gordon, C.A.; Kurscheid, J.; Jones, M.K.; Gray, D.J.; McManus, D.P. Soil-transmitted helminths in Tropical Australia and Asia. Trop. Med. Infect. Dis. 2017, 2, 56. [Google Scholar] [CrossRef] [Green Version]
- Olsen, A.; van Lieshout, L.; Marti, H.; Polderman, T.; Polman, K.; Steinmann, P.; Stothard, R.; Thybo, S.; Verweij, J.J.; Magnussen, P. Strongyloidiasis–the most neglected of the neglected tropical diseases? Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Beknazarova, M.; Whiley, H.; Ross, K. Strongyloidiasis: A disease of socioeconomic disadvantage. Int. J. Environ. Res. Public Health 2016, 13, 517. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.; Page, W.; Speare, R. Strongyloidiasis: An issue in Aboriginal communities. Rural Remote Health 2003, 3, 152. [Google Scholar]
- Johnston, F.H.; Morris, P.S.; Speare, R.; McCarthy, J.; Currie, B.; Ewald, D.; Page, W.; Dempsey, K. Strongyloidiasis: A review of the evidence for Australian practitioners. Aust. J. Rural Health 2005, 13, 247–254. [Google Scholar] [CrossRef]
- Geri, G.; Rabbat, A.; Mayaux, J.; Zafrani, L.; Chalumeau-Lemoine, L.; Guidet, B.; Azoulay, E.; Pène, F. Strongyloides stercoralis hyperinfection syndrome: A case series and a review of the literature. Infection 2015, 43, 691–698. [Google Scholar] [CrossRef]
- Jaleta, T.G.; Zhou, S.; Bemm, F.M.; Schär, F.; Khieu, V.; Muth, S.; Odermatt, P.; Lok, J.B.; Streit, A. Different but overlapping populations of Strongyloides stercoralis in dogs and humans—Dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl. Trop. Dis. 2017, 11, e0005752. [Google Scholar] [CrossRef] [Green Version]
- Nagayasu, E.; Htwe, M.P.P.T.H.; Hortiwakul, T.; Hino, A.; Tanaka, T.; Higashiarakawa, M.; Olia, A.; Taniguchi, T.; Win, S.M.T.; Ohashi, I. A possible origin population of pathogenic intestinal nematodes, Strongyloides stercoralis, unveiled by molecular phylogeny. Sci. Rep. 2017, 7, 4844. [Google Scholar] [CrossRef] [Green Version]
- Beknazarova, M.; Barratt, J.L.; Bradbury, R.S.; Lane, M.; Whiley, H.; Ross, K. Detection of classic and cryptic Strongyloides genotypesby deep amplicon sequencing: A preliminary survey of dog and human specimens collected from remote Australian communities. PLoS Negl. Trop. Dis. 2019, 13, e0007241. [Google Scholar] [CrossRef] [Green Version]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [Green Version]
- Holt, D.C.; McCarthy, J.S.; Carapetis, J.R. Parasitic diseases of remote Indigenous communities in Australia. Int. J. Parasitol. 2010, 40, 1119–1126. [Google Scholar] [CrossRef]
- Palmer, C.S.; Traub, R.J.; Robertson, I.D.; Hobbs, R.P.; Elliot, A.; While, L.; Rees, R.; Thompson, R.A. The veterinary and public health significance of hookworm in dogs and cats in Australia and the status of A. ceylanicum. Vet. Parasitol. 2007, 145, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Hotez, P.; Whitham, M. Helminth infections: A new global women’s health agenda. Obstet. Gynecol. 2014, 123, 155–160. [Google Scholar] [CrossRef]
- Bearup, A. The intensity and type of hookworm infestation in the ingham district of North Queensland. Med. J. Aust. 1931, 2, 65–74. [Google Scholar] [CrossRef]
- Bradbury, R.; Traub, R.J. Hookworm infection in oceania. In Neglected Tropical Diseases-Oceania; Springer: Berlin/Heidelberg, Germany, 2016; pp. 33–68. [Google Scholar]
- Prociv, P.; Luke, R.A. The changing epidemiology of human hookworm infection in Australia. Med. J. Aust. 1995, 162, 150–154. [Google Scholar] [CrossRef]
- Holt, D.C.; Shield, J.; Harris, T.M.; Mounsey, K.E.; Aland, K.; McCarthy, J.S.; Currie, B.J.; Kearns, T.M. Soil-Transmitted Helminths in Children in a Remote Aboriginal Community in the Northern Territory: Hookworm is Rare but Strongyloides stercoralis and Trichuris trichiura Persist. Trop. Med. Infect. Dis. 2017, 2, 51. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.; Majumdar, S.S.; Forbes, R.; Smith, P.; Currie, B.J.; Baird, R.W. Hookworm in the Northern Territory: Down but not out. Med. J. Aust. 2013, 198, 278–281. [Google Scholar] [CrossRef] [Green Version]
- Koehler, A.V.; Bradbury, R.S.; Stevens, M.A.; Haydon, S.R.; Jex, A.R.; Gasser, R.B. Genetic characterization of selected parasites from people with histories of gastrointestinal disorders using a mutation scanning-coupled approach. Electrophoresis 2013, 34, 1720–1728. [Google Scholar] [CrossRef]
- Speare, R.; Bradbury, R.S.; Croese, J. A case of Ancylostoma ceylanicum infection occurring in an Australian soldier returned from Solomon Islands. Korean J. Parasitol. 2016, 54, 533. [Google Scholar] [CrossRef] [PubMed]
- Smout, F.A.; Skerratt, L.F.; Butler, J.R.; Johnson, C.N.; Congdon, B.C.; Thompson, R.A. The hookworm Ancylostoma ceylanicum: An emerging public health risk in Australian tropical rainforests and Indigenous communities. One Health 2017, 3, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.M.; Hobbs, R.P.; Thompson, R.A.; Gracey, M.S.; Spargo, R.M.; Yates, M. The prevalence of hookworm infection, iron deficiency and anaemia in an Aboriginal community in north-west Australia. Med. J. Aust. 1997, 166, 241–244. [Google Scholar] [CrossRef]
- Constable, S.; Dixon, R.; Dixon, R. For the love of dog: The human–dog bond in rural and remote Australian indigenous communities. Anthrozoös 2010, 23, 337–349. [Google Scholar] [CrossRef]
- Traub, R.J.; Robertson, I.D.; Irwin, P.; Mencke, N.; Thompson, R.A. Application of a species-specific PCR-RFLP to identify Ancylostoma eggs directly from canine faeces. Vet. Parasitol. 2004, 123, 245–255. [Google Scholar] [CrossRef]
- Traub, R.J. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int. J. Parasitol. 2013, 43, 1009–1015. [Google Scholar] [CrossRef]
- Bradbury, R.S.; Hii, S.F.; Harrington, H.; Speare, R.; Traub, R. Ancylostoma ceylanicum hookworm in the Solomon Islands. Emerg. Infect. Dis. 2017, 23, 252. [Google Scholar] [CrossRef] [Green Version]
- Inpankaew, T.; Schär, F.; Dalsgaard, A.; Khieu, V.; Chimnoi, W.; Chhoun, C.; Sok, D.; Marti, H.; Muth, S.; Odermatt, P. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg. Infect. Dis. 2014, 20, 976. [Google Scholar] [CrossRef]
- Prociv, P.; Croese, J. Human eosinophilic enteritis caused by dog hookworm Ancylostoma caninum. Lancet 1990, 335, 1299–1302. [Google Scholar] [CrossRef]
- McCarthy, J.; Moore, T.A. Emerging helminth zoonoses. Int. J. Parasitol. 2000, 30, 1351–1359. [Google Scholar] [CrossRef]
- Rusdi, B.; Laird, T.; Abraham, R.; Ash, A.; Robertson, I.D.; Mukerji, S.; Coombs, G.W.; Abraham, S.; O’Dea, M.A. Carriage of critically important antimicrobial resistant bacteria and zoonotic parasites amongst camp dogs in remote Western Australian indigenous communities. Sci. Rep. 2018, 8, 8725. [Google Scholar] [CrossRef]
- Smout, F.; Skerratt, L.; Johnson, C.; Butler, J.; Congdon, B. Zoonotic helminth diseases in dogs and dingoes utilising shared resources in an australian aboriginal community. Trop. Med. Infect. Dis. 2018, 3, 110. [Google Scholar] [CrossRef] [Green Version]
- Walker, N.I.; Croese, J.; Clouston, A.D.; Parry, M.; Loukas, A.; Prociv, P. Eosinophilic enteritis in northeastern Australia. Pathology, association with Ancylostoma caninum, and implications. Am. J. Surg. Pathol. 1995, 19, 328–337. [Google Scholar] [CrossRef]
- Fleming, F.M.; Brooker, S.; Geiger, S.M.; Caldas, I.R.; Correa-Oliveira, R.; Hotez, P.J.; Bethony, J.M. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Trop. Med. Infect. Dis. 2006, 11, 56–64. [Google Scholar] [CrossRef]
- Traub, R.J.; Inpankaew, T.; Sutthikornchai, C.; Sukthana, Y.; Thompson, R.A. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet. Parasitol. 2008, 155, 67–73. [Google Scholar] [CrossRef]
- Schad, G.A.; Page, M.R. Ancylostoma caninum: Adult worm removal, corticosteroid treatment, and resumed development of arrested larvae in dogs. Exp. Parasitol. 1982, 54, 303–309. [Google Scholar] [CrossRef]
- Beveridge, I. Australian hookworms (Ancylostomatoidea): A review of the species present, their distributions and biogeographical origins. Parassitologia 2002, 44, 83–88. [Google Scholar]
- Gibbs, H.; Gibbs, K. The effects of temperature on the development of the free-living stages of Dochmoides stenocephala (Railliet, 1884)(Ancylostomidae: Nematoda). Can. J. Zool. 1959, 37, 247–257. [Google Scholar] [CrossRef]
- Stewart, L. The Taxonomy of Ancylostoma Species in the Townsville Region of North Queensland. Master‘s Thesis, James Cook University, Townsville, Australia, 1994. [Google Scholar]
- Schär, F.; Odermatt, P.; Khieu, V.; Panning, M.; Duong, S.; Muth, S.; Marti, H.; Kramme, S. Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop. 2013, 126, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Verweij, J.J.; Canales, M.; Polman, K.; Ziem, J.; Brienen, E.A.; Polderman, A.M.; van Lieshout, L. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 342–346. [Google Scholar] [CrossRef]
- Gasser, R.; Cantacessi, C.; Loukas, A. DNA technological progress toward advanced diagnostic tools to support human hookworm control. Biotechnol. Adv. 2008, 26, 35–45. [Google Scholar] [CrossRef]
- Hii, S.F.; Senevirathna, D.; Llewellyn, S.; Inpankaew, T.; Odermatt, P.; Khieu, V.; Muth, S.; McCarthy, J.; Traub, R.J. Development and evaluation of a multiplex quantitative real-time polymerase chain reaction for hookworm species in human stool. Am. J. Trop. Med. Hyg. 2018, 99, 1186–1193. [Google Scholar] [CrossRef] [Green Version]
- Massetti, L.; Colella, V.; Zandejas, P.A.; Ng-Nguyen, D.; Harriott, L.; Marwedel, L.; Wiethoelter, A.; Traub, R.J. High-throughput multiplex qPCRs for the surveillance of zoonotic species of canine hookworms. PLoS Negl. Trop. Dis. 2020. [Google Scholar] [CrossRef]
- Sultana, Y.; Jeoffreys, N.; Watts, M.R.; Gilbert, G.L.; Lee, R. Real-time polymerase chain reaction for detection of Strongyloides stercoralis in stool. Am. J. Trop. Med. Hyg. 2013, 88, 1048–1051. [Google Scholar] [CrossRef] [Green Version]
- Brooker, S.; Miguel, E.A.; Moulin, S.; Louba, A.I.; Bundy, D.A.; Kremer, M. Epidemiology of single and multiple species of helminth infections among school children in Busia District, Kenya. East Afr. Med. J. 2000, 77, 77. [Google Scholar] [CrossRef]
- Thompson, R.A.; Roberts, M.G. Does pet helminth prophylaxis increase the rate of selection for drug resistance? Trends Parasitol. 2001, 17, 576–578. [Google Scholar] [CrossRef]
- Traub, R.J.; Robertson, I.D.; Irwin, P.; Mencke, N.; Thompson, R.A. The prevalence, intensities and risk factors associated with geohelminth infection in tea-growing communities of Assam, India. Trop. Med. Int. Health 2004, 9, 688–701. [Google Scholar] [CrossRef]
- Willis, E.; Ross, K. Review of principles governing dog health education in remote Aboriginal communities. Aust. Veter. J. 2019, 97, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Alaeddini, R. Forensic implications of PCR inhibition—A review. Forensic Sci. Int. Genet. 2012, 6, 297–305. [Google Scholar] [CrossRef]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR inhibitors–occurrence, properties and removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef]
- Beknazarova, M.; Millsteed, S.; Robertson, G.; Whiley, H.; Ross, K. Validation of DESS as a DNA Preservation method for the detection of strongyloides spp. in canine feces. Int. J. Environ. Res. Public Health 2017, 14, 624. [Google Scholar] [CrossRef] [Green Version]


| Primer/Probe | Amplicon | Sequence | Reaction Conditions |
|---|---|---|---|
| Stro18S-1530F Stro18S-1630R Stro18S-1586T FAM | rDNA 101 bp | 5′-GAATTCCAAGTAAACGTAAGTCATTAGC-3′ 5′-TGCCTCTGGATATTGCTCAGTTC-3′ 5′-FAM-ACACACCGGCCGTCGCTGC-3′-BHQ1 | Step 1: 95 °C for 15 min, Step 2: 95 °C for 15 s, Step 3: 60 °C for 30 s. Repeat steps two and three 40 times. |
| A. cancey F A. cancey R Ahumanceylanicum probe Acantub probe | ITS1 region | 5′- GGGAAGGTTGGGAGTATCG-3′ 5′- CGAACTTCGCACAGCAATC-3′ 5′- Cy5/CCGTTC+CTGGGTGGC/3IABkRQSp/-3′ 5′-HEX/ AG+T+CGT+T+A+C+TGG/3IABkRFQ/-3′ | Step 1: 95 °C for 2 min, Step 2: 95 °C for 15 s, Step 3: 60°C for 60 s. Repeat steps two and three 40 times. |
| Uncbraz F Uncbraz R Unc Probe Abra probe | ITS1 region | 5′- GAG CTT TAG ACT TGA TGA GCA TTG-3′ 5′- GCA GAT CAT TAA GGT TTC CTG AC-3′ 5’-/5HEX/CAT TAG GCG /ZEN/GCA ACG TCT GGT G/3IABkFQ/-3′ 5’-/56FAM/TGA GCG CTA /ZEN/GGC TAA CGC CT/3IABkFQ/-3’ | Step 1: 95 °C for 2 min, Step 2: 95 °C for 15 s, Step 3: 64 °C for 60 s. Repeat steps two and three 40 times. |
| EMV F ENV R ENV probe | Equine herpesvirus type 4 | 5′-GATGACACTAGCG-ACTTCGA-3′ 5′-CAGGGCAGAAACC-ATAGACA-3′ 5′-TEX-TTTCGCGTGC-CTCCTCCAG-IBRQ-3′ | Step 1: 95 °C for 2 min, Step 2: 95 °C for 15 s, Step 3: 60 °C for 60 s. Repeat steps two and three 40 times. |
| Dog F Dog R Dog probe | mtDNA | 5′-CGACCTCGATGTTGGATCAG-3′ 5′-GAACTCAGATCACGTAGGACTTT-3′ 5′-FAM/ CCTAATGGT/ ZEN/ GCAGCAGCTATTAA/ LABKFQ-3′ | Step 1: 95 °C for 2 min, Step 2: 95 °C for 15 s, Step 3: 60 °C for 60 s. Repeat steps two and three 40 times. |
| Species | GenBank Accession Number | Sequence |
|---|---|---|
| Ancylostoma ceylanicum | DQ780009.1 | CGTGCTAGTCTTCAGGACTTTGTCGGGAAGGTTGGGAGTATCGCCCCCCGTTACAGCCCTACGTGAGGTGTCTATGTGCAGCAAGAGCCGTTCCTGGGTGGCGGCAGTGATTGCTGTGCGAAGTTCGCGTTTCGCTGAGCTTTAGACTTGAG |
| Ancylostoma duodenale/Ancylostoma caninum | EU344797.1 | CGTGCTAGTCTTCACGACTTTGTCGGGAAGGTTGGGAGTATCGCCCCCCGTTATAGCCCTACGTAAGGTGTCTATGTGCAGCAAGAGTCGTTACTGGGTGACGGCAGTGATTGCTGTGCGAAGTTCGCGTTTCGCTGAGCTTTAGACTTGAT |
| Ancylostoma braziliense | JQ812692.1 | TGTACGAAGCTCGCGGTTTCGTCAGAGCTTTAGACTTGATGAGCATTGCTAGAATGCCGCCTTACCTGCTTGTGTTGGTGGTTGAGCGCTAGGCTAACGCCTGGTGCGGCACCTGTCTGTCAGGAAACCTTAATGATCTGCTAACGCGGACGCCAGCACAGCAAT |
| Uncinaria stenocephala | HQ262054.1 | GCTGTGCGAAGTTCGCGTTTCGCTGAGCTTTAGACTTGATGAGCATTGCTGGAATGCCGCCTTACTGTTTGTGTTGGTGGTTGGGCATTAGGCGGCAACGTCTGGTGCGACACCTGTTTGTCAGGAAACCTTAATGATCTGCTCACGTGGACGCCAATACAGCACT |
| Equid herpesvirus | KT324745.1 | ATGAAAGCTCTATACCCAATAACAACCAGGAGCCTTAAAAACAAAGCCAAAGCCTCATACGGCCAAAACGACGATGATGACACTAGCGACTTCGATGAAGCCAAGCTGGAGGAGGCACGCGAAATGATCAAATATATGTCTATGGTTTCTGCCCTGGAAAAACAGGAAAAAAAGGCAATGAAGAAAAACAAGGGGGTTGGACTTATTGCC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beknazarova, M.; Whiley, H.; Traub, R.; Ross, K. Opportunistic Mapping of Strongyloides stercoralis and Hookworm in Dogs in Remote Australian Communities. Pathogens 2020, 9, 398. https://doi.org/10.3390/pathogens9050398
Beknazarova M, Whiley H, Traub R, Ross K. Opportunistic Mapping of Strongyloides stercoralis and Hookworm in Dogs in Remote Australian Communities. Pathogens. 2020; 9(5):398. https://doi.org/10.3390/pathogens9050398
Chicago/Turabian StyleBeknazarova, Meruyert, Harriet Whiley, Rebecca Traub, and Kirstin Ross. 2020. "Opportunistic Mapping of Strongyloides stercoralis and Hookworm in Dogs in Remote Australian Communities" Pathogens 9, no. 5: 398. https://doi.org/10.3390/pathogens9050398





