Anti-Leishmania major Properties of Nuphar lutea (Yellow Water Lily) Leaf Extracts and Purified 6,6′ Dihydroxythiobinupharidine (DTBN)
Abstract
:1. Introduction
2. Materials and Methods
2.1. L. major Promastigote Culture
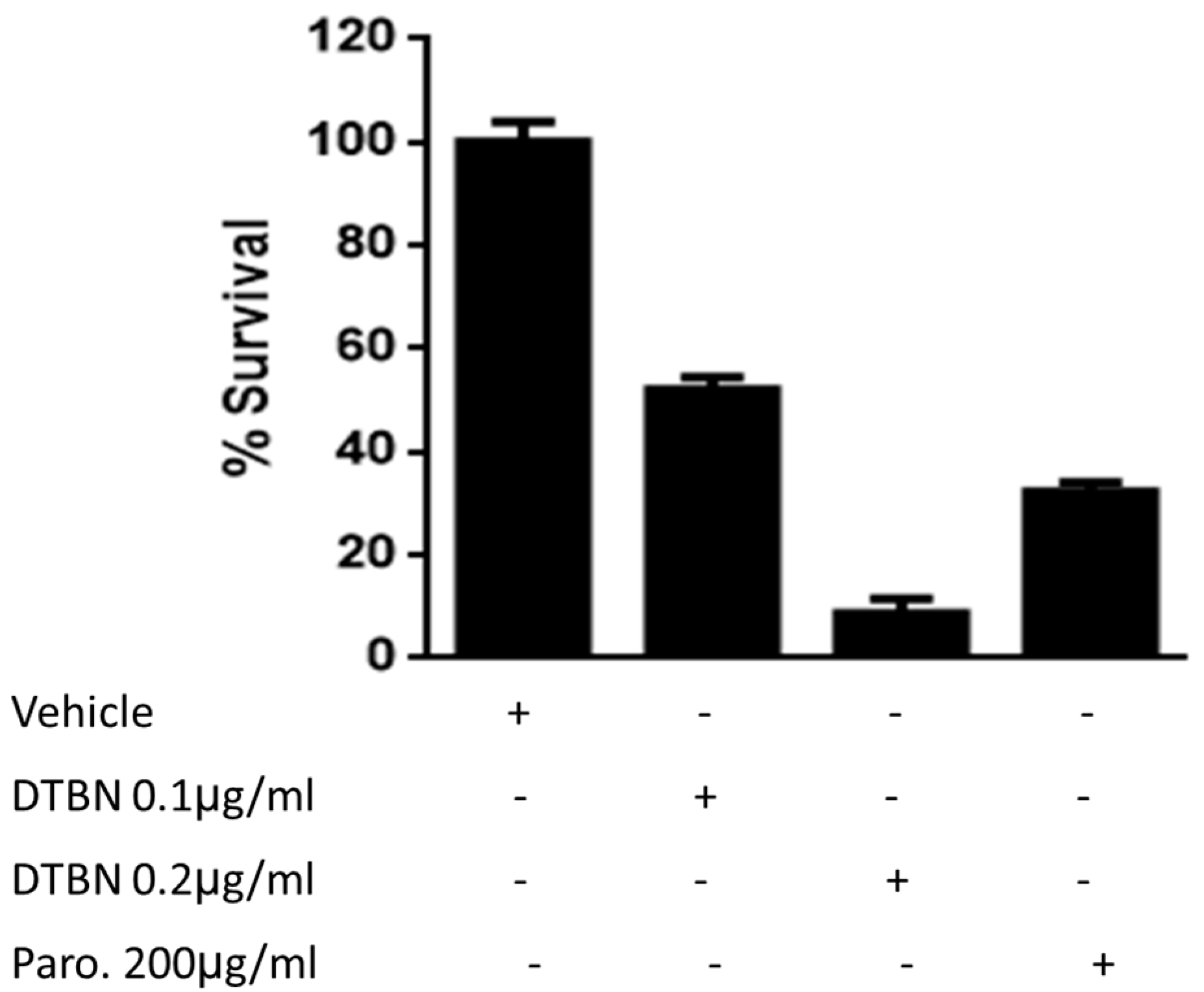
2.2. The Survival Rate of L. major Promastigotes Treated with DTBN
2.3. Macrophage Isolation from C3H Mice
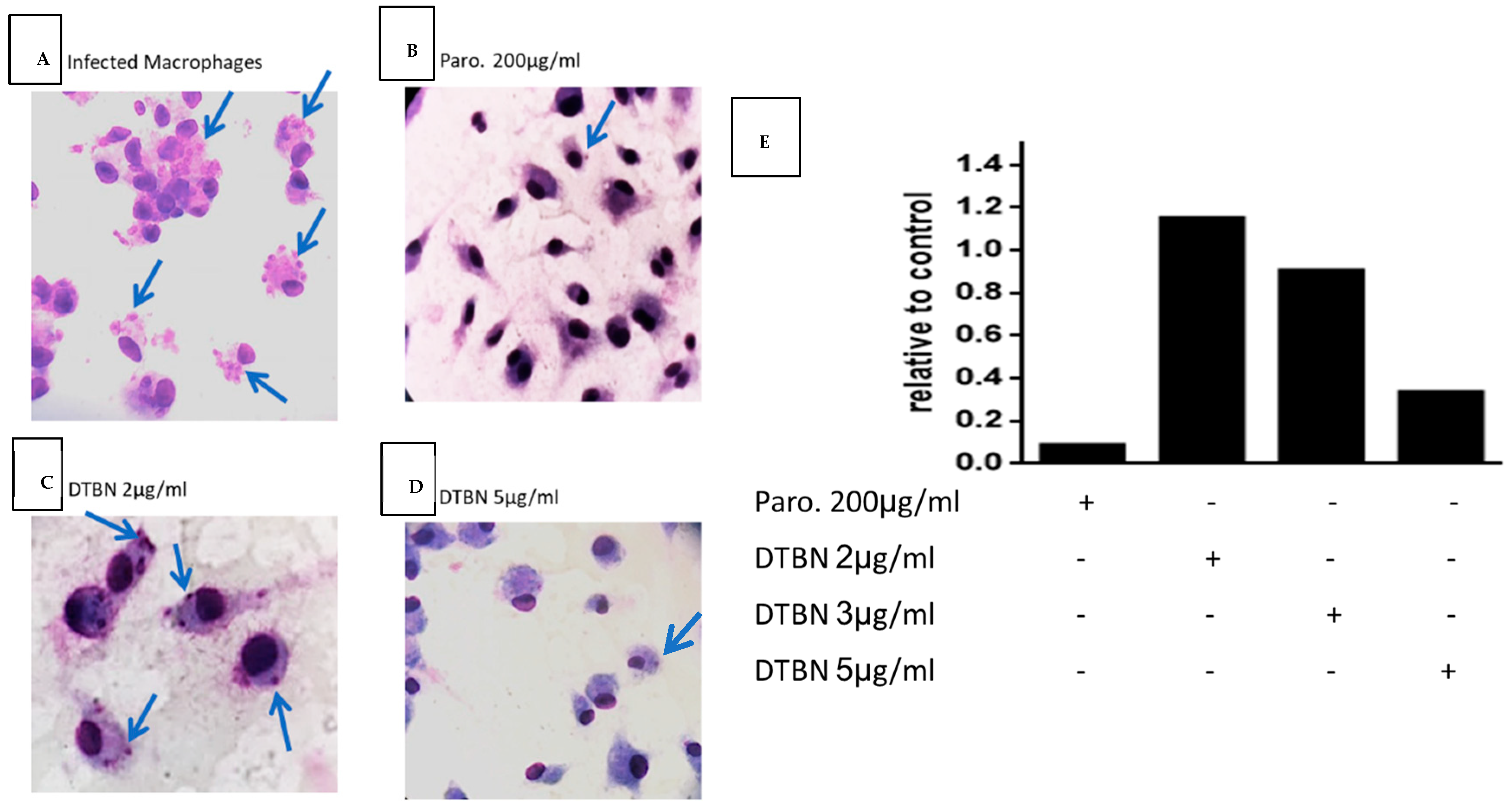
2.4. Macrophage Preparation, Infection with Leishmania Major, and Treatments
2.5. Preparation of Promastigotes for Electron Microscopy
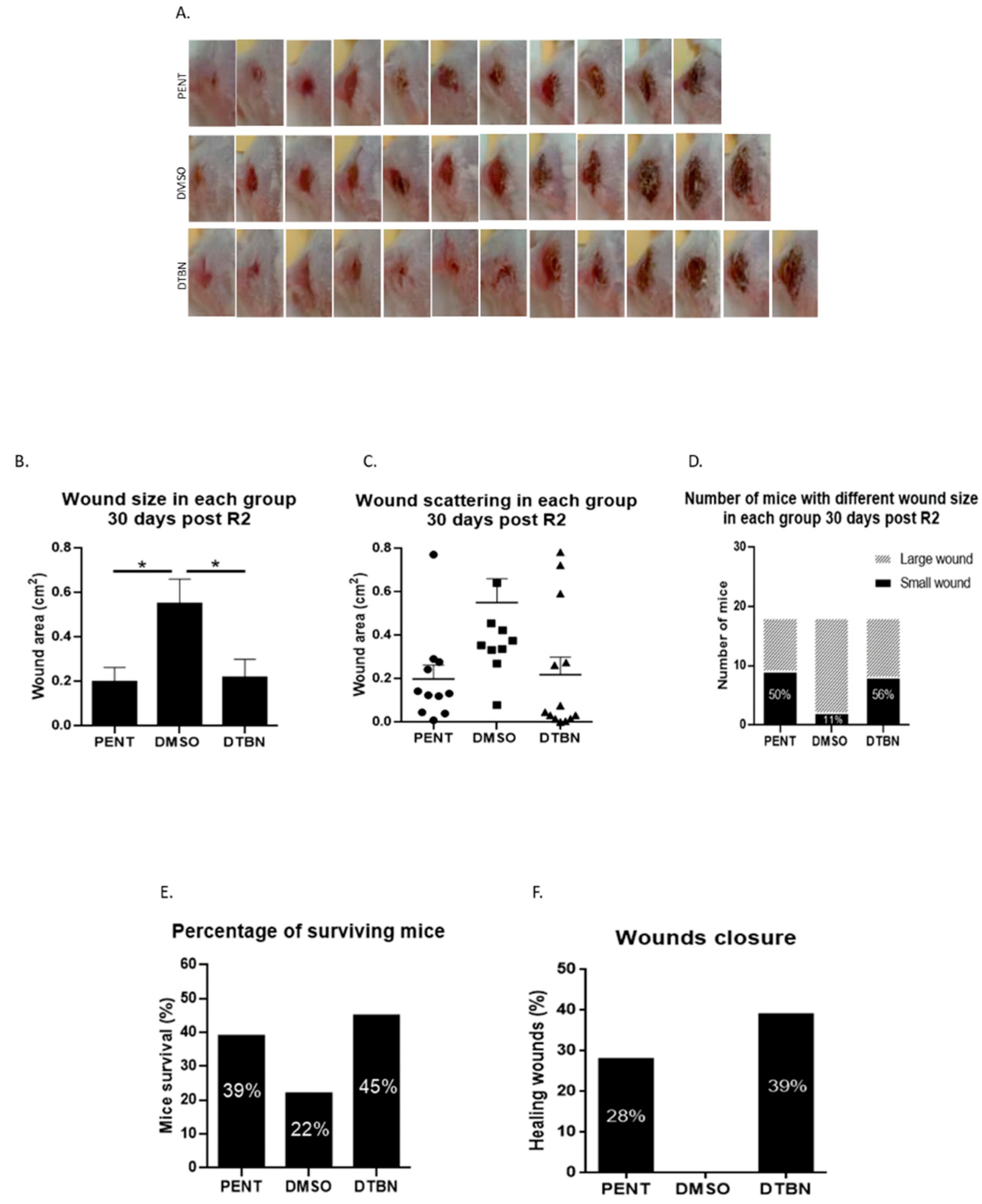
2.6. In Vivo Experiments
2.6.1. Lm Injection
2.6.2. Intraperitoneal Treatment
2.6.3. Intralesional Treatment
3. Results
3.1. Survival of DTBN-Treated Lm Promastigotes
3.2. Transmission (TEM) and Scanning Electron Microscopy (SEM) of Lm Promastigotes Treated with DTBN
3.3. DTBN Treatment of Lm-Infected Macrophages Reduces the Number of Intracellular Amastigotes
3.4. Intraperitoneal Injection of NUP.E Decreases Leishmanial Wound Size in Mice
3.5. Intra-Lesion Injection of Purified DTBN Reduces Wound Size and Partially Cures L. major Infected Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corman, H.N.; McNamara, C.W.; Bakowski, M.A. Drug Discovery for Cutaneous Leishmaniasis: A Review of Developments in the Past 15 Years. Microorganisms 2023, 11, 2845. [Google Scholar] [CrossRef]
- De Vries, H.J.C.; Schallig, H.D.; De Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 12 February 2024).
- Dayakar, A.; Chandrasekaran, S.; Kuchipudi, S.V.; Kalangi, S.K. Cytokines: Key Determinants of Resistance or Disease Progression in Visceral Leishmaniasis: Opportunities for Novel Diagnostics and Immunotherapy. Front. Immunol. 2019, 1, 670. [Google Scholar] [CrossRef] [PubMed]
- Porta, E.O.; Gao, L.; Denny, P.W.; Steel, P.G.; Kalesh, K. Inhibition of HSP90 distinctively modulates the global phosphoproteome of Leishmania mexicana developmental stages. Microbiol. Spectr. 2023, 11, e0296023. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan persister-like cells and drug treatment failure. Nat. Rev. Microbiol. 2019, 17, 607. [Google Scholar] [CrossRef]
- Chaves, M.; Savio, L.E.; Coutinho-Silva, R. Purinergic signaling: A new front-line determinant of resistance and susceptibility in leishmaniasis. Biomed. J. 2022, 45, 109–117. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Neglected Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Ozer, J.; Eisner, N.; Ostrozhenkova, E.; Bacher, A.; Eisenreich, W.; Benharroch, D.; Golan-Goldhirsh, A.; Gopas, J. Nuphar lutea thioalkaloids inhibit the nuclear factor κappaB pathway, potentiate apoptosis and are synergistic with cisplatin and etoposide. Cancer Biol. Ther. 2009, 8, 1860–1868. [Google Scholar] [CrossRef]
- El-On, J.; Ozer, L.; Gopas, J.; Sneir, R.; Golan-Goldhirsh, A. Nuphar lutea: In vitro anti-leishmanial activity against Leishmania major promastigotes and amastigotes. Phytomedicine 2009, 16, 788–792. [Google Scholar] [CrossRef]
- El-On, J.; Ozer, L.; Gopas, J.; Sneir, R.; Enav, H.; Luft, N.; Davidov, G.; Golan-Goldhirsh, A. Antileishmanial activity in Israeli plants. Ann. Trop. Med. Parasitol. 2009, 103, 297–306. [Google Scholar] [CrossRef]
- Ozer, L.; El-On, J.; Golan-Goldhirsh, A.; Gopas, J. Leishmania major: Anti-leishmanial activity of Nuphar lutea extract mediated by the activation of transcription factor NF-κB. Exp. Parasitol. 2010, 126, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Ozer, J.; Levi, T.; Golan-Goldhirsh, A.; Gopas, J. Anti-inflammatory effect of a Nuphar lutea partially purified leaf extract in murine models of septic shock. J. Ethnopharmacol. 2015, 161, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Ozer, J.; Fishman, D.; Eilam, B.; Golan-Goldhirsh, A.; Gopas, J.; Segal, S. Anti-Metastatic Effect of Semi-Purified Nuphar Lutea Leaf Extracts. J. Cancer 2017, 8, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Winer, H.; Ozer, J.; Shemer, Y.; Reichenstein, I.; Eilam-Frenkel, B.; Benharroch, D.; Golan-Goldhirsh, A.; Gopas, J. Nuphar lutea Extracts Exhibit Anti-Viral Activity against the Measles Virus. Molecules 2020, 25, 1657. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Shapira, L. Nupharidine Enhances Aggregatibacter actinomycetemcomitans Clearance by Priming Neutrophils and Augmenting Their Effector Functions. Artic. J. Clin. Periodontol. 2018, 46, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Muduli, S.; Golan-Goldhirsh, A.; Gopas, J.; Danilenko, M. Cytotoxicity of Thioalkaloid-Enriched Nuphar lutea Extract and Purified 6,6′-Dihydroxythiobinupharidine in Acute Myeloid Leukemia Cells: The Role of Oxidative Stress and Intracellular Calcium. Pharmaceuticals 2022, 15, 410. [Google Scholar] [CrossRef] [PubMed]
- Dalvie, E.D.; Gopas, J.; Golan-Goldhirsh, A.; Osheroff, N. 6,6′-Dihydroxythiobinupharidine as a poison of human type II topoisomerases. Bioorganic Med. Chem. Lett. 2019, 29, 1881–1885. [Google Scholar] [CrossRef]
- Waidha, K.; Anto, N.P.; Jayaram, D.R.; Golan-Goldhirsh, A.; Rajendran, S.; Livneh, E.; Gopas, J. 6,6′-Dihydroxythiobinupharidine (DTBN) Purified from Nuphar lutea Leaves Is an Inhibitor of Protein Kinase C Catalytic Activity. Molecules 2021, 26, 2785. [Google Scholar] [CrossRef] [PubMed]
- Waidha, K.; Zurgil, U.; Ben-zeev, E.; Gopas, J.; Rajendran, S.; Golan-goldhirsh, A. Inhibition of Cysteine Proteases by 6,6′-Dihydroxythiobinupharidine (DTBN) from Nuphar lutea. Molecules 2021, 26, 4743. [Google Scholar] [CrossRef]
- Weiss, S.; Waidha, K.; Rajendran, S.; Benharroch, D.; Khalilia, J.; Levy, H.; Bar-David, E.; Golan-Goldhirsh, A.; Gopas, J.; Ben-Shmuel, A. In Vitro and In Vivo Therapeutic Potential of 6,6′-Dihydroxythiobinupharidine (DTBN) from Nuphar lutea on Cells and K18-hACE2 Mice Infected with SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 8327. [Google Scholar] [CrossRef]
- Sagi, O.; Berkowitz, A.; Codish, S.; Novack, V.; Rashti, A.; Akad, F.; Shemer-Avni, Y. Sensitive Molecular Diagnostics for Cutaneous Leishmaniasis. Open Forum Infect. Dis. 2017, 4, ofx037. [Google Scholar] [CrossRef]
- Khayeka-Wandabwa, C.; Kutima, H.L.; Nyambati, V.C.S.; Ingonga, J.; Oyoo–Okoth, E.; Karani, L.W.; Jumba, B.; Githuku, K.S.; O Anjili, C. Combination therapy using Pentostam and Praziquantel improves lesion healing and parasite resolution in BALB/c mice co-infected with Leishmania major and Schistosoma mansoni. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef]
- Duraffour, P.; Mehanna, C.; Hoogewoud, F.; Touboul, A.; Monnet, D.; Brézin, A.P. Comparison between the areas of scarred and active toxoplasmic retinochoroiditis. Eye 2021, 35, 2733–2739. [Google Scholar] [CrossRef]
- Minodier, P.; Parola, P. Cutaneous leishmaniasis treatment. Travel Med. Infect. Dis. 2007, 5, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, M.G.; Márcia, A.; Pereira, M.; Fernandes, A.P.; Antonio, L.; Ferreira, M. Reductions in Skin and Systemic Parasite Burdens as a Combined Effect of Topical Paromomycin and Oral Miltefosine Treatment of Mice Experimentally Infected with Leishmania (Leishmania) amazonensis. Antimicrob. Agents Chemother. 2010, 54, 4699–4704. [Google Scholar] [CrossRef]
- Ghazanfari, T.; Hassan, Z.M.; Ebtekar, M.; Ahmadiani, A.; Naderi, G.; Azar, A. Garlic Induces a Shift in Cytokine Pattern in Leishmania major-Infected BALB/c Mice. Scand. J. Immunol. 2000, 52, 491–495. [Google Scholar] [CrossRef]
- Ghazanfari, T.; Hassan, Z.M.; Khamesipour, A. Enhancement of peritoneal macrophage phagocytic activity against Leishmania major by garlic (Allium sativum) treatment. J. Ethnopharmacol. 2006, 103, 333–371. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; El-Agroudy, E.; Helmy, A.; Ibrahim, T.M.; Shavandi, A.; Bekhit, A.E.D.A. Leishmania treatment and prevention: Natural and synthesized drugs. Eur. J. Med. Chem. 2018, 160, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Sakthianandeswaren, A.; Elso, C.M.; Simpson, K.; Curtis, J.M.; Kumar, B.; Speed, T.P.; Handman, E.; Foote, S.J. The wound repair response controls outcome to cutaneous leishmaniasis. Proc. Natl. Acad. Sci. USA 2005, 102, 15551. [Google Scholar] [CrossRef]
- Satoskar, A.; Laskay, T.; Alexander, J.; Tacchini-Cottier, F.; Passelli, K.; Billion, O. The Impact of Neutrophil Recruitment to the Skin on the Pathology Induced by Leishmania Infection. Front. Immunol. 2021, 1, 649348. [Google Scholar] [CrossRef]
- Escrivani, D.O.; Charlton, R.L.; Caruso, M.B.; Burle-Caldas, G.A.; Borsodi, M.P.G.; Zingali, R.B.; Arruda-Costa, N.; Palmeira-Mello, M.V.; de Jesus, J.B.; Souza, A.M.T.; et al. Chalcones identify cTXNPx as a potential antileishmanial drug target. PLoS Neglected Trop. Dis. 2021, 15, e0009951. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, T.A. Mitochondrial Topoisomerase II Activity Is Essential for Kinetoplast DNA Minicircle Segregation. Mol. Cell. Biol. 1994, 14, 3660–3667. [Google Scholar] [PubMed]
- Chowdhury, S.R.; Godinho, J.L.P.; Vinayagam, J.; Zuma, A.A.; Silva, S.T.D.M.; Jaisankar, P.; Rodrigues, J.C.F.; De Souza, W.; Majumder, H.K. Isobenzofuranone derivative JVPH3, an inhibitor of L. donovani topoisomerase II, disrupts mitochondrial architecture in trypanosomatid parasites. Sci. Rep. 2018, 8, 11940. [Google Scholar] [CrossRef] [PubMed]
- Okamura, S.; Nishiyama, E.; Yamazaki, T.; Otsuka, N.; Taniguchi, S.; Ogawa, W.; Hatano, T.; Tsuchiya, T.; Kuroda, T. Action mechanism of 6,6′-dihydroxythiobinupharidine from Nuphar japonicum, which showed anti-MRSA and anti-VRE activities. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Roy, S.; Patidar, A.; Dandapat, J.; Saha, B.; Sarkar, A. TLR2 dimer-specific ligands selectively activate protein kinase C isoforms in Leishmania infection. Immunology 2021, 164, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Rawat, A.; Kaushik, S.; Jyoti, A.; Srivastava, V.K. Endogenous cysteine protease inhibitors in upmost pathogenic parasitic protozoa. Microbiol. Res. 2022, 261, 127061. [Google Scholar] [CrossRef] [PubMed]
- Silva-Almeida, M.; Pereira, B.A.S.; Ribeiro-Guimarães, M.L.; Alves, C.R. Proteinases as virulence factors in Leishmania spp. infection in mammals. Parasites Vectors 2012, 5, 160. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Debnath, A.; McCall, L.-I.; Bernatchez, J.A.; Ndao, M.; Reed, S.L.; Rosenthal, P.J. Cysteine proteases in protozoan parasites. PLoS Neglected Trop. Dis. 2018, 12, e0006512. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmuel, O.; Rasti, A.; Zaknoun, M.; Astman, N.; Golan-Goldhirsh, A.; Sagi, O.; Gopas, J. Anti-Leishmania major Properties of Nuphar lutea (Yellow Water Lily) Leaf Extracts and Purified 6,6′ Dihydroxythiobinupharidine (DTBN). Pathogens 2024, 13, 384. https://doi.org/10.3390/pathogens13050384
Shmuel O, Rasti A, Zaknoun M, Astman N, Golan-Goldhirsh A, Sagi O, Gopas J. Anti-Leishmania major Properties of Nuphar lutea (Yellow Water Lily) Leaf Extracts and Purified 6,6′ Dihydroxythiobinupharidine (DTBN). Pathogens. 2024; 13(5):384. https://doi.org/10.3390/pathogens13050384
Chicago/Turabian StyleShmuel, Orit, Aviv Rasti, Melodie Zaknoun, Nadav Astman, Avi Golan-Goldhirsh, Orly Sagi, and Jacob Gopas. 2024. "Anti-Leishmania major Properties of Nuphar lutea (Yellow Water Lily) Leaf Extracts and Purified 6,6′ Dihydroxythiobinupharidine (DTBN)" Pathogens 13, no. 5: 384. https://doi.org/10.3390/pathogens13050384




