Different Dose-Dependent Modes of Action of C-Type Natriuretic Peptide on Pseudomonas aeruginosa Biofilm Formation
Abstract
:1. Introduction
2. Results
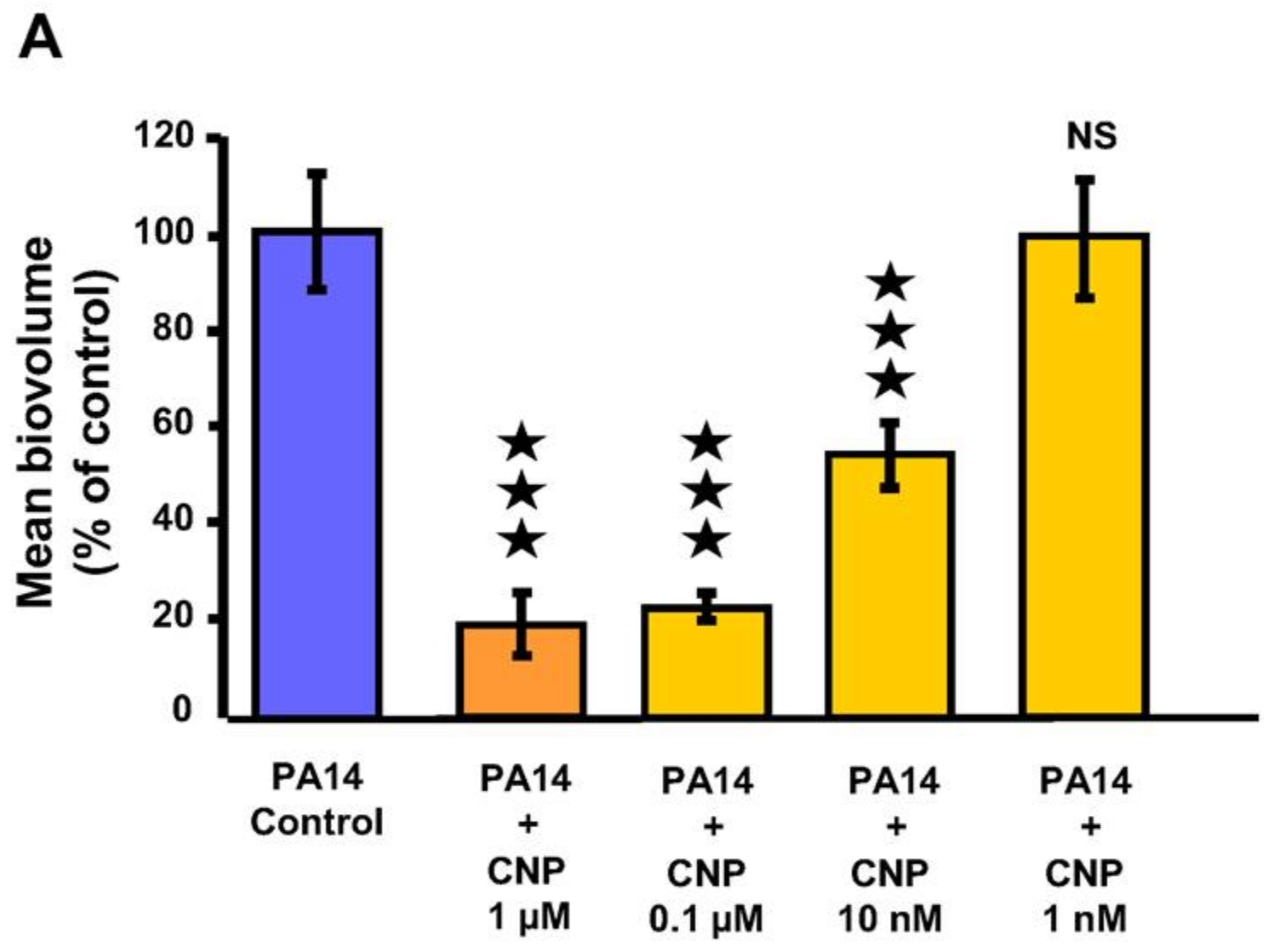
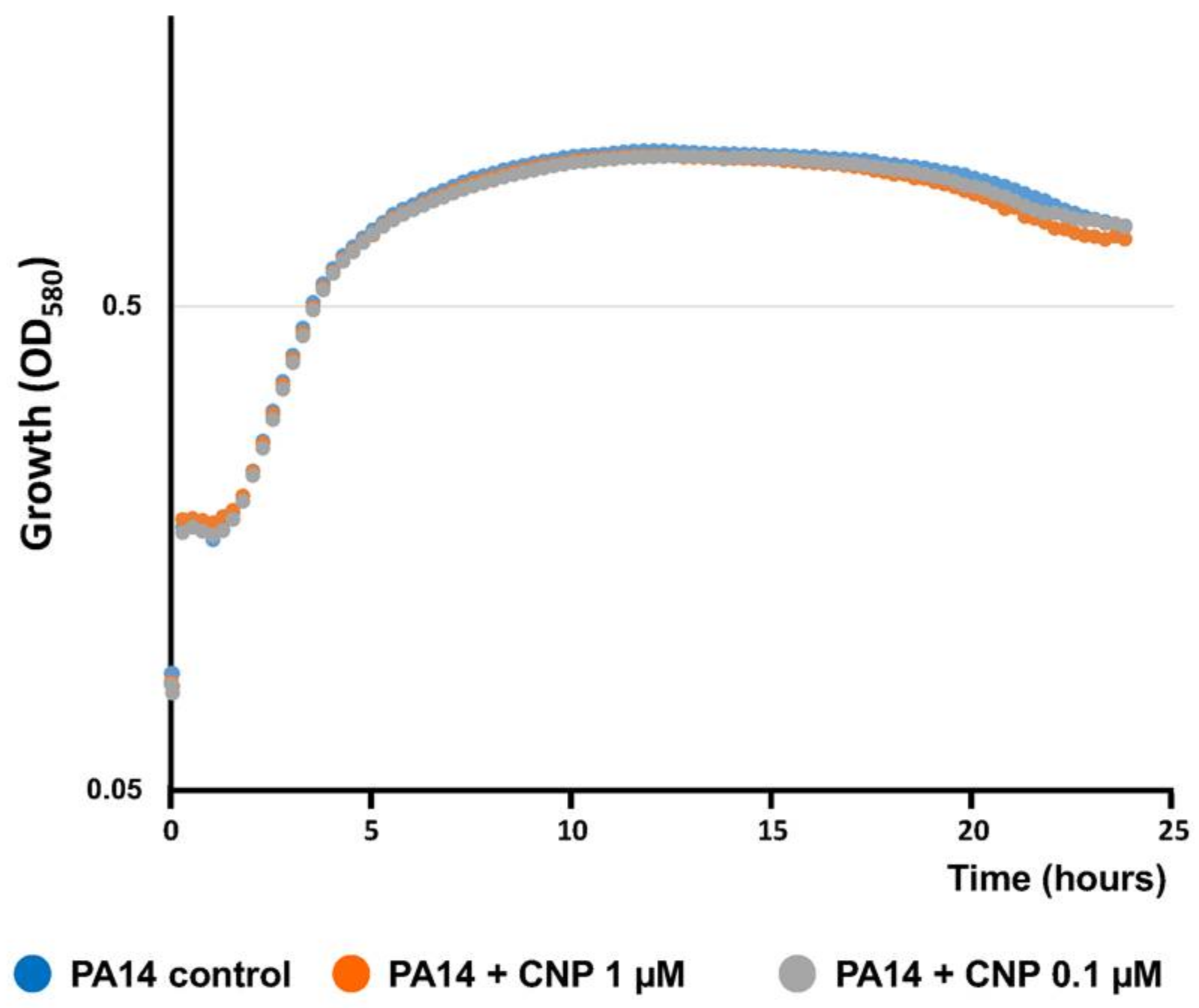
2.1. Effect of CNP on P. aeruginosa Biofilm Formation in Dynamic Conditions
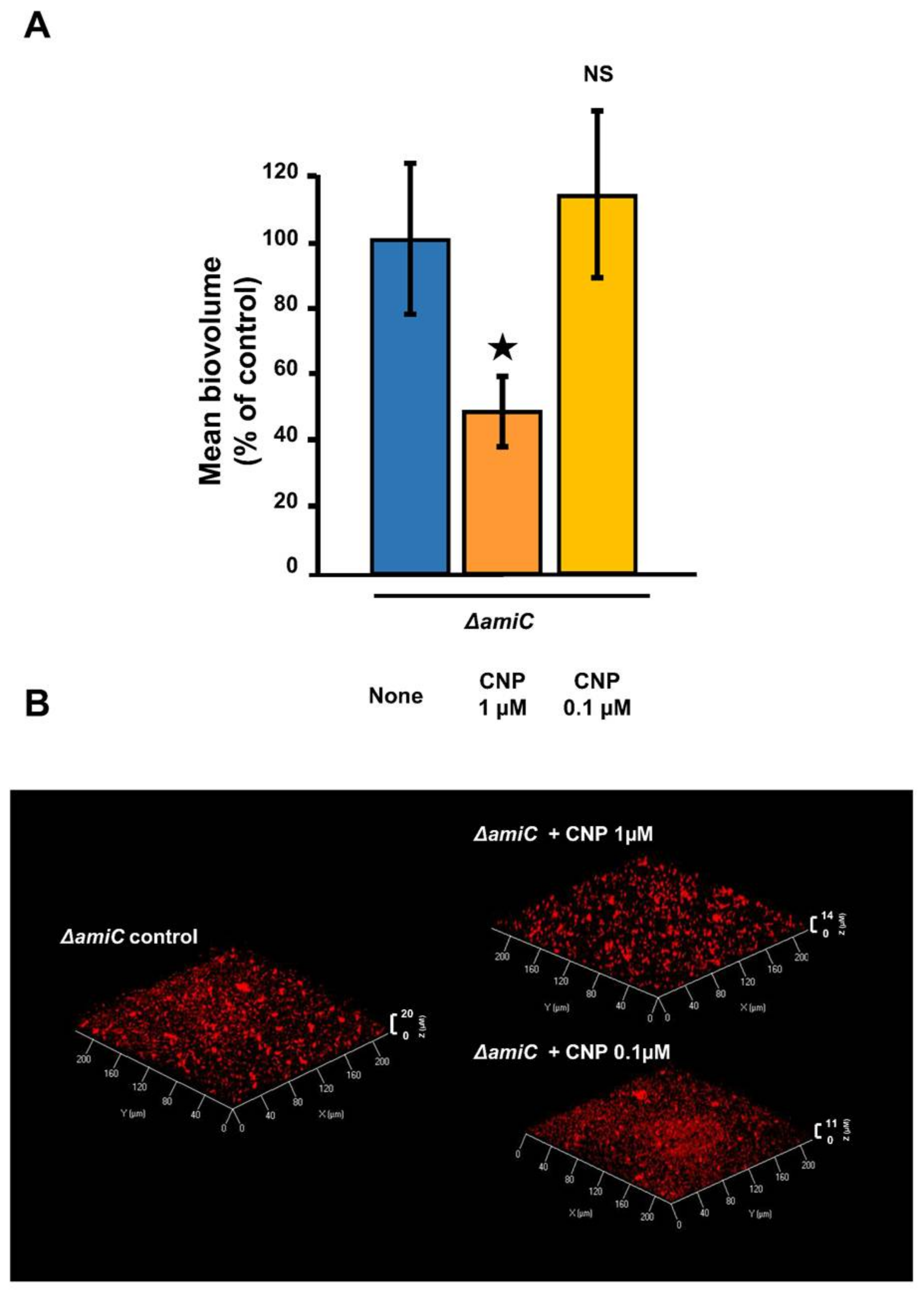
2.2. Involvement of AmiC in the CNP Effect on P. aeruginosa
2.3. Effect of CNP on P. aeruginosa Adhesion Properties
2.4. Effect of CNP on P. aeruginosa Di-Rhamnolipid Production
2.5. Effect of CNP on P. aeruginosa Membrane Fluidity
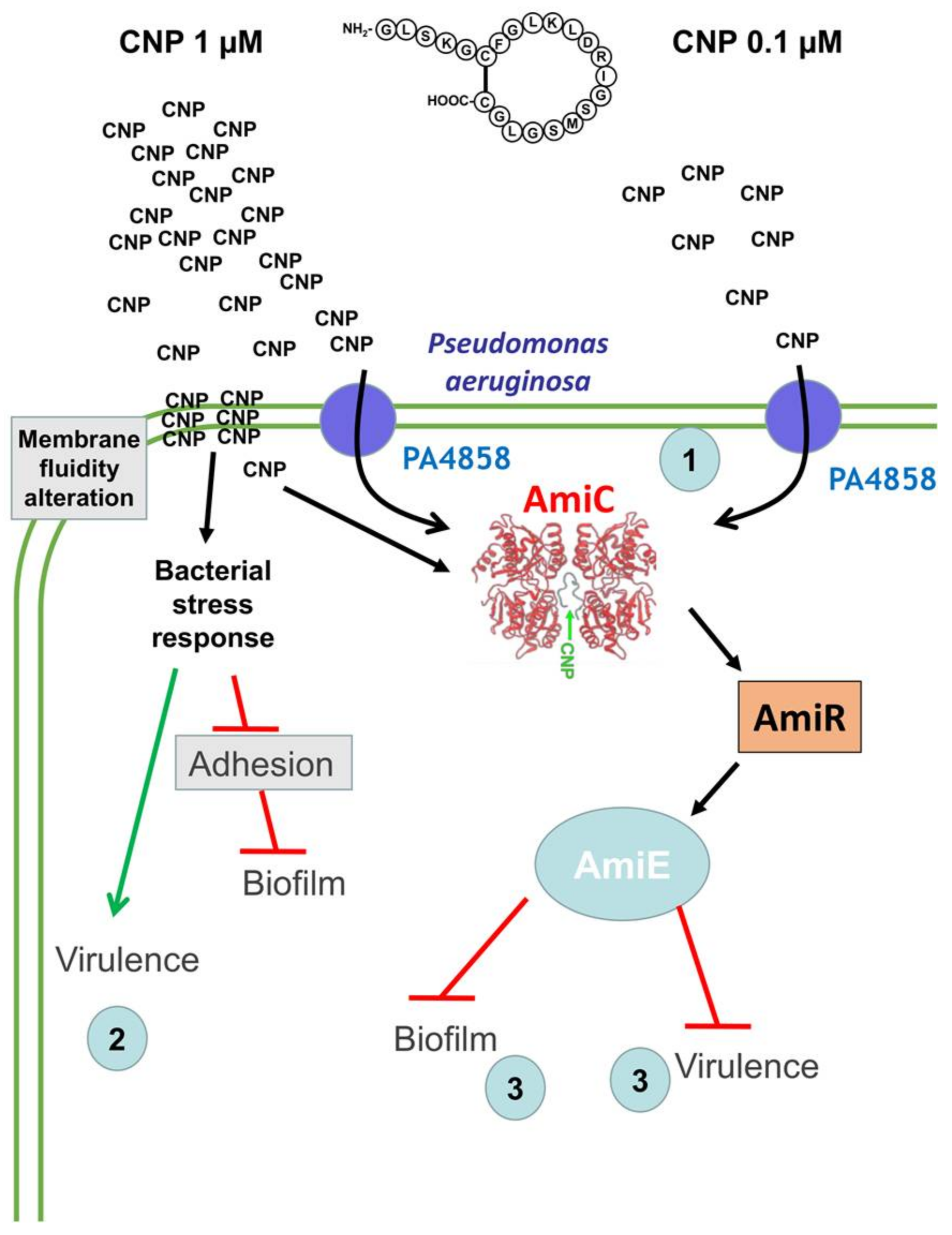
3. Discussion
4. Materials and Methods
4.1. Bacterial Cultures and Tested Molecules
4.2. Kinetics of Bacterial Growth
4.3. Biofilms Formation on Glass Slides Under Dynamic Conditions and Adhesion Analysis
4.4. Fluorescence Anisotropy Microplate Assays
4.5. Rhamnolipid Quantification
5. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Reffuveille, F.; de la Fuente-Núñez, C.; Mansour, S.; Hancock, R.E.W. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob. Agents Chemother. 2014, 58, 5363–5371. [Google Scholar] [CrossRef] [PubMed]
- Pletzer, D.; Hancock, R.E.W. Anti-biofilm peptides: Potential as broad-spectrum agents. J. Bacteriol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Wang, G. Individual and combined effects of engineered peptides and antibiotics on Pseudomonas aeruginosa biofilms. Pharmaceuticals 2017, 10, 58. [Google Scholar] [CrossRef]
- McPhee, J.B.; Lewenza, S.; Hancock, R.E.W. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol. Microbiol. 2003, 50, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Anaya-López, J.L.; López-Meza, J.E.; Ochoa-Zarzosa, A. Bacterial resistance to cationic antimicrobial peptides. Crit. Rev. Microbiol. 2013, 39, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.D.; Groisman, E.A. The biology of the PmrA/PmrB two-component system: The major regulator of lipopolysaccharide modifications. Annu. Rev. Microbiol. 2013, 67, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Ernst, S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992, 50, 203–212. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol. 2004, 12, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lesouhaitier, O.; Veron, W.; Chapalain, A.; Madi, A.; Blier, A.S.; Dagorn, A.; Connil, N.; Chevalier, S.; Orange, N.; Feuilloley, M. Gram-negative bacterial sensors for eukaryotic signal molecules. Sensors 2009, 9, 6967–6990. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.M.; Sperandio, V. What a dinner party! Mechanisms and functions of interkingdom signaling in host-pathogen associations. mBio 2016, 7, e01748. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lustri, B.C.; Sperandio, V.; Moreira, C.G. Bacterial chat: intestinal metabolites and signals in host-microbiota-pathogen interactions. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, A.; Mijouin, L.; Hillion, M.; Diaz, S.; Konto-Ghiorghi, Y.; Percoco, G.; Chevalier, S.; Lefeuvre, L.; Harmer, N.J.; Lesouhaitier, O.; et al. Effect of Substance P in Staphylococcus aureus and Staphylococcus epidermidis virulence: implication for skin homeostasis. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijouin, L.; Hillion, M.; Ramdani, Y.; Jaouen, T.; Duclairoir-Poc, C.; Follet-Gueye, M.-L.; Lati, E.; Yvergnaux, F.; Driouich, A.; Lefeuvre, L.; et al. Effects of a skin neuropeptide (Substance P) on cutaneous microflora. PLoS ONE 2013, 8, e78773. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, A.R.; Leclerc, C.; Kentache, T.; Hardouin, J.; Poc, C.D.; Konto-Ghiorghi, Y.; Chevalier, S.; Lesouhaitier, O.; Feuilloley, M.G.J. Skin-bacteria communication: Involvement of the neurohormone Calcitonin Gene Related Peptide (CGRP) in the regulation of Staphylococcus epidermidis virulence. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Englert, D.; Lee, J.; Hegde, M.; Wood, T.K.; Jayaraman, A. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect. Immun. 2007, 75, 4597–4607. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Meng, J.; Huang, Y.; Ye, L.; Li, G.; Huang, J.; Chen, H. The role of the QseC quorum-sensing sensor kinase in epinephrine-enhanced motility and biofilm formation by Escherichia coli. Cell Biochem. Biophys. 2014, 70, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, S.M.; Ernst, R.K.; Miller, S.I. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J. Bacteriol. 2004, 186, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Jensen, P.Ø.; Fiandaca, M.J.; Pedersen, J.; Hansen, C.R.; Andersen, C.B.; Pressler, T.; Givskov, M.; Høiby, N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009, 44, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Camilli, A.; Bassler, B.L. Bacterial small-molecule signaling pathways. Science 2006, 311, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Seal, J.B.; Alverdy, J.C.; Zaborina, O.; An, G. Agent-based dynamic knowledge representation of Pseudomonas aeruginosa virulence activation in the stressed gut: Towards characterizing host-pathogen interactions in gut-derived sepsis. Theor. Biol. Med. Model. 2011, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Planamente, S.; Mondy, S.; Hommais, F.; Vigouroux, A.; Moréra, S.; Faure, D. Structural basis for selective GABA binding in bacterial pathogens. Mol. Microbiol. 2012, 86, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Dagorn, A.; Hillion, M.; Chapalain, A.; Lesouhaitier, O.; Duclairoir Poc, C.; Vieillard, J.; Chevalier, S.; Taupin, L.; Le Derf, F.; Feuilloley, M.G.J. Gamma-aminobutyric acid acts as a specific virulence regulator in Pseudomonas aeruginosa. Microbiology 2013, 159, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Dagorn, A.; Chapalain, A.; Mijouin, L.; Hillion, M.; Duclairoir-Poc, C.; Chevalier, S.; Taupin, L.; Orange, N.; Feuilloley, M.G.J. Effect of GABA, a bacterial metabolite, on Pseudomonas fluorescens surface properties and cytotoxicity. Int. J. Mol. Sci. 2013, 14, 12186–12204. [Google Scholar] [CrossRef] [PubMed]
- Zaborina, O.; Lepine, F.; Xiao, G.; Valuckaite, V.; Chen, Y.; Li, T.; Ciancio, M.; Zaborin, A.; Petrof, E.O.; Petroff, E.; et al. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog. 2007, 3, e35. [Google Scholar] [CrossRef] [Green Version]
- Wright, M.H.; Fetzer, C.; Sieber, S.A. Chemical probes unravel an antimicrobial defense response triggered by binding of the human opioid dynorphin to a bacterial sensor kinase. J. Am. Chem. Soc. 2017, 139, 6152–6159. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Estrada, O.; Zaborina, O.; Bains, M.; Shen, L.; Kohler, J.E.; Patel, N.; Musch, M.W.; Chang, E.B.; Fu, Y.X.; et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science 2005, 309, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef] [PubMed]
- Fito-Boncompte, L.; Chapalain, A.; Bouffartigues, E.; Chaker, H.; Lesouhaitier, O.; Gicquel, G.; Bazire, A.; Madi, A.; Connil, N.; Veron, W.; et al. Full virulence of Pseudomonas aeruginosa requires OprF. Infect. Immun. 2011, 79, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Bouffartigues, E.; Moscoso, J.A.; Duchesne, R.; Rosay, T.; Fito-Boncompte, L.; Gicquel, G.; Maillot, O.; Bénard, M.; Bazire, A.; Brenner-Weiss, G.; et al. The absence of the Pseudomonas aeruginosa OprF protein leads to increased biofilm formation through variation in c-di-GMP level. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.A.; Giles, W.R. Natriuretic peptide C receptor signalling in the heart and vasculature. J. Physiol. 2008, 586, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Vila, G.; Resl, M.; Stelzeneder, D.; Struck, J.; Maier, C.; Riedl, M.; Hülsmann, M.; Pacher, R.; Luger, A.; Clodi, M. Plasma NT-proBNP increases in response to LPS administration in healthy men. J. Appl. Physiol. 2008, 105, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Suga, S.; Itoh, H.; Komatsu, Y.; Ogawa, Y.; Hama, N.; Yoshimasa, T.; Nakao, K. Cytokine-induced C-type natriuretic peptide (CNP) secretion from vascular endothelial cells—Evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology 1993, 133, 3038–3041. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Liepke, C.; Meyer, M.; Adermann, K.; Forssmann, W.G.; Maronde, E. Human natriuretic peptides exhibit antimicrobial activity. Eur. J. Med. Res. 2001, 6, 215–218. [Google Scholar] [PubMed]
- Blier, A.S.; Veron, W.; Bazire, A.; Gerault, E.; Taupin, L.; Vieillard, J.; Rehel, K.; Dufour, A.; Le Derf, F.; Orange, N.; et al. C-type natriuretic peptide modulates quorum sensing molecule and toxin production in Pseudomonas aeruginosa. Microbiology 2011, 157, 1929–1944. [Google Scholar] [CrossRef] [PubMed]
- Rosay, T.; Bazire, A.; Diaz, S.; Clamens, T.; Blier, A.S.; Mijouin, L.; Hoffmann, B.; Sergent, J.A.; Bouffartigues, E.; Boireau, W.; et al. Pseudomonas aeruginosa expresses a functional human natriuretic peptide receptor ortholog: involvement in biofilm formation. mBio 2015, 6, e01033-15. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Pamp, S.J.; Tolker-Nielsen, T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.E.; Caiazza, N.C.; O’Toole, G.A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003, 185, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Bazire, A.; Diab, F.; Taupin, L.; Rodrigues, S.; Jebbar, M.; Dufour, A. Effects of osmotic stress on rhamnolipid synthesis and time-course production of cell-to-cell signal molecules by Pseudomonas aeruginosa. Open Microbiol. J. 2009, 3, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Brissonnet, F.; Trotier, E.; Briandet, R. The biofilm lifestyle involves an increase in bacterial membrane saturated fatty acids. Front. Microbiol. 2016, 7, 1673. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; England, L.S.; Trevors, J.T. Cytoplasmic membrane polarization in Gram-positive and Gram-negative bacteria grown in the absence and presence of tetracycline. Biochim. Biophys. Acta 2004, 1672, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Tajima, F.; Itoh, H.; Nakata, Y.; Hama, N.; Nakagawa, O.; Nakao, K.; Kawai, T.; Torikata, C.; Suga, T.; et al. Expression of C-type natriuretic peptide during development of rat lung. Am. J. Physiol. 1999, 277, L996–L1002. [Google Scholar] [CrossRef] [PubMed]
- Veron, W.; Lesouhaitier, O.; Pennanec, X.; Rehel, K.; Leroux, P.; Orange, N.; Feuilloley, M.G. Natriuretic peptides affect Pseudomonas aeruginosa and specifically modify lipopolysaccharide biosynthesis. FEBS J. 2007, 274, 5852–5864. [Google Scholar] [CrossRef] [PubMed]
- Clamens, T.; Rosay, T.; Crepin, A.; Grandjean, T.; Kentache, T.; Hardouin, J.; Bortolotti, P.; Neidig, A.; Mooij, M.; Hillion, M.; et al. The aliphatic amidase AmiE is involved in regulation of Pseudomonas aeruginosa virulence. Sci. Rep. 2017, 7, 41178. [Google Scholar] [CrossRef] [PubMed]
- Kourie, J.I.; Hanna, E.A.; Henry, C.L. Properties and modulation of alpha human atrial natriuretic peptide (alpha-hANP)-formed ion channels. Can. J. Physiol. Pharmacol. 2001, 79, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Kourie, J.I. Synthetic mammalian C-type natriuretic peptide forms large cation channels. FEBS Lett. 1999, 445, 57–62. [Google Scholar] [CrossRef]
- Sharma, S.; Sahoo, N.; Bhunia, A. Antimicrobial peptides and their pore/ion channel properties in neutralization of pathogenic microbes. Curr. Top. Med. Chem. 2016, 16, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Oguri, T.; Yeo, W.-S.; Bae, T.; Lee, H. Identification of EnvC and its cognate amidases as novel determinants of intrinsic resistance to cationic antimicrobial peptides. Antimicrob. Agents Chemother. 2016, 60, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Raya, A.; Sodagari, M.; Pinzon, N.M.; He, X.; Zhang Newby, B.-M.; Ju, L.-K. Effects of rhamnolipids and shear on initial attachment of Pseudomonas aeruginosa PAO1 in glass flow chambers. Environ. Sci. Pollut. Res. Int. 2010, 17, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, M.; Claverol, S.; Lomenech, A.-M.; Le Sénéchal, C.; Costaglioli, P.; Barthe, C.; Garbay, B.; Bonneu, M.; Vilain, S. Pseudomonas aeruginosa cells attached to a surface display a typical proteome early as 20 min of incubation. PLoS ONE 2017, 12, e0180341. [Google Scholar] [CrossRef] [PubMed]
- Bohn, Y.-S.T.; Brandes, G.; Rakhimova, E.; Horatzek, S.; Salunkhe, P.; Munder, A.; van Barneveld, A.; Jordan, D.; Bredenbruch, F.; Häußler, S.; et al. Multiple roles of Pseudomonas aeruginosa TBCF10839 PilY1 in motility, transport and infection. Mol. Microbiol. 2008, 71, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Heiniger, R.W.; Winther-Larsen, H.C.; Pickles, R.J.; Koomey, M.; Wolfgang, M.C. Infection of human mucosal tissue by Pseudomonas aeruginosa requires sequential and mutually dependent virulence factors and a novel pilus-associated adhesin. Cell. Microbiol. 2010, 12, 1158–1173. [Google Scholar] [CrossRef] [PubMed]
- Boechat, A.L.; Kaihami, G.H.; Politi, M.J.; Lépine, F.; Baldini, R.L. A novel role for an ECF sigma factor in fatty acid biosynthesis and membrane fluidity in Pseudomonas aeruginosa. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.L.; Liu, Y.; Yam, J.K.H.; Chen, Y.; Vejborg, R.M.; Tan, B.G.C.; Kjelleberg, S.; Tolker-Nielsen, T.; Givskov, M.; Yang, L. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat. Commun. 2014, 5, 4462. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Murphy, K.; Krieger, J.R.; Brewer, D.; Taylor, P.; Habash, M.; Khursigara, C.M. A temporal examination of the planktonic and biofilm proteome of whole cell Pseudomonas aeruginosa PAO1 using quantitative mass spectrometry. Mol. Cell. Proteom. 2014, 13, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Murphy, K.; Surette, M.D.; Bandoro, C.; Krieger, J.R.; Taylor, P.; Khursigara, C.M. Tracking the Dynamic Relationship between Cellular Systems and Extracellular Subproteomes in Pseudomonas aeruginosa Biofilms. J. Proteome Res. 2015, 14, 4524–4537. [Google Scholar] [CrossRef] [PubMed]
- Colucci, W.S.; Elkayam, U.; Horton, D.P.; Abraham, W.T.; Bourge, R.C.; Johnson, A.D.; Wagoner, L.E.; Givertz, M.M.; Liang, C.S.; Neibaur, M.; et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N. Engl. J. Med. 2000, 343, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Edelson, J.D.; Makhlina, M.; Silvester, K.R.; Vengurlekar, S.S.; Chen, X.; Zhang, J.; Koziol-White, C.J.; Cooper, P.R.; Hallam, T.J.; Hay, D.W.; et al. In vitro and in vivo pharmacological profile of PL-3994, a novel cyclic peptide (Hept-cyclo(Cys-His-Phe-d-Ala-Gly-Arg-d-Nle-Asp-Arg-Ile-Ser-Cys)-Tyr-[Arg mimetic]-NH(2)) natriuretic peptide receptor-A agonist that is resistant to neutral endopeptidase and acts as a bronchodilator. Pulm. Pharmacol. Ther. 2013, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Nojiri, T.; Hino, J.; Hosoda, H.; Miura, K.; Shintani, Y.; Inoue, M.; Zenitani, M.; Takabatake, H.; Miyazato, M.; et al. C-type natriuretic peptide ameliorates pulmonary fibrosis by acting on lung fibroblasts in mice. Respir. Res. 2016, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Liberati, N.T.; Urbach, J.M.; Miyata, S.; Lee, D.G.; Drenkard, E.; Wu, G.; Villanueva, J.; Wei, T.; Ausubel, F.M. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. USA 2006, 103, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Pamp, S.J.; Sternberg, C.; Tolker-Nielsen, T. Insight into the microbial multicellular lifestyle via flow-cell technology and confocal microscopy. Cytometry A 2009, 75, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Tolker-Nielsen, T.; Sternberg, C. Growing and analyzing biofilms in flow chambers. Curr. Protoc. Microbiol. 2011. [Google Scholar] [CrossRef]
- Heydorn, A.; Ersboll, B.K.; Hentzer, M.; Parsek, M.R.; Givskov, M.; Molin, S. Experimental reproducibility in flow-chamber biofilms. Microbiology 2000, 146, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Bazire, A.; Dheilly, A.; Diab, F.; Morin, D.; Jebbar, M.; Haras, D.; Dufour, A. Osmotic stress and phosphate limitation alter production of cell-to-cell signal molecules and rhamnolipid biosurfactant by Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2005, 253, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Déziel, E.; Lépine, F.; Milot, S.; Villemur, R. Mass spectrometry monitoring of rhamnolipids from a growing culture of Pseudomonas aeruginosa strain 57RP. Biochim. Biophys. Acta 2000, 1485, 145–152. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desriac, F.; Clamens, T.; Rosay, T.; Rodrigues, S.; Tahrioui, A.; Enault, J.; Roquigny, L.; Racine, P.-J.; Taupin, L.; Bazire, A.; et al. Different Dose-Dependent Modes of Action of C-Type Natriuretic Peptide on Pseudomonas aeruginosa Biofilm Formation. Pathogens 2018, 7, 47. https://doi.org/10.3390/pathogens7020047
Desriac F, Clamens T, Rosay T, Rodrigues S, Tahrioui A, Enault J, Roquigny L, Racine P-J, Taupin L, Bazire A, et al. Different Dose-Dependent Modes of Action of C-Type Natriuretic Peptide on Pseudomonas aeruginosa Biofilm Formation. Pathogens. 2018; 7(2):47. https://doi.org/10.3390/pathogens7020047
Chicago/Turabian StyleDesriac, Florie, Thomas Clamens, Thibaut Rosay, Sophie Rodrigues, Ali Tahrioui, Jérémy Enault, Lucille Roquigny, Pierre-Jean Racine, Laure Taupin, Alexis Bazire, and et al. 2018. "Different Dose-Dependent Modes of Action of C-Type Natriuretic Peptide on Pseudomonas aeruginosa Biofilm Formation" Pathogens 7, no. 2: 47. https://doi.org/10.3390/pathogens7020047




