Candida albicans Shed Msb2 and Host Mucins Affect the Candidacidal Activity of Salivary Hst 5
Abstract
:1. Introduction
2. Results
2.1. Sap 6 Affects Hst 5 Candidacidal Activity
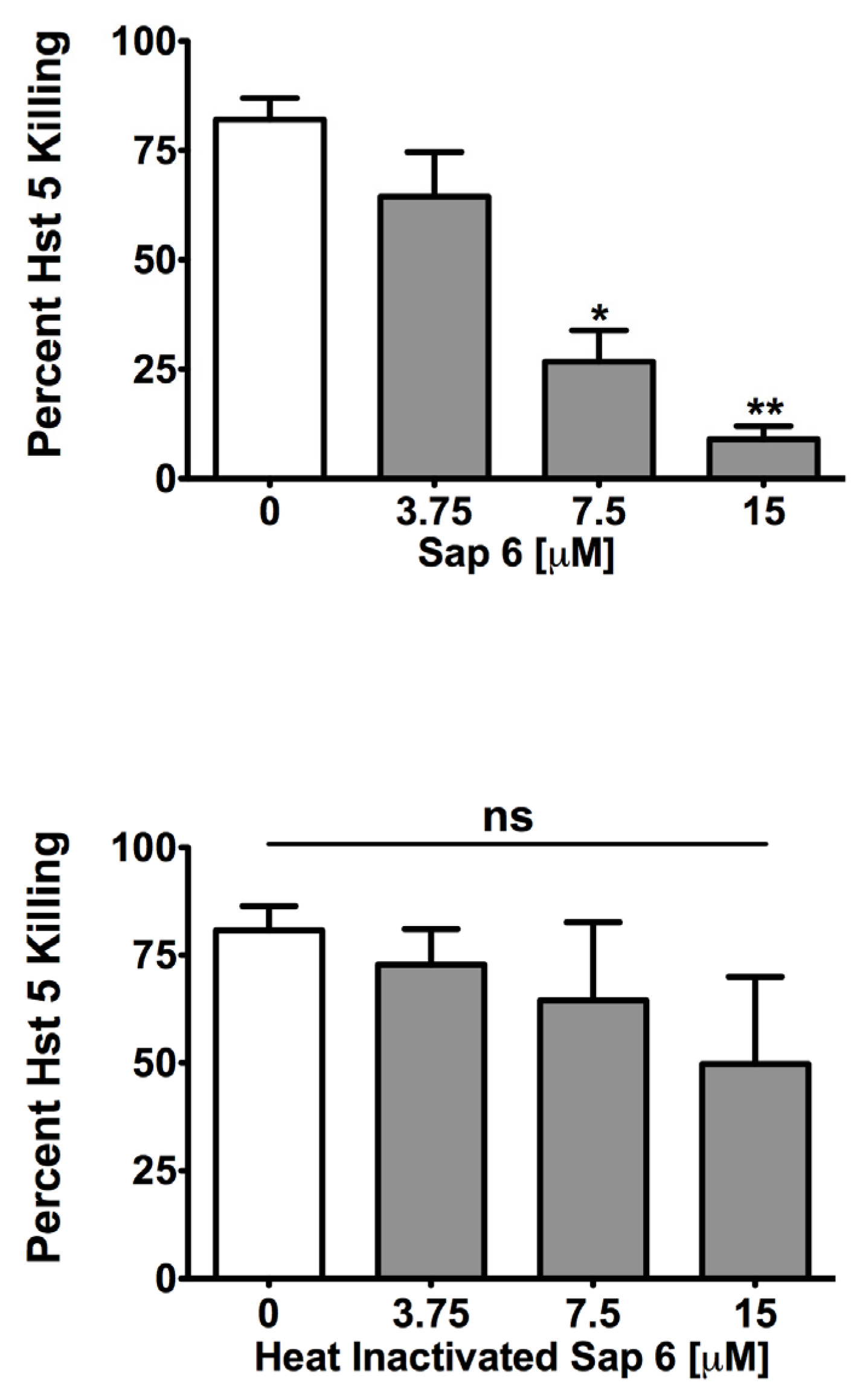
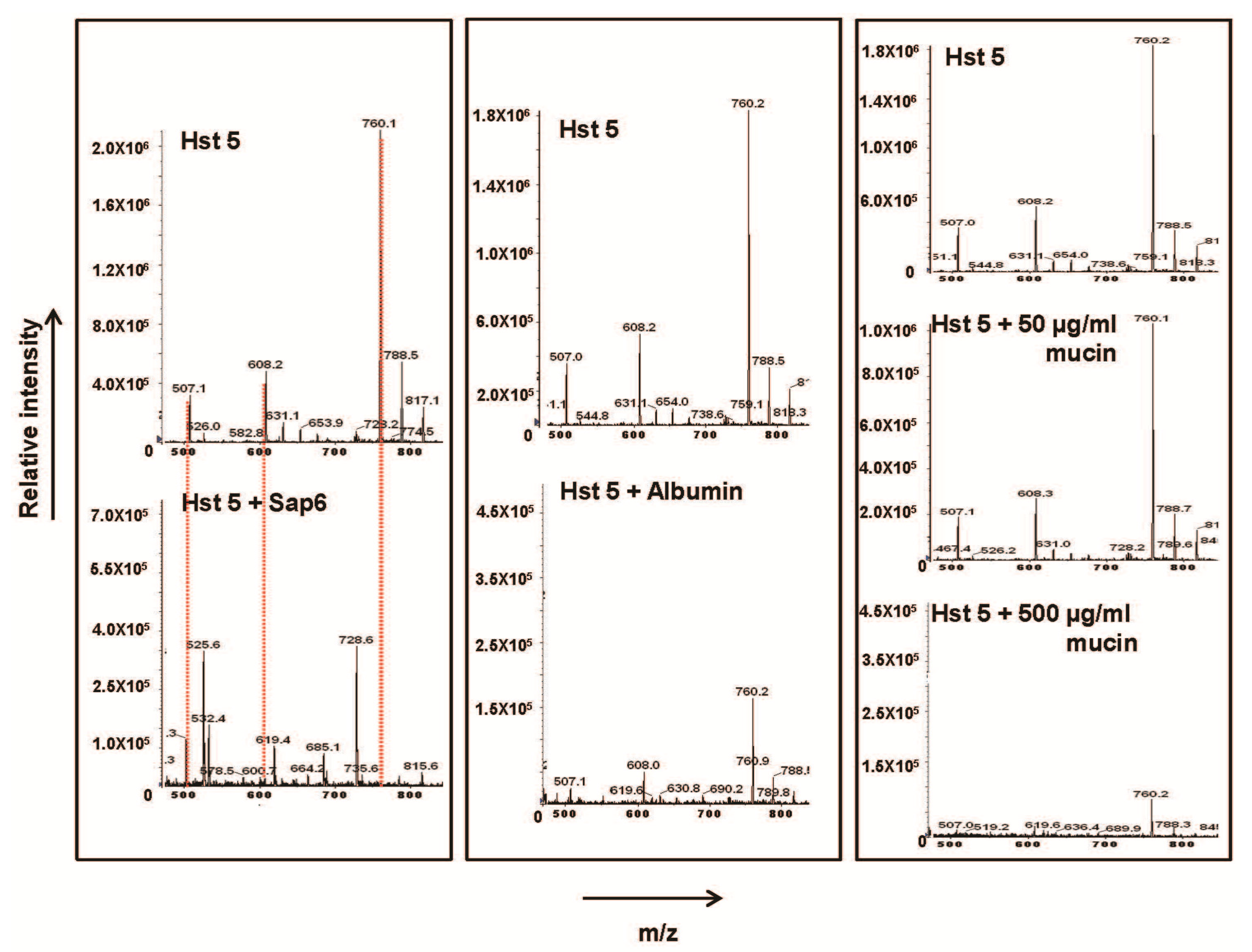
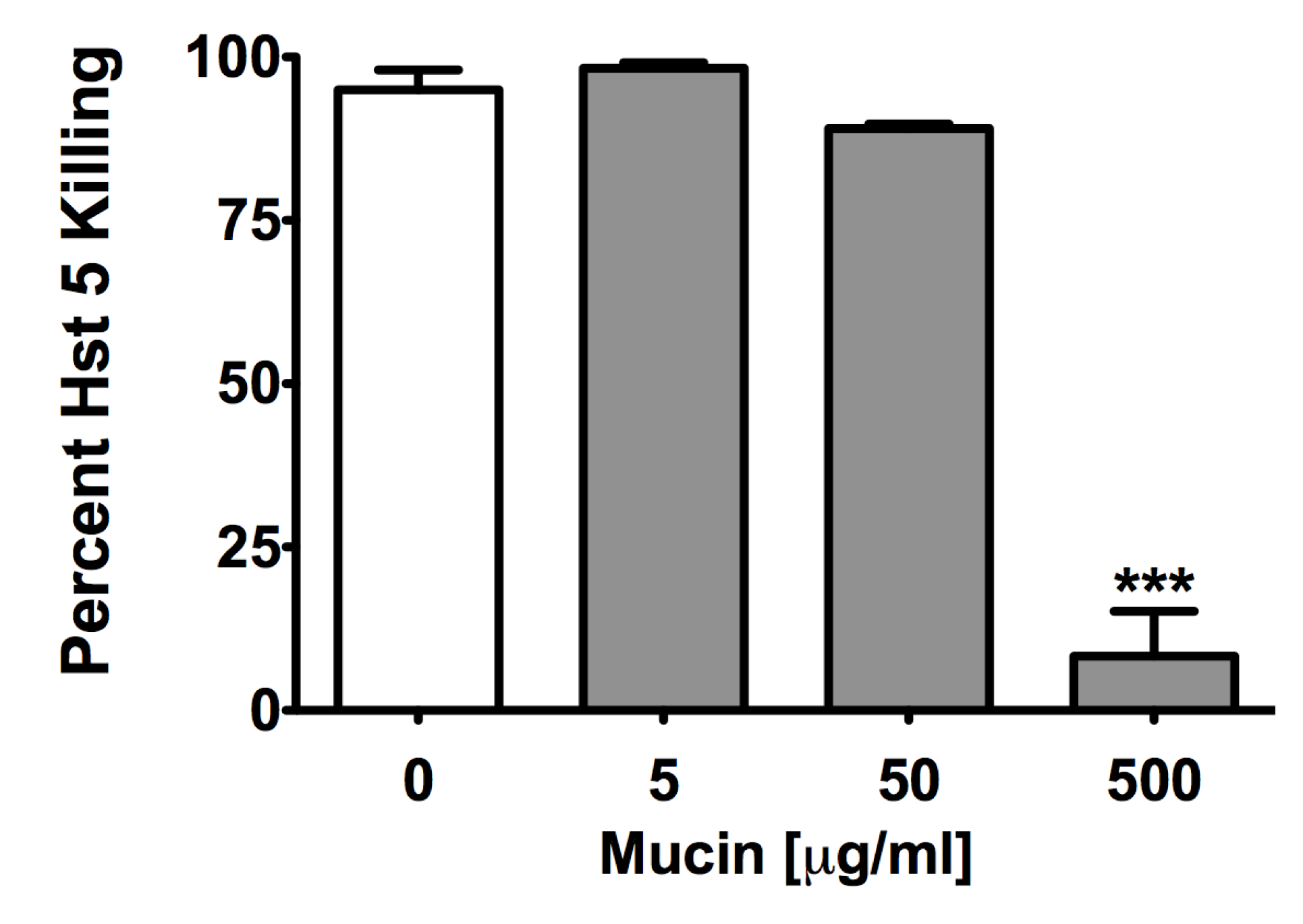
2.2. Glycoproteins Affect Hst 5 Activity and C. albicans Growth Characteristics
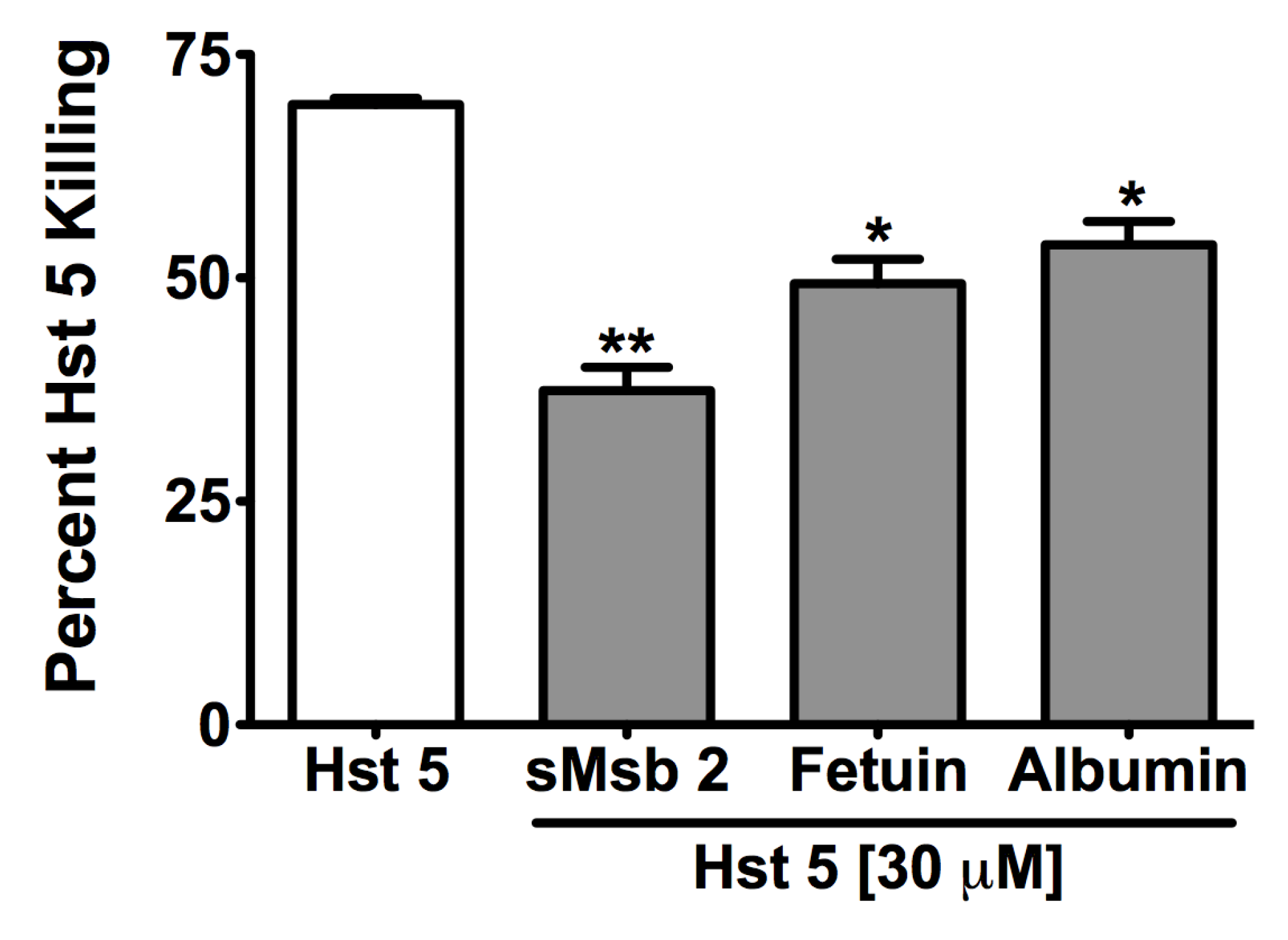
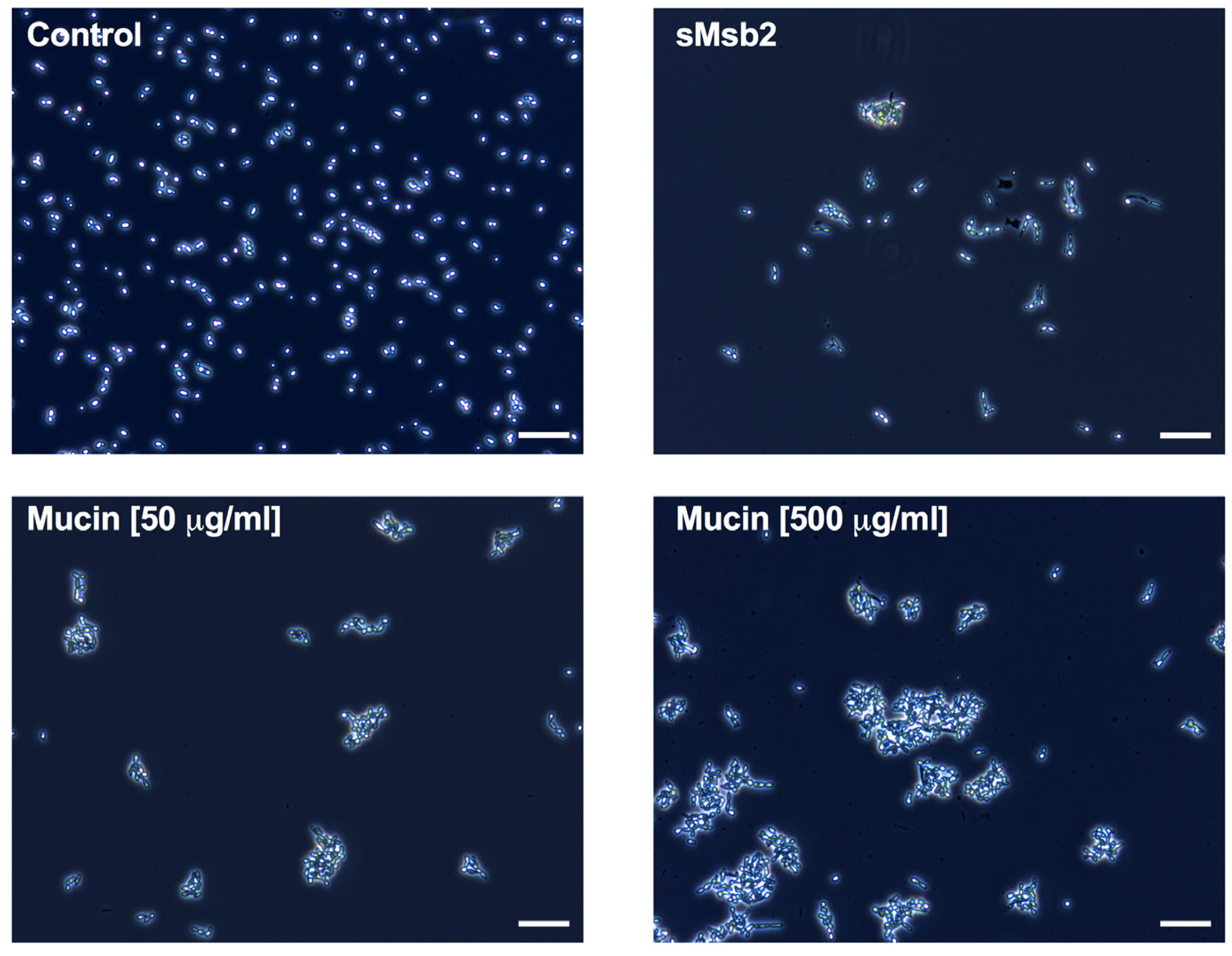
2.3. Msb2 Shed by C. albicans Does Not Uniformly Protect Cells against Hst 5
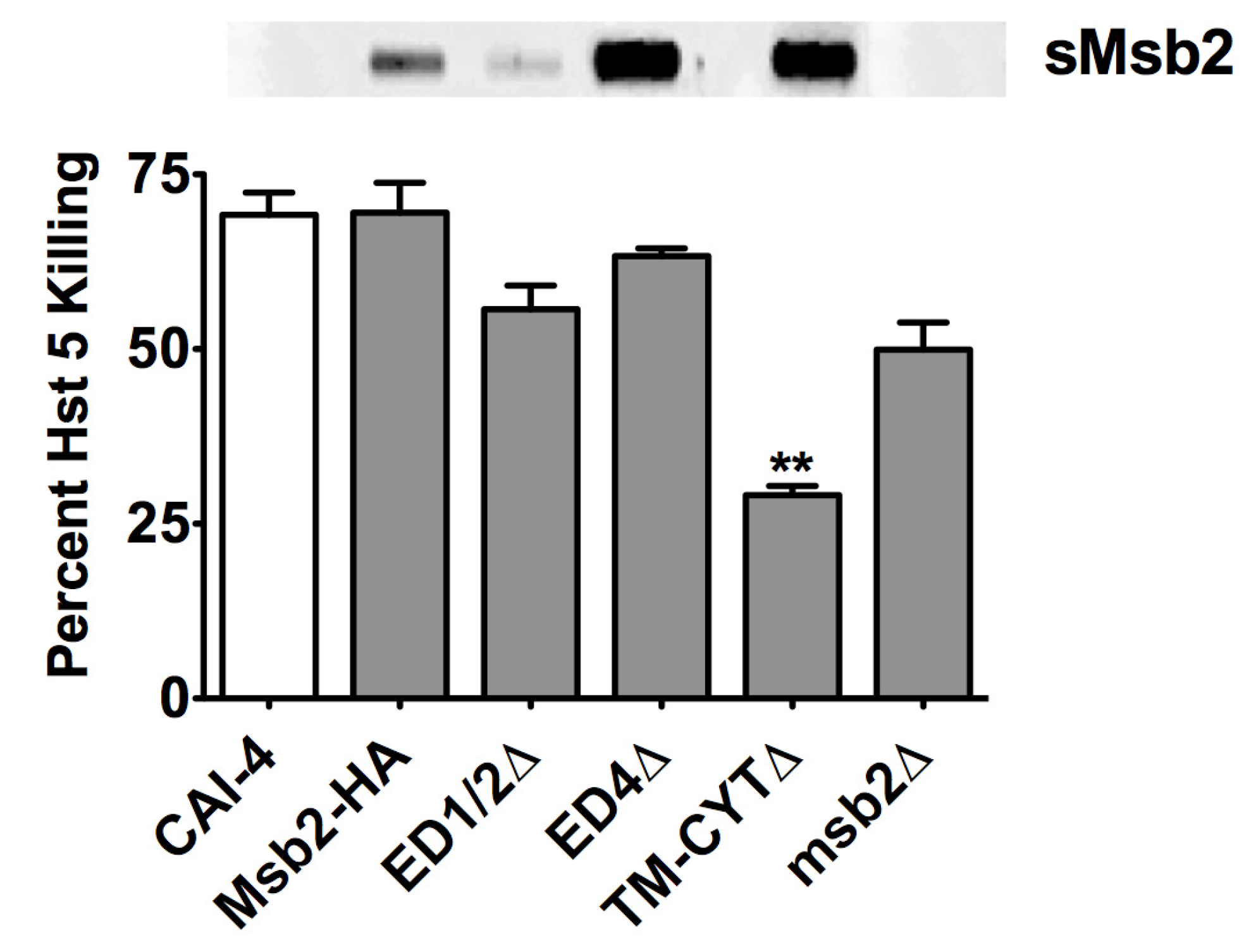
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1998, 263, 7472–7477. [Google Scholar]
- Xu, T.; Levitz, S.M.; Diamond, R.D.; Oppenheim, F.G. Anticandidal activity of major human salivary histatins. Infect. Immun. 1991, 59, 2549–2554. [Google Scholar] [PubMed]
- Puri, S.; Li, R.; Ruszaj, D.; Tati, S.; Edgerton, M. Iron binding modulates candidacidal properties of salivary histatin 5. J. Dent. Res. 2015, 94, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.J.; Flora, B.; Troxler, R.F.; Oppenheim, F.G. Dialysis unmasks the fungicidal properties of glandular salivary secretions. Infect. Immun. 2004, 72, 2703–2709. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, W.L.; Lee, Y.H.; Xiao, Y.; Held, K.; Wong, W. Identification and characterization of histatin 1 salivary complexes by using mass spectrometry. Proteomics 2012, 12, 3426–3435. [Google Scholar] [CrossRef] [PubMed]
- Iontcheva, I.; Oppenheim, F.G.; Troxler, R.F. Human salivary mucin MG1 selectively forms heterotypic complexes with amylase, proline-rich proteins, statherin, and histatins. J. Dent. Res. 1997, 76, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Iontcheva, I.; Oppenheim, F.G.; Offner, G.D.; Troxler, R.F. Molecular mapping of statherin- and histatin-binding domains in human salivary mucin MG1 (MUC5B) by the yeast two-hybrid system. J. Dent. Res. 2000, 79, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Sorgo, A.G.; Heilmann, C.J.; Dekker, H.L.; Brul, S.; de Koster, C.G.; Klis, F.M. Mass spectrometric analysis of the secretome of Candida albicans. Yeast 2010, 27, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. R 2003, 67, 400–428. [Google Scholar] [CrossRef]
- Naglik, J.; Albrecht, A.; Bader, O.; Hube, B. Candida albicans proteinases and host/pathogen interactions. Cell. Microbiol. 2004, 6, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Meiller, T.F.; Hube, B.; Schild, L.; Shirtiff, M.E.; Scheper, M.A.; Winkler, R.; Ton, A.; Jabra-Rizk, M.A. A novel immune evasion strategy of Candida albicans: Proteolytic cleavage of a salivary antimicrobial peptide. PLOS ONE 2009, 4, e5039. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Kumar, R.; Chadha, S.; Tati, S.; Conti, H.R.; Hube, B.; Cullen, P.J.; Edgerton, M. Secreted aspartic protease cleavage of Candida albicans Msb2 activates Cek1 MAPK signaling affecting biofilm formation and oropharyngeal candidiasis. PLOS ONE 2012, 7, e46020. [Google Scholar] [CrossRef] [PubMed]
- Szafranski-Schneider, E.; Swidergall, M.; Cottier, F.; Tielker, D.; Roman, E.; Pla, J.; Ernst, J.F. Msb2 Shedding protects Candida albicans against Antimicrobial Peptides. PLOS Pathog. 2012, 8, e1002501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swidergall, M.; Ernst, A.M.; Ernst, J.F. Candida albicans mucin Msb2 is a broad-range protectant against antimicrobial peptides. Antimicrob. Agents Chemother. 2013, 57, 3917–3922. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, S. The scientific exploration of saliva in the post-proteomic era: From database back to basic function. Expert Rev. Proteom. 2012, 9, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Messana, I.; Cabras, T.; Iavarone, F.; Manconi, B.; Huang, L.; Martelli, C.; Olianas, A.; Sanna, M.T.; Pisano, E.; Sanna, M.; et al. Chrono-proteomics of human saliva: Variations of the salivary proteome during human development. J. Proteom. Res. 2015, 14, 1666–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, L.S.; Li, X.; Wang, L.; Soares, R.V.; Siqueira, C.C.; Oppenheim, F.G.; Troxler, R.F.; Offner, G.D. Two-hybrid analysis of human salivary mucin MUC7 interactions. Biochim. Biophys. Acta 2005, 1746, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Saraswat, D.; Tati, S.; Edgerton, M. Novel aggregation properties of Candida albicans Secreted Aspartyl Proteinase Sap6 mediate virulence in oral Candidiasis. Infect. Immun. 2015, 83, 2614–2626. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, D.; Kumar, R.; Pande, T.; Edgerton, M.; Cullen, P. Signaling mucin Msb2 regulates adaptation to thermal stress in Candida albicans. 2015; in press. [Google Scholar]
- Li, L.D.; Crouzier, T.; Sarkar, A.; Dunphy, L.; Han, J.; Ribbeck, K. Spatial configuration and composition of charge modulates transport into a mucin hydrogel barrier. Biophys J. 2013, 105, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Edgerton, M. How does it kill—Understanding the candidacidal mechanism of salivary Histatin 5. Eukaryot Cell. 2014, 13, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Biesbrock, A.R.; Reddy, M.S.; Levine, M.J. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect. Immun. 1991, 59, 3492–3497. [Google Scholar] [PubMed]
- Kavanaugh, N.L.; Zhang, A.Q.; Nobile, C.J.; Johnson, A.D.; Ribbeck, K. Mucins suppress virulence traits of Candida albicans. mBio 2014, 5, e01911. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B.; Davis, D.; Enloe, B.M.; Mitchell, A.P. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruptions. Yeast 2000, 16, 65–70. [Google Scholar] [CrossRef]
- Fonzi, W.A.; Irwin, M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar] [PubMed]
- Liu, T.T.; Znaidi3, S.; Barker, K.S.; Xu, L.; Homayouni, R.; Saidane, S.; Morschhäuser, J.; Nantel, A.; Raymond, M.; Rogers, P.D. Genome-wide expression and location analyses of the Candida albicans Tac1p regulon. Eukaryot. Cell. 2007, 6, 2122–2138. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Reddy, M.S.; Baev, D.; Edgerton, M. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J. Biol. Chem. 2003, 278, 28553–28561. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puri, S.; Friedman, J.; Saraswat, D.; Kumar, R.; Li, R.; Ruszaj, D.; Edgerton, M. Candida albicans Shed Msb2 and Host Mucins Affect the Candidacidal Activity of Salivary Hst 5. Pathogens 2015, 4, 752-763. https://doi.org/10.3390/pathogens4040752
Puri S, Friedman J, Saraswat D, Kumar R, Li R, Ruszaj D, Edgerton M. Candida albicans Shed Msb2 and Host Mucins Affect the Candidacidal Activity of Salivary Hst 5. Pathogens. 2015; 4(4):752-763. https://doi.org/10.3390/pathogens4040752
Chicago/Turabian StylePuri, Sumant, Justin Friedman, Darpan Saraswat, Rohitashw Kumar, Rui Li, Donna Ruszaj, and Mira Edgerton. 2015. "Candida albicans Shed Msb2 and Host Mucins Affect the Candidacidal Activity of Salivary Hst 5" Pathogens 4, no. 4: 752-763. https://doi.org/10.3390/pathogens4040752





