Legionella pneumophila Carbonic Anhydrases: Underexplored Antibacterial Drug Targets
Abstract
:1. Introduction
2. Cloning and Biochemical Properties of LpCA1 and LpCA2, the β-CAs from L. pneumophila
3. Sulfonamide/Sulfamate Inhibition Studies of LpCA1 and LpCA2
- (i)
- In the case of the slow isoform LpCA1, the less effective inhibitors were SAC and HCT which showed affinities in the micromolar range (KIs of 15.8–20.5 µM), whereas compounds 1–3, 5–11, 14–18, DCP, DZA, ZNS, IND, and CLX were poorly effective as CAIs against LpCA1 (KIs in the range of 734–3540 nM, Table 3). Benzenesulfonamides with a more complicated substitution pattern (DCP, IND, or CLX) were also weak—medium potency inhibitors of this enzyme, together with heterocyclic sulfonamides such as 14, DZA, and ZNS.
- (ii)
- A better inhibitory power against LpCA1, with inhibition constants ranging between 101 and 665 nM was observed for the following derivatives: 12, 13, 19, 21, 22, MZA, BRZ, BZA, TPM, SLP, VLX, and SLT. An increase of LpCA1 inhibitory activity was seen for 12 (compared to the structurally related 11) or 13 over the structurally related 14. In fact the two pairs of compounds are rather similar: 12 has a chlorine instead the CF3 moiety of 11, but their KIs differ by a factor of 1.44. Thus, rather small structural changes in the scaffold of the inhibitors lead to important changes in the inhibitory power of the compound against the LpCA1 enzyme.
- (iii)
- The best LpCA1 inhibitors were 20, 23, 24, AAZ, and EZA (KIs in the range of 40.3–90.5 nM, Table 3). In this case the SAR is very interesting. In addition to the heterocyclic derivatives AAZ and EZA, which are usually highly potent CAIs of most investigated Cas [18], acting thus as promiscuous inhibitors, the potent LpCA1 inhibitors possessed a rather similar structure of elongated, sulfonylated sulfonamide type. Aminobenzolamide 20 (and benzolamide BZA) are the prototype of such compounds, but interestingly, the aromatic compounds 23 and 24 were the best LpCA1 inhibitors. Furthermore, LpCA1 inhibition clearly increased with an increase in the spacer between the two aminobenzenesulfonyl fragments from the molecules. Indeed, 23 and 24 showed KIs of 59.8 and 40.3 nM, respectively (Table 3).
- (iv)
- The fast Legionella isoform, LpCA2 was also inhibited by all sulfonamides/sulfamates investigated so far (Table 3). The inhibition range was however not as wide as for the previous isoform (LpCA1), as the best LpCA2 inhibitor showed a KI of 25.2 nM and the worst one of 933 nM. A small number of the investigated sulfonamides/sulfamates were quite weak LpCA2 inhibitors, among which compound 3, TPM, ZNS, VLX, and HCT (KIs in the range of 745–933 nM, Table 3).
- (v)
- Many of the investigated sulfonamides were medium potency LpCA2 inhibitors, with KIs in the range of 103–721 nM. They include: 1, 2, 4–19, EZA, DZA, BRZ, BZA, SLP, IND, CLX, SLT, and SAC (Chart 1 and Table 3). Simple benzenesulfonamide incorporating one or two substituents of the amino, aminoalkyl, hydroxy, hydroxyalkyl, halogens, sufamoyl etc. type, were not very different in their behavior as LpCA2 inhibitors, all of them leading to the medium potency inhibition profiles. The various aromatic/heterocyclic scaffolds present in the clinically used drugs EZA, DZA, BRZ, BZA, SLP, IND, CLX, SLT, and SAC were also comparable to the simple scaffolds present in compounds 1, 2, and 3–19.
- (vi)
- The best LpCA2 inhibitors were 20–24, AAZ, MZA, and DCP (KIs in the range of 25.2–88.5 nM, Table 3). SAR is highly interesting here. Except the clinically used drugs (AAZ, MZA, and DCP) which have not much in common, all the other effective LpCA2 inhibitors possess the same scaffold, of the arylsulfonylated aminosulfonamide type. Thus, aminobenzolamide 20 was 3.3 times more effective as LpCA2 inhibitor compared to benzolamide BZA, whereas for the aromatic componds 22–24, as for LpCA1, the activity increases with the increase of the molecule length, the best LpCA2 inhibitor being 24 (this was also the best LpCA1 inhibitor detected so far).
- (vii)
- Except for some of the effective CAIs detected here, which showed a good activity against both LpCA1 and LpCA2 (e.g., 22–24 and AAZ), generally the two isoforms had a rather different affinity for these inhibitors. For example SAC was a very weak LpCA1 inhibitor but a medium potency LpCA2 inhibitor. The same behavior was observed for DCP. Most of the time, these compounds showed an enhanced inhibition of LpCA2 over LpCA1, although several compounds with the reverse profile (e.g., 4, EZA, VLX) were also detected.
- (viii)
- The inhibition profiles of the two Legionella enzymes is very different compared to that of other bacterial β-CAs (e.g., HypCA) or the off-target, human isoforms hCA I and II (Table 3). This is of interest in case some of these compounds should be used for targeting the bacterial over the human isofoms in experimental or clinical settings.
4. Inorganic Anions and Other Small Molecule LpCA1/LpCA2 Inhibitors
- (i)
- Perchlorate and tetrafluoroborate did not inhibit the two new β-CAs reported here (KI > 200 mM). Similar results have been observed in most of the CAs examined to date: only HpyCA was effectively inhibited by perchlorate, with a KI of 6.5 mM [45]. Sulfate was also an ineffective LpCA1/LpCA2 inhibitor, with KI values between 77.9–96.5 mM (Table 4). Iodide and nitrate were also quite weak LpCA2 inhibitors, with inhibition constants of 59.1 and 30.1 mM, respectively.
- (ii)
- Another group of anions inhibited LpCA1 and LpCA2 weakly, with inhibition constants in the range of 3.5–9.1 mM. They include bicarbonate, carbonate, nitrate, nitrite, hydrogen sulfite, selenate and fluorosulfonate against LpCA1, whereas for LpCA2, the weak inhibitors included bromide, bicarbonate, carbonate, nitrite, and hydrogen sulfite (Table 4).
- (iii)
- A large number of the anions acted as submillimolar inhibitors against both these enzymes. All of the halides, cyanate, thiocyanate, stannate, tellurate, pyrophosphate, divanadate, tetraborate, perrhenate, perruthenate, peroxydisulfate, selenocyanate, and trithiocarbonate inhibited LpCA1 with KI values from 0.24–0.98 mM. Iminodisulfonate was less effective as an LpCA1 inhibitor (KI of 1.17 mM). The effective, submillimolar LpCA2 inhibitors were fluoride, chloride, cyanate, thiocyanate, cyanide, azide, hydrogen sulfide, stannate, tellurate, pyrophosphate, divanadate, tetraborate, perrhenate, perruthenate, peroxydisulfate, selenocyanate, trithiocarbonate, fluorosulfonate, and iminodisulfonate (KI values ranging from 0.29–0.96 mM, Table 4). The best inhibitor in this subseries was tellurate, with KI values of 0.24 and 0.29 mM against LpCA1 and LpCA2, respectively.
- (iv)
- The best anionic LpCA1 inhibitors were cyanide, azide, hydrogen sulfide, diethyldithiocarbamate, sulfamide, sulfamate, phenylboronic acid, and phenylarsonic acid (KI of 6–94 µM). N,N-diethyldithiocarbamate had a much higher affinity for LpCA1, with a low micromolar value for KI of 6 µM. However, all of the small molecules were low micromolar inhibitors of LpCA2, with KI values ranging between 2 and 13 µM (Table 4).
- (v)
- There are net differences in the behavior of the two Legionella enzymes towards the anionic inhibitors investigated here. LpCA1 showed higher affinity for some poisonous metal anions such as cyanide, azide, and hydrogen sulfide, which are around one order of magnitude more potent inhibitors against LpCA1 than LpCA2. However, LpCA2 showed higher affinity for N,N-diethyldithiocarbamate, sulfamide, sulfamate, phenylboronic acid, and phenylarsonic acid compared to LpCA1.
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Furtado, G.H.; Nicolau, D.P. Overview perspective of bacterial resistance. Expert Opin. Ther. Pat. 2010, 20, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Dye, C. Doomsday postponed? Preventing and reversing epidemics of drug-resistant tuberculosis. Nat. Rev. Microbiol. 2009, 7, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, A.M. Emerging drugs for active tuberculosis. Semin. Respir. Crit. Care Med. 2008, 29, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Inhibition of bacterial carbonic anhydrases and zinc proteases: From orphan targets to innovative new drugs? Curr. Med. Chem. 2012, 19, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Bacterial carbonic anhydrases as drug targets: Toward novel antibiotics? Front. Pharmacol. 2011, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin. Ther. Targets 2015, 19, 1689–1704. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An Overview of the Selectivity and Efficiency of the Bacterial Carbonic Anhydrase Inhibitors. Curr. Med. Chem. 2015, 22, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzym. Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Sulfa and trimethoprim-like drugs—Antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J. Enzym. Inhib. Med. Chem. 2014, 29, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Anti-infective carbonic anhydrase inhibitors: a patent and literature review. Expert Opin. Ther. Pat. 2013, 23, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Macielag, M.J. New β-lactam antibiotics and β-lactamase inhibitors. Expert Opin. Ther. Pat. 2010, 20, 1277–1293. [Google Scholar] [CrossRef] [PubMed]
- Cloeckaert, A.; Schwarz, S. Molecular characterization, spread and evolution of multidrug resistance in Salmonella enterica typhimurium DT104. Vet. Res. 2001, 32, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Showalter, H.D.; Denny, W.A. A roadmap for drug discovery and its translation to small molecule agents in clinical development for tuberculosis treatment. Tuberculosis (Edinb) 2008, 88, S3–S17. [Google Scholar] [CrossRef]
- Suarez Covarrubias, A.; Bergfors, T.; Jones, T.A.; Hogbom, M. Structural mechanics of the pH-dependent activity of the β-carbonic anhydrase from Mycobacterium tuberculosis. J. Biol. Chem. 2006, 281, 4993–4999. [Google Scholar] [CrossRef] [PubMed]
- Suarez Covarrubias, A.; Larsson, A.M.; Hogbom, M.; Lindberg, J.; Bergfors, T.; Bjorkelid, C.; Mowbray, S.L.; Unge, T.; Jones, T.A. Structure and function of carbonic anhydrases from Mycobacterium tuberculosis. J. Biol. Chem. 2005, 280, 18782–18789. [Google Scholar] [CrossRef] [PubMed]
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2002, 347, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Tsolis, R.M.; Young, G.M.; Solnick, J.V.; Bäumler, A.J. From bench to bedside: stealth of enteroinvasive pathogens. Nat. Rev. Microbiol. 2008, 6, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibition/activation: Trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr. Pharm. Des. 2010, 16, 3233–3245. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Scozzafava, A.; Casini, A. Carbonic anhydrase inhibitors. Med. Res. Rev. 2003, 23, 146–189. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016. [Google Scholar] [CrossRef]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J Enzym. Inhib. Med. Chem. 2016, 31, 345–366. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med. Chem. 2011, 3, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; Fisher, G.M.; Andrews, K.T.; Poulsen, S.A.; Capasso, C.; Supuran, C.T. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum-the eta-carbonic anhydrases. Bioorg. Med. Chem. Lett. 2014, 24, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. The eta-class carbonic anhydrases as drug targets for antimalarial agents. Expert Opin. Ther. Targets 2015, 19, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.A.; Ferry, J.G.; Supuran, C.T. Inhibition of the Archaeal β-Class (Cab) and γ-Class (Cam) Carbonic Anhydrases. Curr. Top. Med. Chem. 2007, 7, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Langella, E.; Viparelli, F.; Vullo, D.; Ascione, G.; Dathan, N.A.; Morel, F.M.M.; Supuran, C.T.; de Simone, G.; Monti, S.M. Structural and inhibition insights into carbonic anhydrase CDCA1 from the marine diatom Thalassiosira weissflogii. Biochimie 2012, 94, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Ferraroni, M.; del Prete, S.; Vullo, D.; Capasso, C.; Supuran, C.T. Crystal structure and kinetic studies of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Acta Crystall. D 2015, 71, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; de Luca, V.; Vullo, D.; Scozzafava, A.; Carginale, V.; Supuran, C.T.; Capasso, C. Biochemical characterization of the gamma-carbonic anhydrase from the oral pathogen Porphyromonas gingivalis, PgiCA. J. Enzym. Inhib. Med. Chem. 2014, 29, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; de Luca, V.; Carginale, V.; Scozzafava, A.; Supuran, C.T.; Capasso, C. A highly catalytically active gamma-carbonic anhydrase from the pathogenic anaerobe Porphyromonas gingivalis and its inhibition profile with anions and small molecules. Bioorg. Med. Chem. Lett. 2013, 23, 4067–4071. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Parkkila, S.; Pastorek, J.; Supuran, C.T. Carbonic anhydrases: Current state of the art, therapeutic applications and future prospects. J. Enzym. Inhib. Med. Chem. 2004, 19, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; de Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Alterio, V.; Supuran, C.T. Exploiting the hydrophobic and hydrophilic binding sites for designing carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2013, 8, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.W.; Tsai, T.R.; Orenstein, W.; Parkin, W.E.; Beecham, H.J.; Sharrar, R.G.; Harris, J.; Mallison, G.F.; Martin, S.M.; McDade, J.E.; et al. Legionnaires’ disease: Description of an epidemic of pneumonia. N. Engl. J. Med. 1977, 297, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, P.H.; Finegold, S.M. Isolation of Legionella pneumophila from a transtracheal aspirate. J. Clin. Microbiol. 1979, 9, 457–458. [Google Scholar] [PubMed]
- Gomez-Valero, L.; Rusniok, C.; Cazalet, C.; Buchrieser, C. Comparative and functional genomics of legionella identified eukaryotic like proteins as key players in host-pathogen interactions. Front. Microbiol. 2011, 2, 208. [Google Scholar] [CrossRef] [PubMed]
- Escoll, P.; Rolando, M.; Gomez-Valero, L.; Buchrieser, C. From Amoeba to Macrophages: Exploring the Molecular Mechanisms of Legionella pneumophila Infection in Both Hosts. Curr. Top. Microbiol. Immunol. 2013, 376, 1–34. [Google Scholar] [PubMed]
- Xu, L.; Luo, Z.Q. Cell biology of infection by Legionella pneumophila. Microbes Infect. 2013, 15, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.; Morozova, I.; Shi, S.; Sheng, H.; Chen, J.; Gomez, S.M.; Asamani, G.; Hill, K.; Nuara, J.; Feder, M.; et al. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 2004, 305, 1966. [Google Scholar] [CrossRef]
- Horwitz, M.A.; Maxfield, F.R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell. Biol. 1984, 99, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Vullo, D.; Minakuchi, T.; Scozzafava, A.; Osman, S.M.; AlOthman, Z.; Capasso, C.; Supuran, C.T. Anion inhibition studies of two new beta-carbonic anhydrases from the bacterial pathogen Legionella pneumophila. Bioorg. Med. Chem. Lett. 2014, 24, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Vullo, D.; Minakuchi, T.; Scozzafava, A.; Capasso, C.; Supuran, C.T. Sulfonamide inhibition studies of two beta-carbonic anhydrases from the bacterial pathogen Legionella pneumophila. Bioorg. Med. Chem. 2014, 22, 2939–2946. [Google Scholar] [CrossRef]
- Nishimori, I.; Minakuchi, T.; Kohsaki, T.; Onishi, S.; Takeuchi, H.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. The β-carbonic anhydrase from Helicobacter pylori is a new target for sulfonamide and sulfamate inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 3585–3594. [Google Scholar] [CrossRef]
- Joseph, P.; Turtaut, F.; Ouahrani-Bettache, S.; Montero, J.L.; Nishimori, I.; Minakuchi, T.; Vullo, D.; Scozzafava, A.; Köhler, S.; Winum, J.Y.; et al. Cloning, characterization and inhibition studies of a β-carbonic anhydrase from Brucella suis. J. Med. Chem. 2010, 53, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Ouahrani-Bettache, S.; Montero, J.L.; Nishimori, I.; Vullo, D.; Scozzafava, A.; Winum, J.Y.; Köhler, S.; Supuran, C.T. A new Brucella suis β-carbonic anhydrase, bsCA II: inhibition of bsCA I and II with sulfonamides and sulfamates inhibits the pathogen growth. Bioorg. Med. Chem. 2011, 19, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Minakuchi, T.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Inhibition studies of the β-carbonic anhydrases from the bacterial pathogen Salmonella enterica serovar Typhimurium with sulfonamides and sulfamates. Bioorg. Med. Chem. 2011, 19, 5023–5030. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Nishimori, I.; Minakuchi, T.; Scozzafava, A.; Supuran, C.T. Inhibition studies with anions and small molecules of two novel β-carbonic anhydrases from the bacterial pathogen Salmonella enterica serovar Typhimurium. Bioorg. Med. Chem. Lett. 2011, 21, 3591–3595. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, C.; Hall, R.A.; Vullo, D.; Middelhaufe, S.; Gertz, M.; Supuran, C.T.; Mühlschlegel, F.A.; Steegborn, C. Structure and inhibition of the CO2 sensing carbonic anhydrase Can2 from the pathogenic fungus Cryptococcus neoformans. J. Mol. Biol. 2009, 385, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Hall, R.A.; Schlicker, C.; Mühlschlegel, F.A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of the beta-class enzymes from the fungal pathogens Candida albicans and Cryptococcus neoformans with aliphatic and aromatic carboxylates. Bioorg. Med. Chem. 2009, 17, 2654–2657. [Google Scholar] [CrossRef] [PubMed]
- Isik, S.; Kockar, F.; Aydin, M.; Arslan, O.; Guler, O.O.; Innocenti, A.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of the β-class enzyme from the yeast Saccharomyces cerevisiae with sulfonamides and sulfamates. Bioorg. Med. Chem. 2009, 17, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.; Innocenti, A.; Casini, A.; Ferry, J.G.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of the prokariotic beta and gamma-class enzymes from Archaea with sulfonamides. Bioorg. Med. Chem. Lett. 2004, 14, 6001–6006. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Carta, F.; Scozzafava, A.; Supuran, C.T. Antiglaucoma carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Scozzafava, A.; Supuran, C.T. Which carbonic anhydrases are targeted by the antiepileptic sulfonamides and sulfamates? Chem. Biol. Drug Des. 2009, 74, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Supuran, C.T. Diuretics with carbonic anhydrase inhibitory action: A patent and literature review (2005–2013). Expert Opin. Ther. Pat. 2013, 23, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Minakuchi, T.; Morimoto, K.; Sano, S.; Onishi, S.; Takeuchi, H.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: DNA cloning and inhibition studies of the alpha-carbonic anhydrase from Helicobacter pylori, a new target for developing sulfonamide and sulfamate gastric drugs. J. Med. Chem. 2006, 49, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Buzas, G.M.; Supuran, C.T. The history and rationale of using carbonic anhydrase inhibitors in the treatment of peptic ulcers. In memoriam Ioan Puşcaş (1932–2015). J. Enzym. Inhib. Med. Chem. 2016, 31, 527–533. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Supuran, C.T. (In) organic anions as carbonic anhydrase inhibitors. J. Inorg. Biochem. 2012, 111, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hainzl, T.; Grundström, C.; Forsman, C.; Samuelsson, G.; Sauer-Eriksson, A.E. Structural studies of β-carbonic anhydrase from the green alga Coccomyxa: Inhibitor complexes with anions and acetazolamide. PLoS ONE 2011, 6, e28458. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.A.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Temperini, C.; Innocenti, A.; Scozzafava, A.; Kaila, K.; Supuran, C.T. Polyamines inhibit carbonic anhydrases by anchoring to the zinc-coordinated water molecule. J. Med. Chem. 2010, 53, 5511–5522. [Google Scholar] [CrossRef] [PubMed]
- Tars, K.; Vullo, D.; Kazaks, A.; Leitans, J.; Lends, A.; Grandane, A.; Zalubovskis, R.; Scozzafava, A.; Supuran, C.T. Sulfocoumarins (1,2-benzoxathiine 2,2-dioxides): A class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J. Med. Chem. 2013, 56, 293–300. [Google Scholar] [CrossRef] [PubMed]
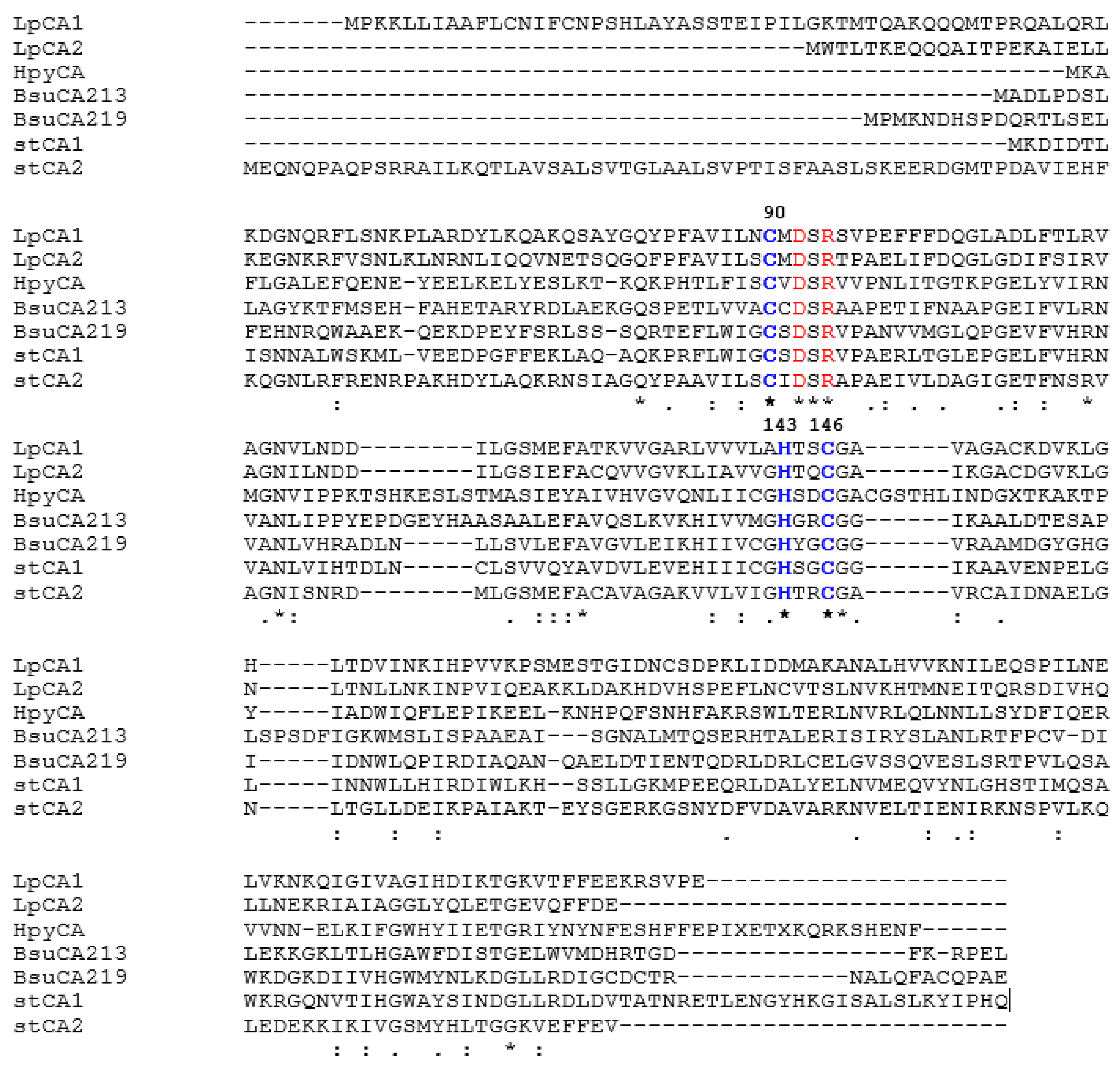


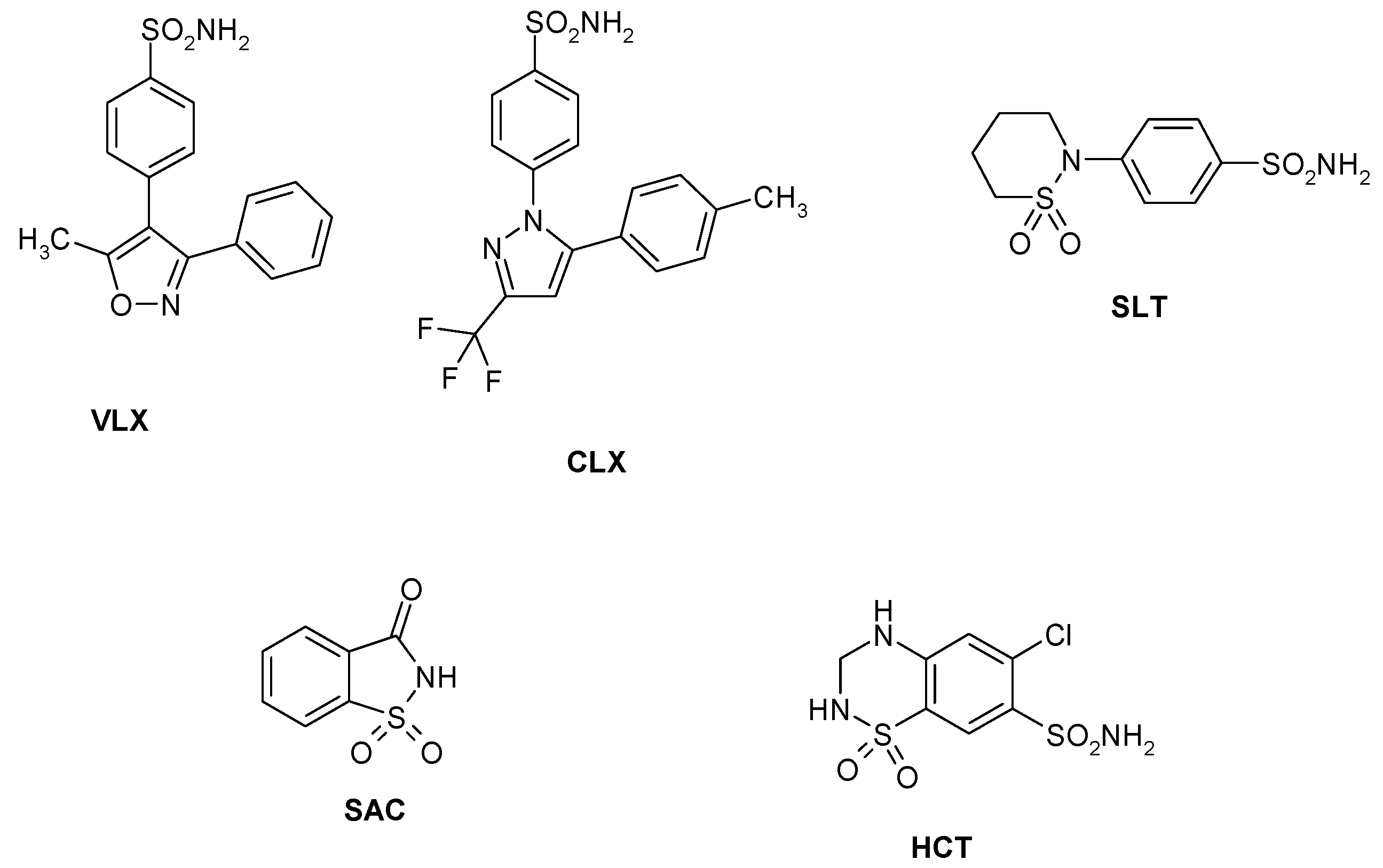
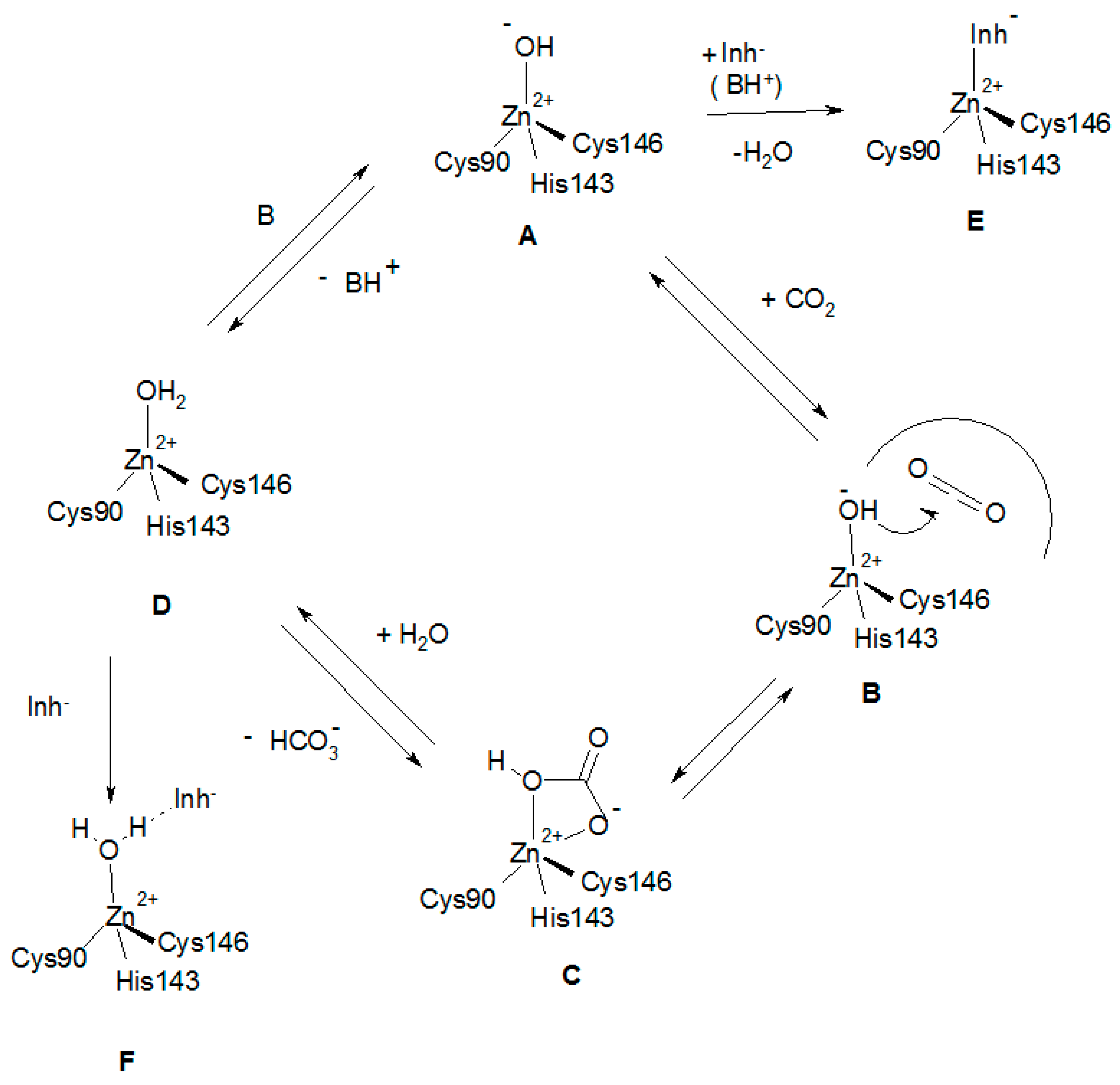
| Isozyme | Activity Level | Class (s−1) | kcat (M−1·s−1) | kcat/Km (nM) | KI (AAZ) | Ref. |
|---|---|---|---|---|---|---|
| hCA I | moderate | α | 2.0 × 105 | 5.0 × 107 | 250 | [18] |
| hCA II | very high | α | 1.4 × 106 | 1.5 × 108 | 12 | [18] |
| Can2 | moderate | β | 3.9 × 105 | 4.3 × 107 | 10.5 | [50] |
| CalCA | high | β | 8.0 × 105 | 9.7 × 107 | 132 | [51] |
| SceCA | high | β | 9.4 × 105 | 9.8 × 107 | 82 | [52] |
| HpyCA | moderate | β | 7.1 × 105 | 4.8 × 107 | 40 | [45] |
| BsuCA219 | moderate | β | 6.4 × 105 | 3.9 × 107 | 63 | [46] |
| BsuCA213 | high | β | 1.1 × 106 | 8.9 × 107 | 303 | [47] |
| LpCA1 | moderate | β | 3.4 × 105 | 4.7 × 107 | 76.8 | [43,44] |
| LpCA2 | high | β | 8.3 × 105 | 8.5 × 107 | 72.1 | [43,44] |
| Domain | Species | Accession Number | Cryptonym |
|---|---|---|---|
| Bacteria | Legionella pneumophila | WP_014844650.1 | LpCA1 |
| Legionella pneumophila | WP_014842179.1 | LpCA2 | |
| Myroides injenensis | ZP_10784819.1 | MinCA | |
| Porphyromonas gingivalis | YP_001929649.1 | PgiCA | |
| Acinetobacter baumannii | YP_002326524 | AbaCA | |
| Escherichia coli | ACI70660 | EcoCa | |
| Helicobacter pylori | BAF34127.1 | HpyCA | |
| Burkholderia thailandensis Bt4 | ZP_02386321.1 | BthCA | |
| Brucella suis 1330 | NP_699962.1 | BsuCA | |
| Archaea | Methanobacterium thermoautotrophicum | GI:13786688 | Cab |
| Eukaryota (fungus) | Saccharomyces cerevisiae | GAA26059 | SceCA |
| Dekkera bruxellensis AWRI1499 | EIF49256 | DbrCA | |
| Schizosaccharomyces pombe | CAA21790 | SpoCA | |
| Eukaryota (green alga) | Coccomyxa sp. | AAC33484.1 | CspCA |
| Chlamydomonas reinhardtii | XP_001699151.1 | CreCA | |
| Eukaryota (green plant) | Vigna radiata | AAD27876 | VraCA |
| Flaveria bidentis, isoform I | AAA86939.2 | FbiCA | |
| Zea mays | NP_001147846.1 | ZmaCA | |
| Arabidopsis thaliana | AAA50156 | AthCA |
| Inhibitor | KI (nM) | ||||
|---|---|---|---|---|---|
| Enzyme Class | hCA I | hCA II | HpyCA | LpCA1 | LpCA2 |
| α | α | β | β | β | |
| 1 | 28,000 | 300 | nt | 939 | 455 |
| 2 | 25,000 | 240 | 1845 | 946 | 277 |
| 3 | 79 | 8 | nt | 1060 | 933 |
| 4 | 78,500 | 320 | 2470 | 556 | 624 |
| 5 | 25,000 | 170 | 2360 | 757 | 516 |
| 6 | 21,000 | 160 | 3500 | 734 | 375 |
| 7 | 8300 | 60 | 1359 | 770 | 592 |
| 8 | 9800 | 110 | 1463 | 866 | 396 |
| 9 | 6500 | 40 | 1235 | 988 | 181 |
| 10 | 7300 | 54 | nt | 913 | 622 |
| 11 | 5800 | 63 | 973 | 929 | 593 |
| 12 | 8400 | 75 | 640 | 642 | 496 |
| 13 | 8600 | 60 | 2590 | 541 | 382 |
| 14 | 9300 | 19 | 768 | 913 | 391 |
| 15 | 5500 | 80 | nt | 969 | 280 |
| 16 | 9500 | 94 | 236 | 2260 | 631 |
| 17 | 21,000 | 125 | 218 | 3540 | 721 |
| 18 | 164 | 46 | 450 | 2390 | 476 |
| 19 | 109 | 33 | 38 | 472 | 321 |
| 20 | 6 | 2 | 64 | 90.5 | 45.1 |
| 21 | 69 | 11 | nt | 101 | 78.9 |
| 22 | 164 | 46 | nt | 319 | 52.3 |
| 23 | 109 | 33 | 87 | 59.8 | 50.1 |
| 24 | 95 | 30 | 71 | 40.3 | 25.2 |
| AAZ | 250 | 12 | 40 | 76.8 | 72.1 |
| MZA | 50 | 14 | 176 | 201 | 88.5 |
| EZA | 25 | 8 | 33 | 71.4 | 103 |
| DCP | 1200 | 38 | 105 | 1670 | 64.1 |
| DZA | 50,000 | 9 | 73 | 2070 | 336 |
| BRZ | 45,000 | 3 | 128 | 648 | 467 |
| BZA | 15 | 9 | 54 | 159 | 148 |
| TPM | 250 | 10 | 32 | 665 | 882 |
| ZNS | 56 | 35 | 254 | 831 | 820 |
| SLP | 1200 | 40 | 35 | 253 | 245 |
| IND | 31 | 15 | 143 | 1090 | 525 |
| VLX | 54,000 | 43 | nt | 536 | 879 |
| CLX | 50,000 | 21 | nt | 990 | 421 |
| SLT | 374 | 9 | nt | 485 | 463 |
| SAC | 18,540 | 5959 | nt | 20,500 | 441 |
| HCT | 328 | 290 | nt | 15,800 | 745 |
| Inhibitor § | KI (mM) | |||
|---|---|---|---|---|
| hCA II | HpyCA | LpCA1 | LpCA2 | |
| α | β | β | β | |
| F− | >300 | 0.67 | 0.91 | 0.77 |
| Cl− | 200 | 0.56 | 0.79 | 0.81 |
| Br− | 63 | 0.38 | 0.65 | 8.0 |
| I− | 26 | 0.63 | 0.32 | 59.1 |
| CNO− | 0.03 | 0.37 | 0.66 | 0.96 |
| SCN− | 1.60 | 0.68 | 0.52 | 0.88 |
| CN− | 0.02 | 0.54 | 0.064 | 0.61 |
| N3− | 1.51 | 0.80 | 0.077 | 0.45 |
| HCO3− | 85 | 0.50 | 3.5 | 6.6 |
| CO32− | 73 | 0.42 | 4.7 | 4.8 |
| NO3− | 35 | 0.78 | 7.6 | 30.1 |
| NO2− | 63 | 0.67 | 7.9 | 5.8 |
| HS− | 0.04 | 0.58 | 0.076 | 0.51 |
| HSO3− | 89 | 0.63 | 6.6 | 7.2 |
| SnO32− | 0.83 | 0.48 | 0.57 | 0.63 |
| SeO42− | 112 | 0.65 | 7.3 | 0.66 |
| TeO42− | 0.92 | 0.45 | 0.24 | 0.29 |
| P2O74− | 48.50 | 0.75 | 0.94 | 0.83 |
| V2O74− | 0.57 | 0.18 | 0.39 | 0.47 |
| B4O72− | 0.95 | 0.68 | 0.60 | 0.55 |
| ReO4− | 0.75 | 0.82 | 0.89 | 0.77 |
| RuO4− | 0.69 | 1.10 | 0.82 | 0.86 |
| S2O82− | 0.084 | 0.93 | 0.85 | 0.57 |
| SeCN− | 0.086 | 0.97 | 0.98 | 0.66 |
| CS32− | 0.0088 | 0.21 | 0.53 | 0.62 |
| Et2NCS2− | 3.1 | 0.0074 | 0.006 | 0.002 |
| SO42− | >200 | 0.57 | 77.9 | 96.5 |
| ClO4− | >200 | 6.50 | >200 | >200 |
| BF4− | >200 | >200 | >200 | >200 |
| FSO3− | 0.46 | 0.75 | 9.1 | 0.46 |
| NH(SO3)22− | 0.76 | 0.70 | 1.17 | 0.59 |
| H2NSO2NH2 | 1.13 | 0.072 | 0.094 | 0.009 |
| H2NSO3H | 0.39 | 0.094 | 0.076 | 0.013 |
| Ph-B(OH)2 | 23.1 | 0.073 | 0.065 | 0.006 |
| Ph-AsO3H2 | 49.2 | 0.092 | 0.084 | 0.008 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supuran, C.T. Legionella pneumophila Carbonic Anhydrases: Underexplored Antibacterial Drug Targets. Pathogens 2016, 5, 44. https://doi.org/10.3390/pathogens5020044
Supuran CT. Legionella pneumophila Carbonic Anhydrases: Underexplored Antibacterial Drug Targets. Pathogens. 2016; 5(2):44. https://doi.org/10.3390/pathogens5020044
Chicago/Turabian StyleSupuran, Claudiu T. 2016. "Legionella pneumophila Carbonic Anhydrases: Underexplored Antibacterial Drug Targets" Pathogens 5, no. 2: 44. https://doi.org/10.3390/pathogens5020044






