HACCP-Based Programs for Preventing Disease and Injury from Premise Plumbing: A Building Consensus
Abstract
:1. Introduction
1.1. Building Water Systems are Comprised of a Series of Unit Processes
1.2. History of Hazard Analysis and Critical Control Point
- (1)
- Conduct a Hazard Analysis;
- (2)
- Determine the Critical Control Points;
- (3)
- Establish Critical Limit(s);
- (4)
- Establish a system to monitor control of the Critical Control Points;
- (5)
- Establish Corrective Action(s) to be taken when monitoring indicates that a particular Critical Control Point is not within Critical Limits;
- (6)
- Establish procedures to confirm that the HACCP system is working effectively; and
- (7)
- Establish documentation of all procedures pertaining to these HACCP principles and their application.
- (1)
- Establish a HACCP team;
- (2)
- Describe the system;
- (3)
- Identify intended use(s);
- (4)
- Construct process flow diagrams;
- (5)
- Confirm the accuracy of the process flow diagrams;
- (6)
- List all potential hazards associated with each process step, conduct a hazard analysis and consider measures to control identified hazards at each step;
- (7)
- Determine Critical Control Points, the locations where control must be applied to prevent hazards from causing harm;
- (8)
- Establish Critical Limits for each Critical Control Point;
- (9)
- Establish monitoring procedures for each Critical Control Point and specify frequency of monitoring;
- (10)
- Establish corrective actions to be taken when monitoring indicates that conditions at a Critical Control Point are outside of Critical Limits;
- (11)
- Establish procedures to confirm that the plan is being implemented as designed (verification) and is working effectively (validation); and
- (12)
- Establish documentation and record keeping procedures.
2. HACCP for Protection of the Public Water Supply
3. HACCP-Based Programs for Preventing Disease and Injury from Building Water Systems
- They lacked documentation of building water systems and familiarity with water processes, especially in large, complex systems;
- They lacked a systematic program for identifying, monitoring and controlling factors known to affect microbial growth (e.g., water temperatures, disinfectant residual levels); and
- They lacked inter-disciplinary/inter-departmental communication, e.g., between facility managers and clinicians.
- HACCP-based methodology enjoys the benefit of extensive real-world use in mitigating a range of environmental risks, including microbial hazards associated with public water supplies.
- HACCP-based methodology is well suited to establishing and maintaining appropriate controls of temperature, disinfectant residual and other factors that can reduce environmental exposure of building occupants, especially susceptible persons, to large numbers of plumbing-associated pathogens.
- HACCP-based methodology provides a systematic, standardized framework that can accommodate substantial variation in buildings and building water systems, including differences in purpose, design and propensity for disease transmission.
- HACCP-based methodology provides a systematic, standardized framework for risk characterization, hazard prevention and validation but does not prescribe specific means or methods. It is designed to accommodate future scientific progress and new/improved methods.
- HACCP-based methodology provides a practical, resource-efficient way for the largest number of buildings to accomplish significant risk reduction at reasonable cost. It enables technically competent building personnel to implement an effective hazard-prevention plan without reliance on expensive consultants and other specialists.
- HACCP-based programs for management of water systems in buildings have been developed and proposed by government agencies (VHA), major industry groups (ASHRAE) and prominent public health organizations (NSF International). They share fundamental features of HACCP methodology, with small differences in terminology and level of prescription. Except where noted (Table 1), these programs use conventional HACCP terminology.
| Program Components | NSF Int’l * 444 | WHO * WSP | VHA * Directive 1061 | ASHRAE * 188 |
|---|---|---|---|---|
| Interdisciplinary Team with authority & responsibility | + | + | + | + |
| Water system description (process flow diagrams) | + | + | + | + |
| Hazard analysis and risk characterization based on water system description | + | + Note: Variously called hazard analysis or risk assessment | + Note: Risk characterization includes assessment of clinical and environmental factors | + |
| Critical Control Points are selected based on hazard analysis and risk characterization | + | + | +
Note: Controls are called “Engineering Controls”. Values are prescribed for temperature and oxidant residual levels | +
Note: Critical Control Points are called “Control Locations” |
| Critical Limits are specified and monitored; Corrective actions are required | + | + | + | +
Note: Critical Limits are called “Control Limits” |
| Confirmation that the plan is being implemented according to design (verification) is required | + | + | + | + |
| Confirmation that controls, when applied according to plan, are effectively controlling hazards (validation) is required | +
Note: Both initial and ongoing validation are required | + Note: Validation is variously called monitoring or testing | +
Note: Requires validation by both environmental and clinical testing. Responses to test results are prescribed | + |
3.1. WHO Water Safety Plans
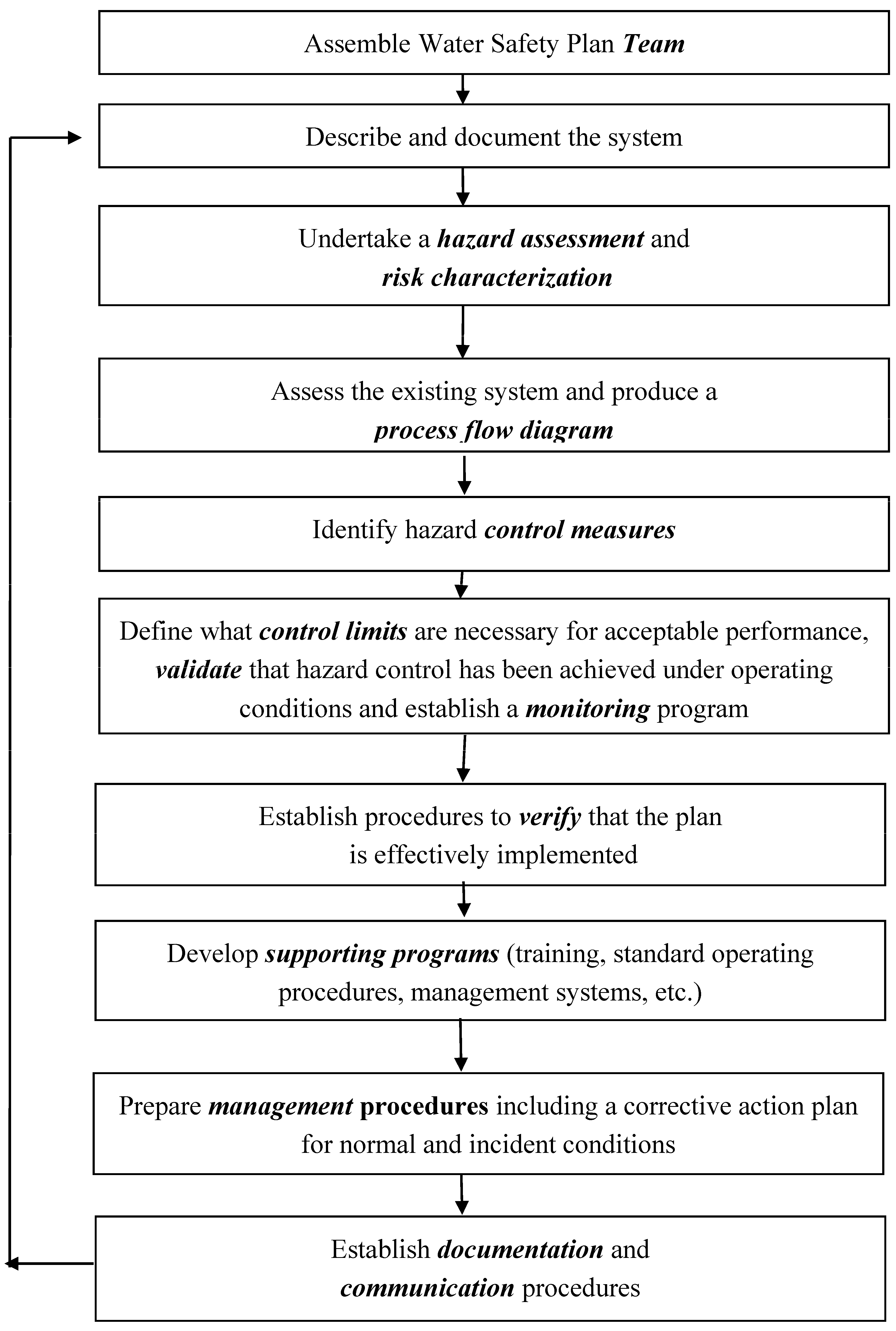
3.2. VHA Directive 1061
3.3. ANSI/ASHRAE 188-2015
3.4. BSR/NSF 444
4. Validation

Negative Screening of Environmental Samples with the PCR for Legionella
| Binary Statistical Parameter | Total (Potable + Utility) Water Samples | Utility Water Samples | Potable Water Samples |
|---|---|---|---|
| True-Positives 1 | 520 | 38 | 482 |
| False-Positives | 765 | 167 | 598 |
| True-Negatives | 2342 | 365 | 1977 |
| False-Negatives | 81 | 9 | 72 |
| Sum | 3708 | 579 | 3129 |
| Accuracy (%) 2 | 77.2 | 69.6 | 78.6 |
| Specificity (%) 3 | 75.4 | 68.6 | 76.8 |
| Sensitivity (%) 4 | 86.5 | 80.9 | 87.0 |
| Positive Predictive Value (%) 5 | 40.5 | 18.5 | 44.6 |
| Negative Predictive Value (%) 6 | 96.7 | 97.6 | 96.5 |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WRF (Water Research Foundation). State of the Science and Research Needs for Opportunistic Pathogens in Premise Plumbing. 2013. Available online: http://www.waterrf.org/PublicReportLibrary/4379.pdf (accessed on 29 March 2015).
- CDC (Centers for Disease Control and Prevention). Surveillance for Waterborne Outbreaks Associated with Drinking Water. 2011. Available online: www.cdc.gov/mmwr/preview/mmwrhtml/ss6012a4.htm (accessed on 29 March 2015). [Google Scholar]
- WHO (World Health Organization). Water Safety Plans. In Guidelines for Drinking Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- WHO (World Health Organization). Legionella and the Prevention of Legionellosis; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- WHO (World Health Organization). Water Safety in Buildings; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- OSHA (Occupational Safety and Health Adminstration). Legionnaires’ disease. Technical Manual, Chapter 7; 1999. Available online: https://www.osha.gov/dts/osta/otm/otm_iii/otm_iii_7.html (accessed on 29 March 2015).
- McCoy, W.F. Preventing Legionellosis; International Water Association Publishing: London, UK, 2005. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). Notifiable diseases. MMWR. 2015. Available online: http://www.cdc.gov/mmwr/index2015.html (accessed on 29 March 2015). [Google Scholar]
- Looney, WJ. Role of Stenotrophomonas maltophilia in hospital-acquired infection. Br. J. Biomed. Sci. 2005, 62, 145–154. [Google Scholar] [PubMed]
- Anaissie, E.J.; Kuchar, R.T.; Rex, J.H.; Francesconi, A.; Kasai, M.; Müller, F.M.; Lozano-Chiu, M.; Summerbell, R.C.; Dignani, M.C.; Chanock, S.J.; et al. Fusariosis associated with pathogenic Fusarium species colonization of a hospital water system: A new paradigm for the epidemiology of opportunistic mold infections. Clin. Infect. Dis. 2001, 33, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Anaissie, E.J.; Stratton, S.L.; Dignani, M.C.; Summerbell, R.C.; Rex, J.H.; Monson, T.P.; Spencer, T.; Kasai, M.; Francesconi, A.; Walsh, T.J. Pathogenic Aspergillus species recovered from a hospital water system: A 3-year prospective study. Clin. Infect. Dis. 2002, 34, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Nour, M.; Duncan, C.; Low, D.E.; Guyard, C. Biofilms: The Stronghold of Legionella pneumophila. Int. J. Mol. Sci. 2013, 14, 21660–21675. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, A.; Pruckler, J.; Benson, R.; Rowbotham, T.; Halablab, M.; Fields, B.S. Legionella-like amoebal pathogens—Phylogenetic status and possible role in respiratory disease. Emerg. Infect. Dis. 1996, 2, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Brown, M.R.W.; Collier, P.J.; Farrell, I.; Gilbert, P. Relationship between Legionella pneumophila and Acathamoeba polyphaga: Physiological status and susceptibility to chemical inactivation. Appl. Environ. Microbiol. 1992, 58, 2420–2425. [Google Scholar] [PubMed]
- Essig, A.; Heinemann, M.; Simnacher, U.; Marre, R. Infection of Acanthamoeba castellanii by Chlamydia pneumoniae. Appl. Environ. Microbiol. 1997, 63, 1396–1399. [Google Scholar] [PubMed]
- Fritsche, T.R.; Horn, M.; Seyedirashti, S.; Gautom, R.K.; Schleifer, K-H.; Wagner, M. In situ detection of novel bacterial endosymbionts of Acanthamoeba spp. phylogenetically related to members of the Order Rickettsiales. Appl. Environ. Microbiol. 1999, 65, 206–212. [Google Scholar]
- Greub, G.; Raoult, D. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 2004, 17, 413–413. [Google Scholar] [CrossRef] [PubMed]
- La Scola, B.; Barrassi, L.; Raoult, D. Isolation of new fastidious α-proteobacteria and Afipia felis from hospital water supplies by direct plating and amoebal co-culture procedures. FEMS Microbiol. Ecol. 2000, 34, 129–137. [Google Scholar] [CrossRef]
- Wang, H.; Masters, S.; Hong, Y.; Stallings, J.; Falkinham, J.O., 3rd; Edwards, M.A.; Pruden, A. Effect of disinfectant, water age, and pipe material on occurrence and persistence of Legionella, mycobacteria, Pseudomonas aeruginosa, and two amoebas. Environ. Sci. Technol. 2012, 46, 11566–11574. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, T.J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 1980, 33, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Short, D.P.G.; O’Donnell, K.; Zhang, N.; Juba, J.H.; Geise, D.M. Widespread occurrence of diverse human pathogenic types of the fungus Fusarium detected in plumbing drains. J. Clin. Microbiol. 2011, 49, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Steinert, M.; Birkness, K.; White, E.; Fields, B.; Quinn, F. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl. Environ. Microbiol. 1998, 64, 2256–2261. [Google Scholar] [PubMed]
- Thomas, J.M.; Ashbolt, N.J. Do free-living amoebae in treated drinking water systems present an emerging health risk? Environ. Sci. Technol. 2011, 45, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Vaerewijck, M.J.; Huys, G.; Palomino, J.C.; Swings, J.; Portaels, F. Mycobacteria in drinking water distribution systems: Ecology and significance for human health. FEMS Microbiol. Rev. 2005, 29, 911–934. [Google Scholar] [CrossRef] [PubMed]
- Loret, J.F.; Robert, S.; Thomas, Y.; Levi, A.; Cooper, A.J.; McCoy, W.F. Comparison of disinfectants for biofilm, protozoa and Legionella control. J. Water Health 2005, 3, 423–433. [Google Scholar] [PubMed]
- Carlson, C.S. Effective FMEAs: Achieving Safe, Reliable, and Economical Products and Processes using Failure Mode and Effects Analysis; Wiley Series in Quality & Reliability Engineering; John Wiley & Sons: Hoboken, NJ, 2012. [Google Scholar]
- WHO (World Health Organization). The Codex Alimentarius. 2015. Available online: http://www.who.int/foodsafety/areas_work/food-standard/en/ (accessed on 30 March 2015).
- Havelaar, A.H. Application of HACCP and Drinking Water Supply. Food Control 1994, 5, 145–152. [Google Scholar] [CrossRef]
- Rosén, L.; Hokstad, P.; Lindhe, A.; Sklet, S.; Røstum, J. Generic framework and methods for integrated risk management in water safety plans. Techneau. 2007. Available online: http://www.techneau.org/fileadmin/files/Publications/Publications/Deliverables/D4.1.3.pdf (accessed on 30 March 2015).
- Dewettinck, T.; Van Houtte, E.; Geenens, D.; Van Hege, K.; Verstraete, W. HACCP (Hazard Analysis and Critical Control Points) to guarantee safe water reuse and drinking water production—a case study. Water Sci. Technol. 2001, 43, 31–38. [Google Scholar] [PubMed]
- Dyck, A.; Exner, M.; Kramer, A. Experimental based experiences with the introduction of a water safety plan for a multi-located university clinic and its efficacy according to WHO recommendations. BMC Public Health 2007, 7. Available online: http://www.biomedcentral.com/content/pdf/1471-2458-7-34.pdf (accessed on 29 March 2015). [Google Scholar] [CrossRef] [PubMed]
- Leoni, E.; Laura Dallolio, L.; Stagni, F.; Sanna, T.; D’Alessandro, G.; Piana, G. Impact of a risk management plan on Legionella contamination of dental unit water. Int. J. Environ. Res. Public Health 2015, 12, 2344–2358. [Google Scholar] [CrossRef] [PubMed]
- Nadebaum, P.; Chapman, M.; Ortisi, S.; Baker, A. Application of quality management systems for drinking water quality. Water Supply 2003, 3, 359–364. [Google Scholar]
- Howard, G. Water safety plans for small systems: A model for applying HACCP concepts for cost-effective monitoring in developing countries. Water Sci. Technol. 2003, 47, 215–220. [Google Scholar] [PubMed]
- Yokoi, H.; Embutsu, I.; Waseda, K. Study on the introduction of hazard analysis and critical control point (HACCP) concept of the water quality management in water supply systems. Water Sci. Technol. 2006, 53, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Jagals, C.; Jagals, P. Application of HACCP principles as a management tool for monitoring and controlling microbiological hazards in water treatment facilities. Water Sci. Technol. 2004, 50, 69–76. [Google Scholar] [PubMed]
- Krageschmidt, D.A.; Kubly, A.F.; Browning, M.S.; Wright, A.J.; Lonneman, J.D.; Detmer, M.J.; McCoy, W.F. A comprehensive water management program for multi-campus healthcare facilities. Infect. Control Hosp. Epidemiol. 2014, 35, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Gillilland, C.; Rosenblatt, A.A.; McCoy, W.F. HACCP for building water systems. Water Cond. Purif. 2014, 56, 40–42. Available online: http://www.wcponline.com/pdf/January_2014_WaterMatters.pdf (accessed on 29 March 2015). [Google Scholar]
- VHA (Veterans Health Administration). VHA Directive 1061—Prevention of healthcare-associated Legionella disease and scald injury from potable water distribution systems. 2014. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3033 (accessed on 29 March 2015). [Google Scholar]
- McCoy, W.F. Preventing legionellosis with hazard analysis and control systems. In Legionella: State of the art 30 years after its recognition, Proceedings of the 6th International Conference on Legionella, Chicago, USA, 16–20 October 2005; Cianciotto, N.P., Kwaik, Y.A., Edelstein, P.H., Fields, B.S., Geary, D.F., Harrison, T.G., Joseph, C.A., Ratcliff, R.M., Stout, J.E., Swanson, M.S., Eds.; ASM Publishing: Washington, DC, USA, 2005. [Google Scholar]
- Lucas, C.E.; Taylor, T.H., Jr.; Fields, B.S. Accuracy and precision of Legionella isolation by US laboratories in the ELITE program pilot study. Water Res. 2011, 45, 4428–4436. [Google Scholar] [CrossRef] [PubMed]
- McCoy, W.F.; Downes, E.L.; Leonidas, L.F.; Cain, M.F.; Sherman, D.L.; Chen, K.; Devender, S.; Neville, M.J. Inaccuracy in Legionella tests of building water systems due to sample holding time. Water Res. 2012, 46, 3497–3506. [Google Scholar] [CrossRef] [PubMed]
- Scheikl, U.; Sommer, R.; Kirschner, A.; Rameder, A.; Schrammel, B.; Zweimüller, I.; Walochnik, J. Free-living amoebae (FLA) co-occurring with legionellae in industrial waters. Eur. J. Protistol. 2014, 50, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Segal, G.; Shuman, H.A. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Inf. Immun. 1999, 67, 2117–2124. [Google Scholar]
- Albers, U.; Reus, K.; Shuman, H.A.; Hilbi, H. The amoebae plate test implicates a paralogue of lpxB in the interaction of Legionella pneumophila with Acanthamoeba castellanii. Microbiology. 2005, 151, 167–182. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCoy, W.F.; Rosenblatt, A.A. HACCP-Based Programs for Preventing Disease and Injury from Premise Plumbing: A Building Consensus. Pathogens 2015, 4, 513-528. https://doi.org/10.3390/pathogens4030513
McCoy WF, Rosenblatt AA. HACCP-Based Programs for Preventing Disease and Injury from Premise Plumbing: A Building Consensus. Pathogens. 2015; 4(3):513-528. https://doi.org/10.3390/pathogens4030513
Chicago/Turabian StyleMcCoy, William F., and Aaron A. Rosenblatt. 2015. "HACCP-Based Programs for Preventing Disease and Injury from Premise Plumbing: A Building Consensus" Pathogens 4, no. 3: 513-528. https://doi.org/10.3390/pathogens4030513
APA StyleMcCoy, W. F., & Rosenblatt, A. A. (2015). HACCP-Based Programs for Preventing Disease and Injury from Premise Plumbing: A Building Consensus. Pathogens, 4(3), 513-528. https://doi.org/10.3390/pathogens4030513





