MultiSero: An Open-Source Multiplex-ELISA Platform for Measuring Antibody Responses to Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. SARS-CoV-2 Positive Samples and Negative Controls
2.2. Generation of SARS-CoV-2 96-Well Plate Arrays
2.2.1. S, RBD, and N Protein Production
2.2.2. Printing of Protein Arrays
2.3. SARS-CoV-2 ELISA Array Assay
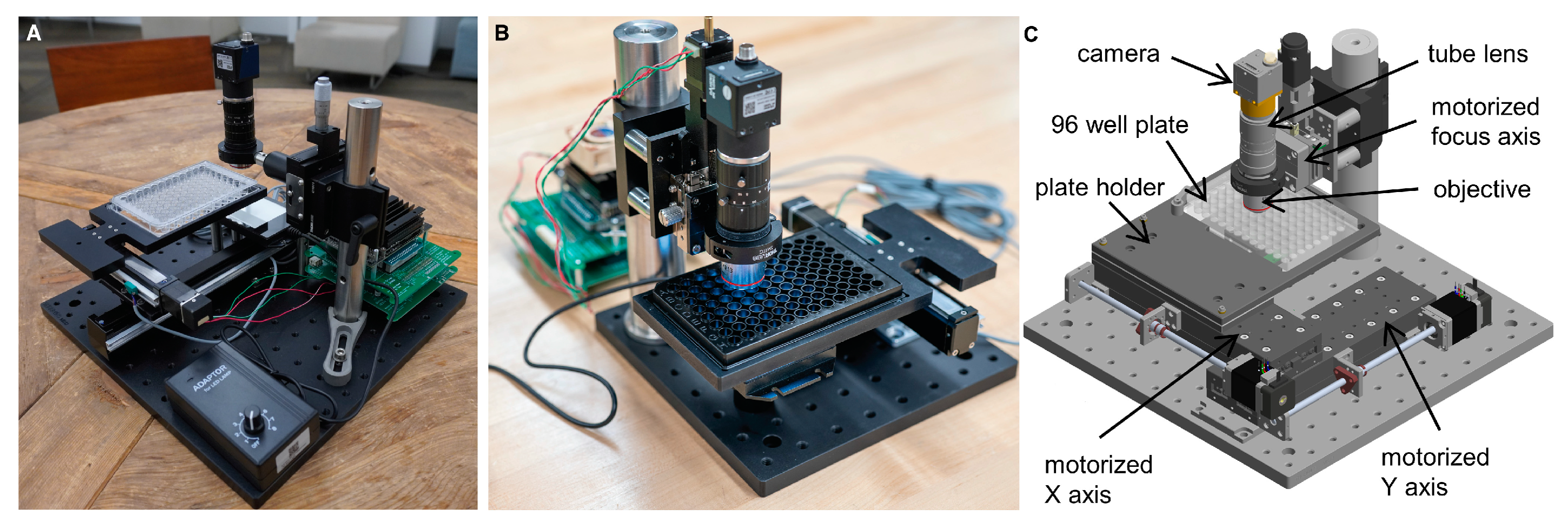
2.4. Open-Source Plate Reader, Nautilus
2.5. Multiplex ELISA Analysis with MultiSero Software
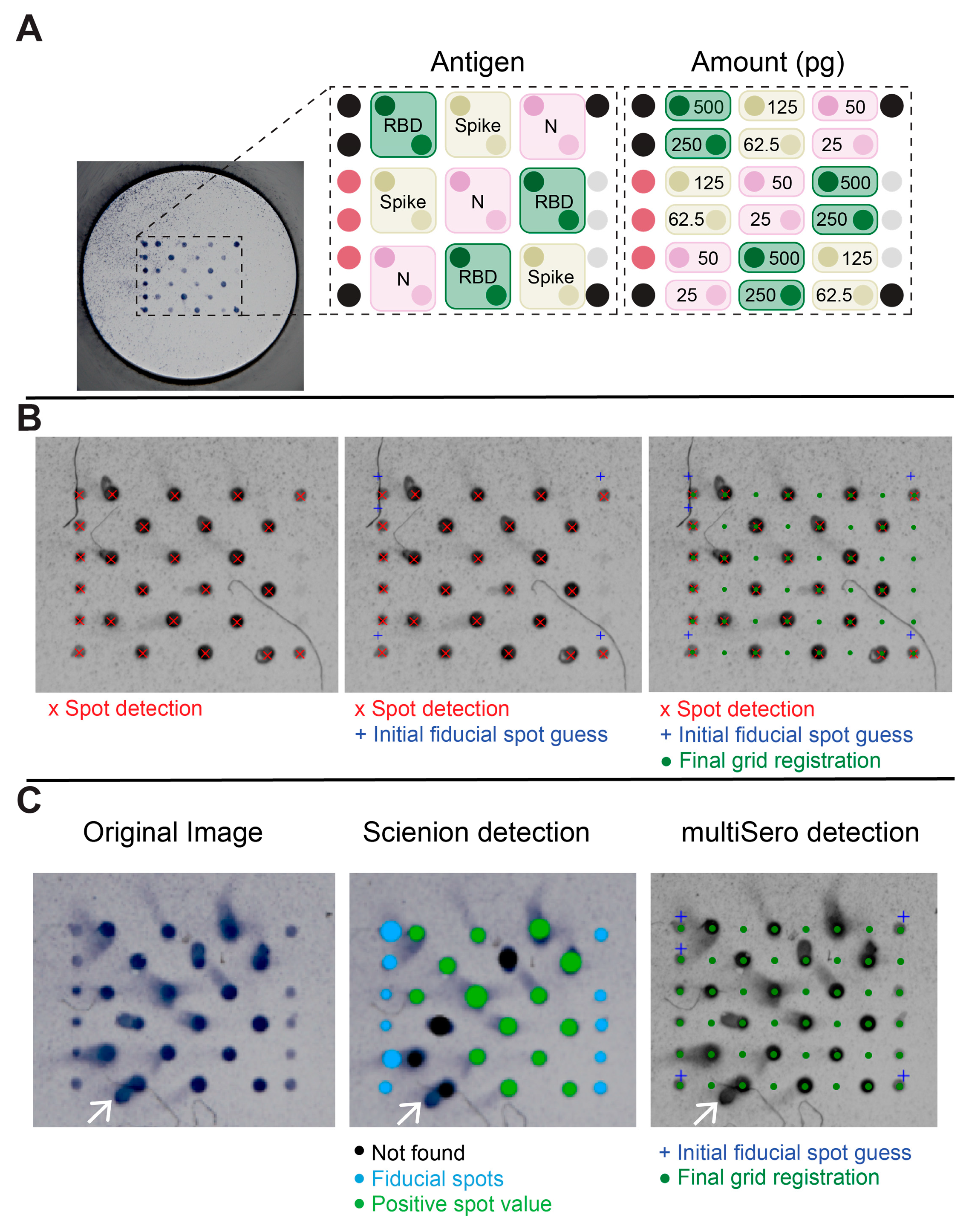
2.5.1. Detection of Spots
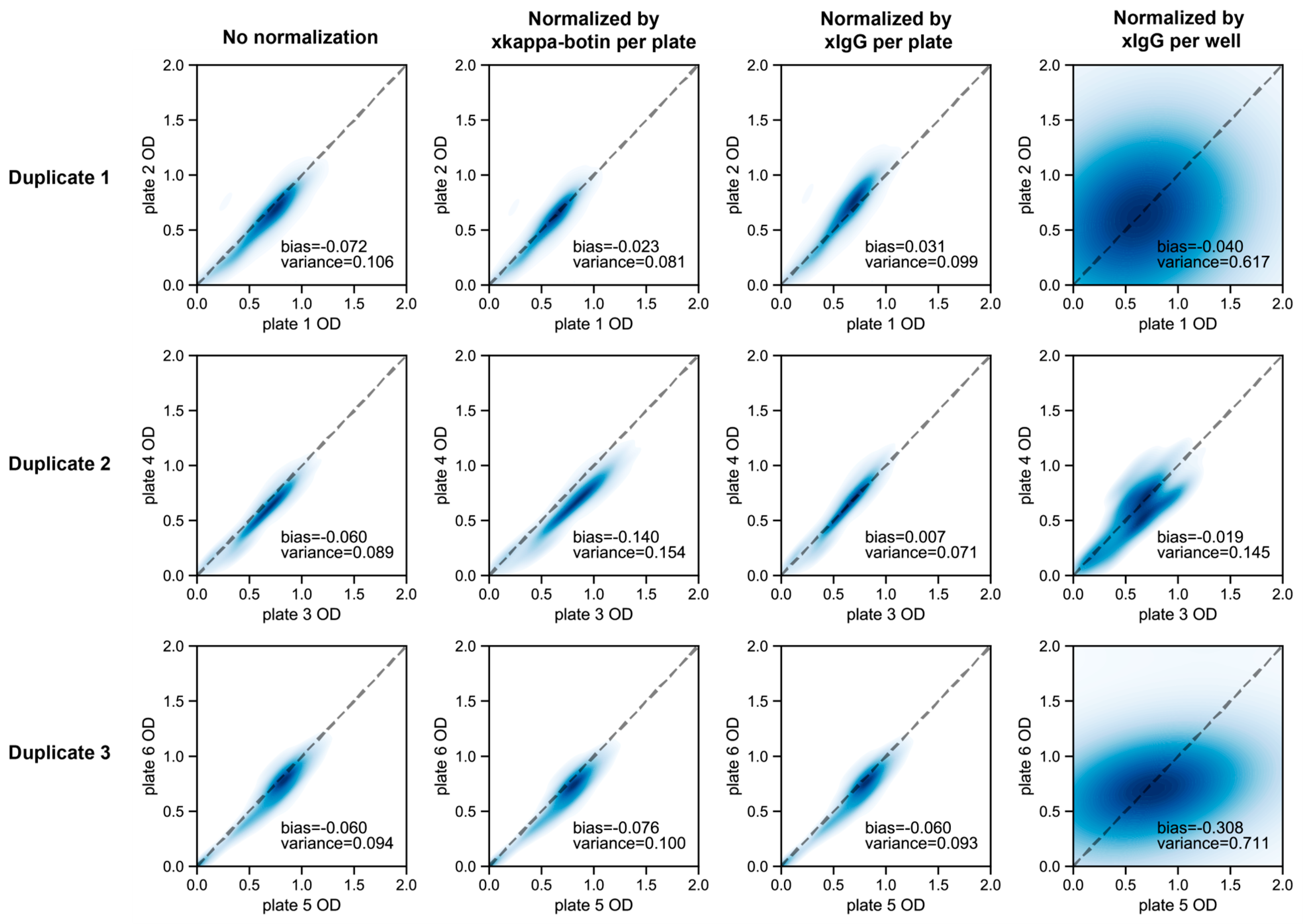
2.5.2. Measurement of OD
3. Results
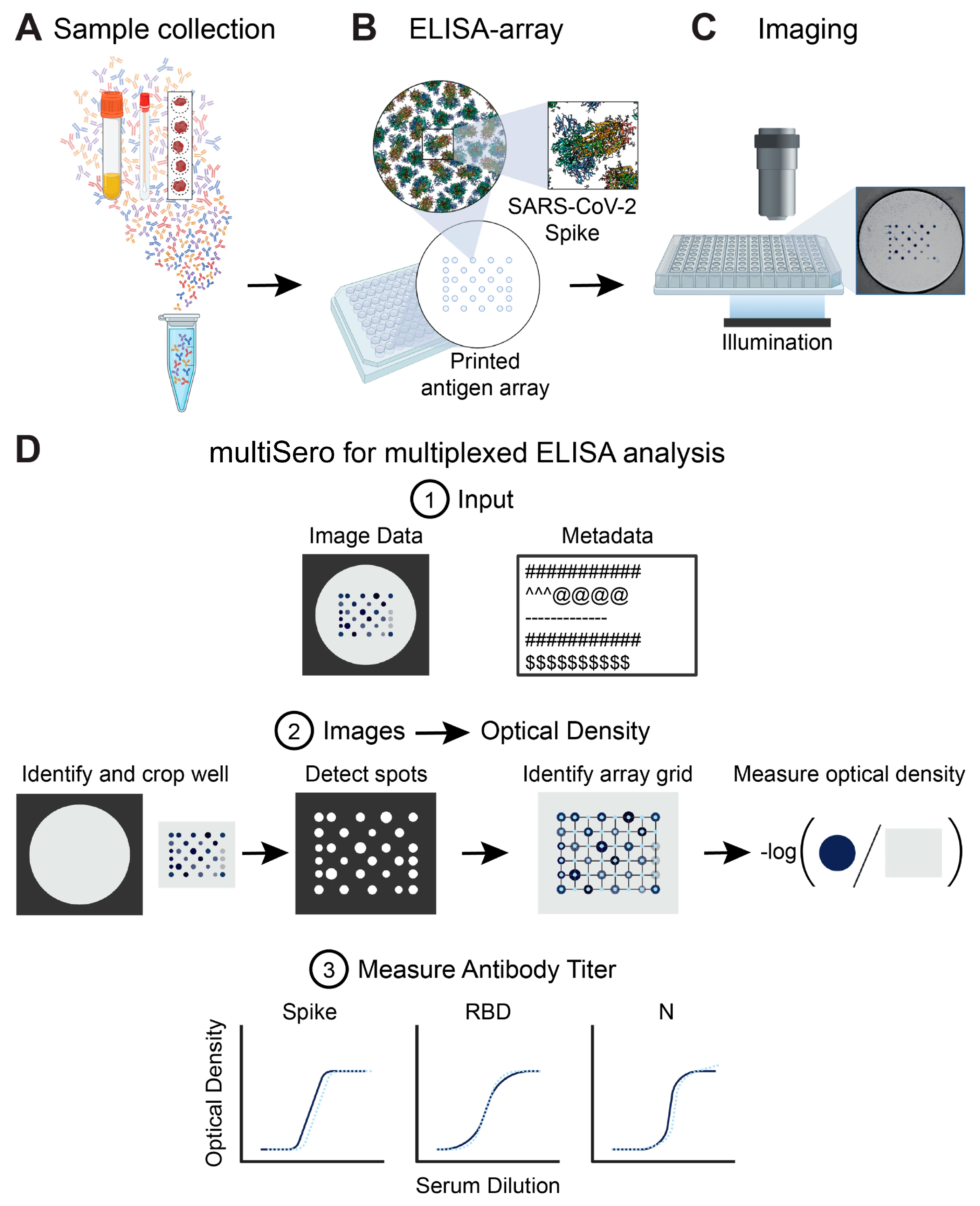
3.1. Overview of the ELISA Array and the Nautilus Plate Reader
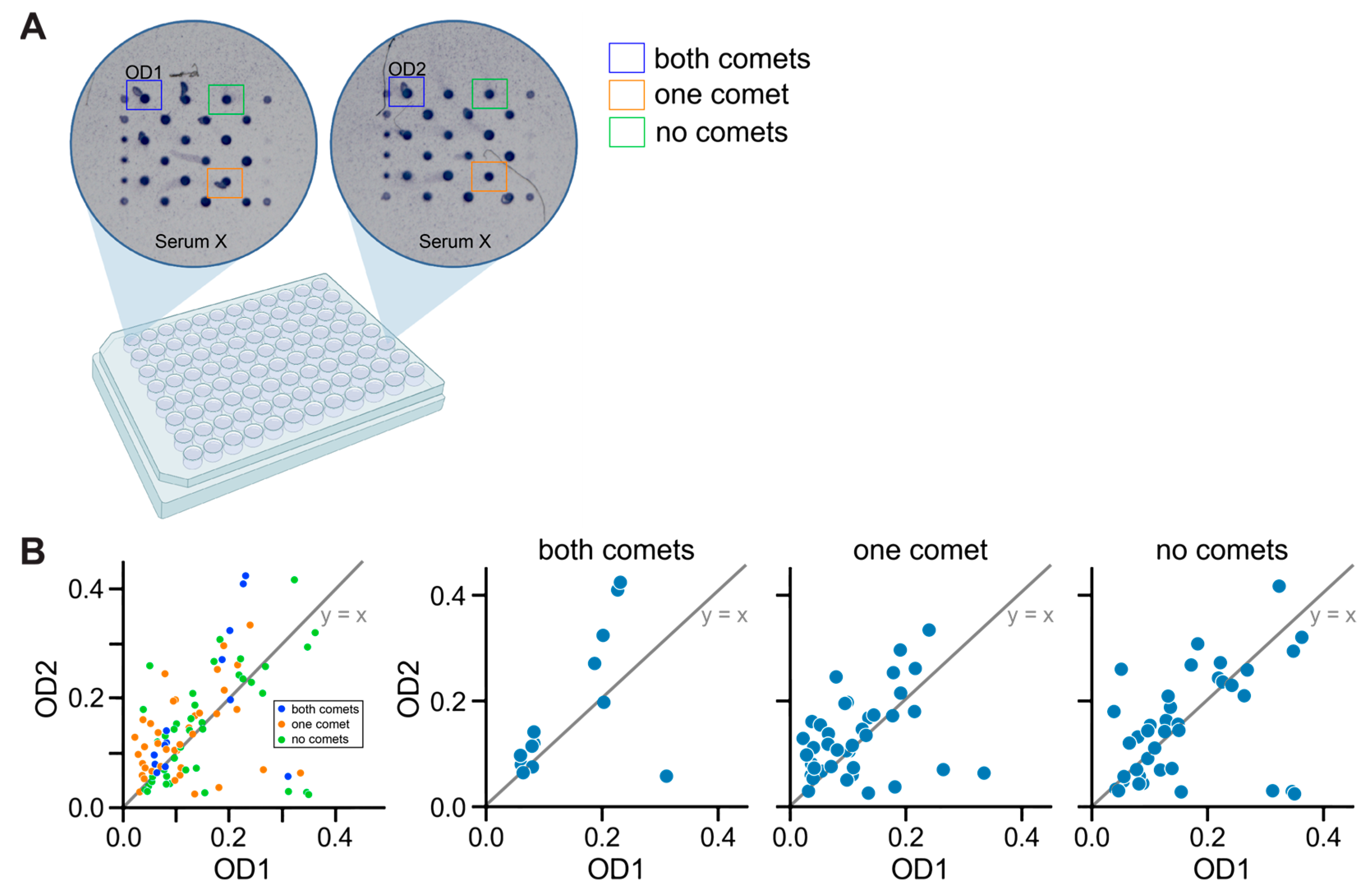
3.2. Analyzing ELISA Arrays
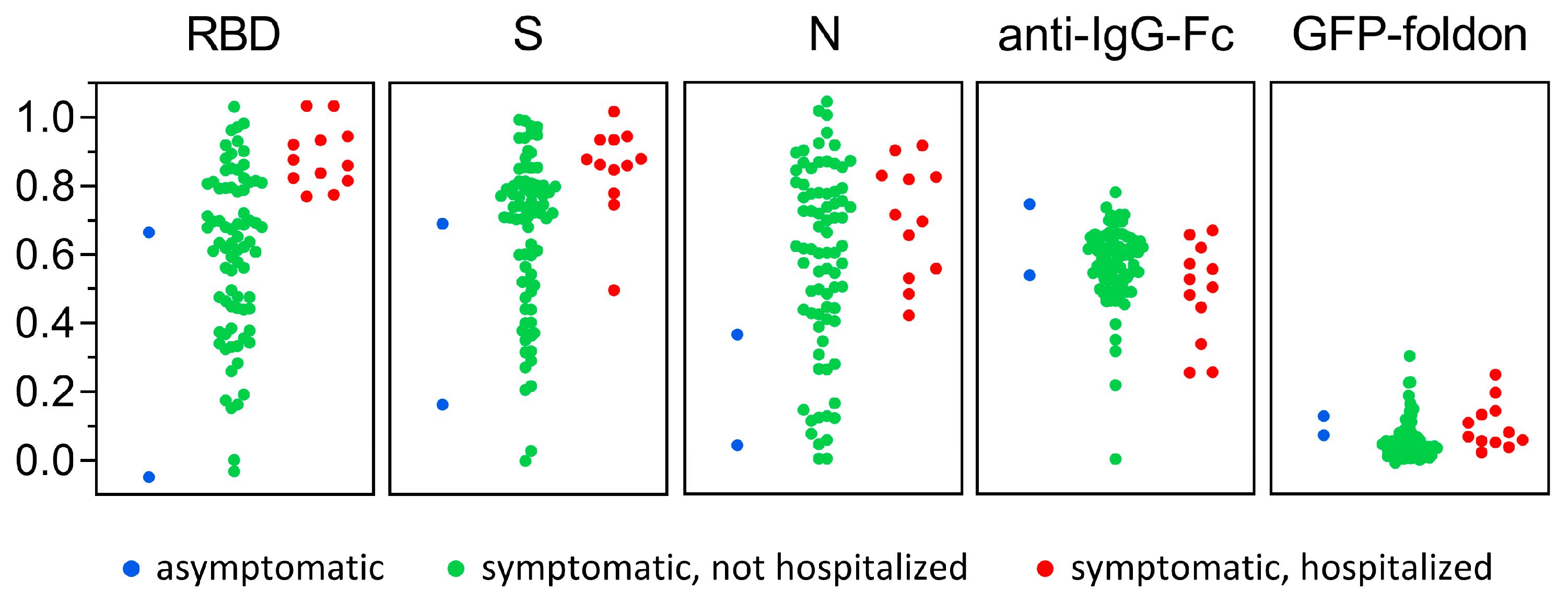
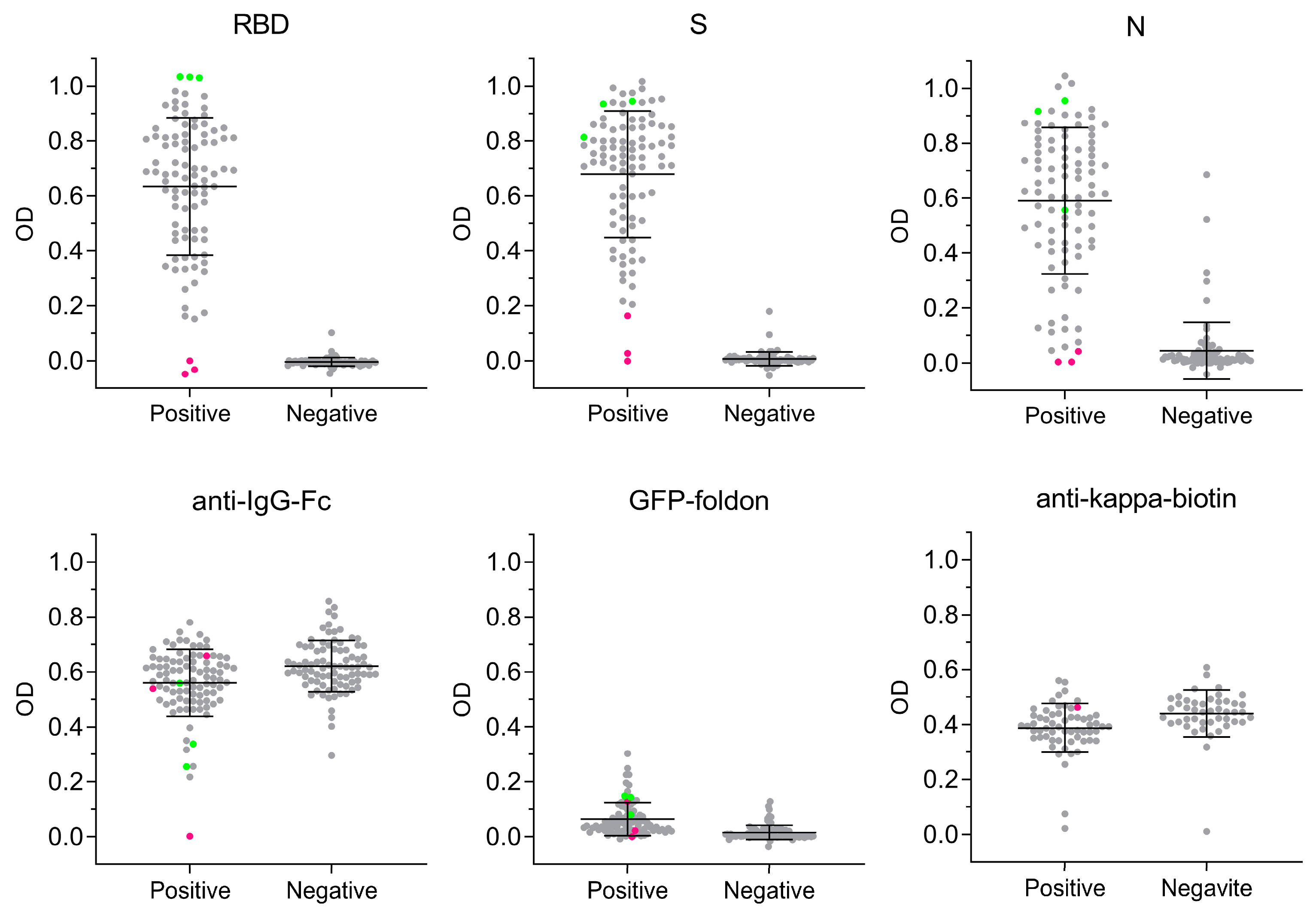
3.3. Antigen-Specific Antibody Responses to SARS-CoV-2 Infection
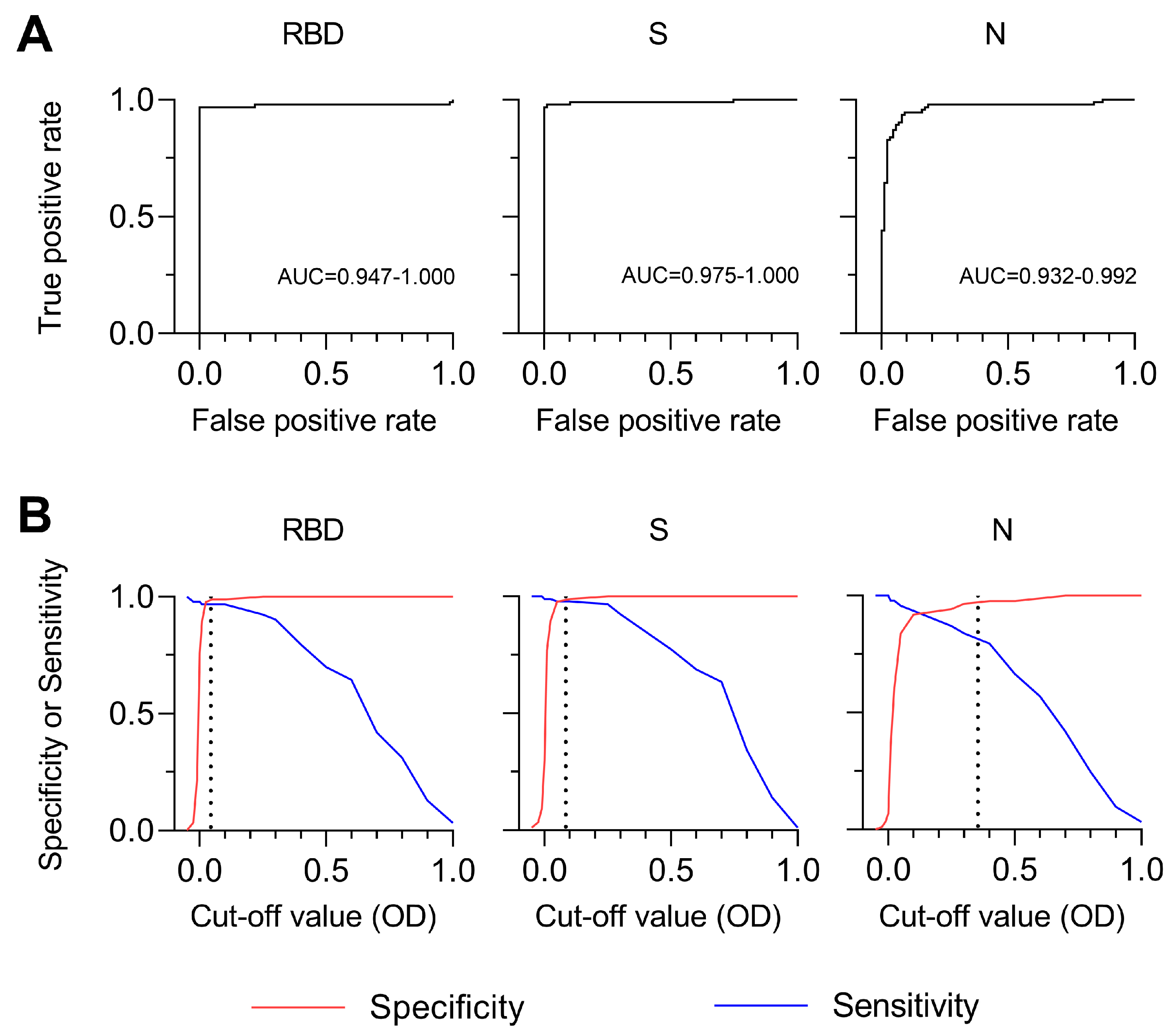
3.4. Sensitivity and Specificity of SARS-CoV-2 MultiSero ELISA
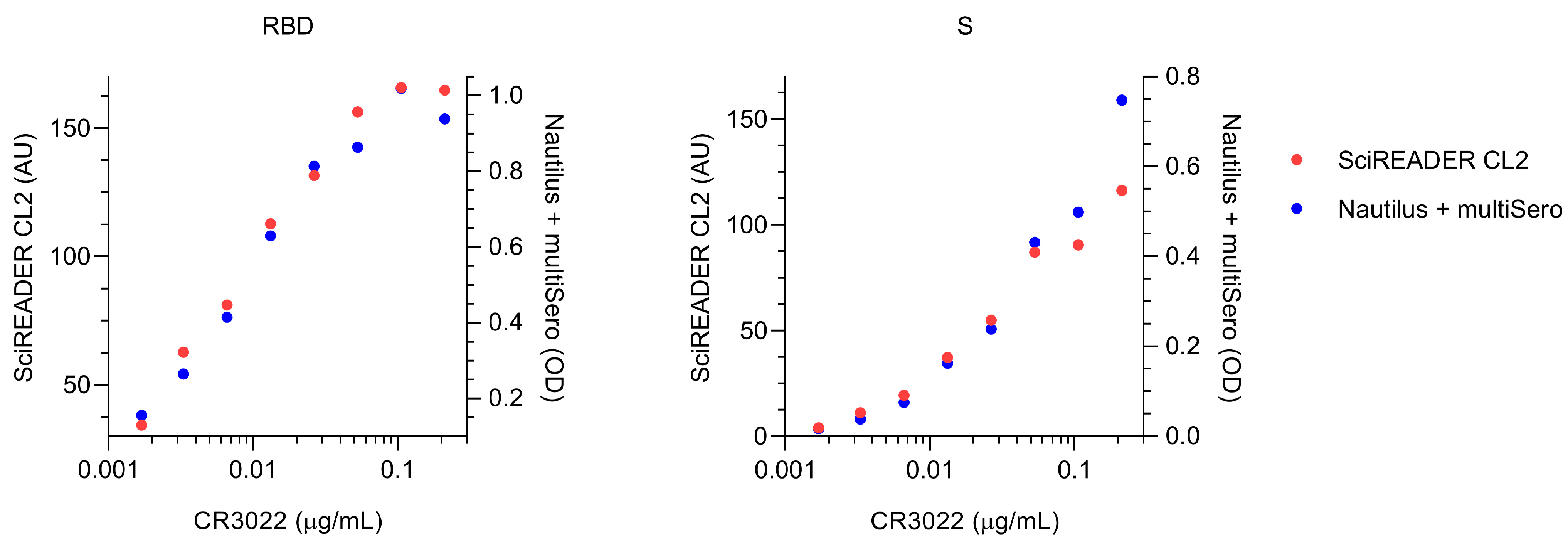
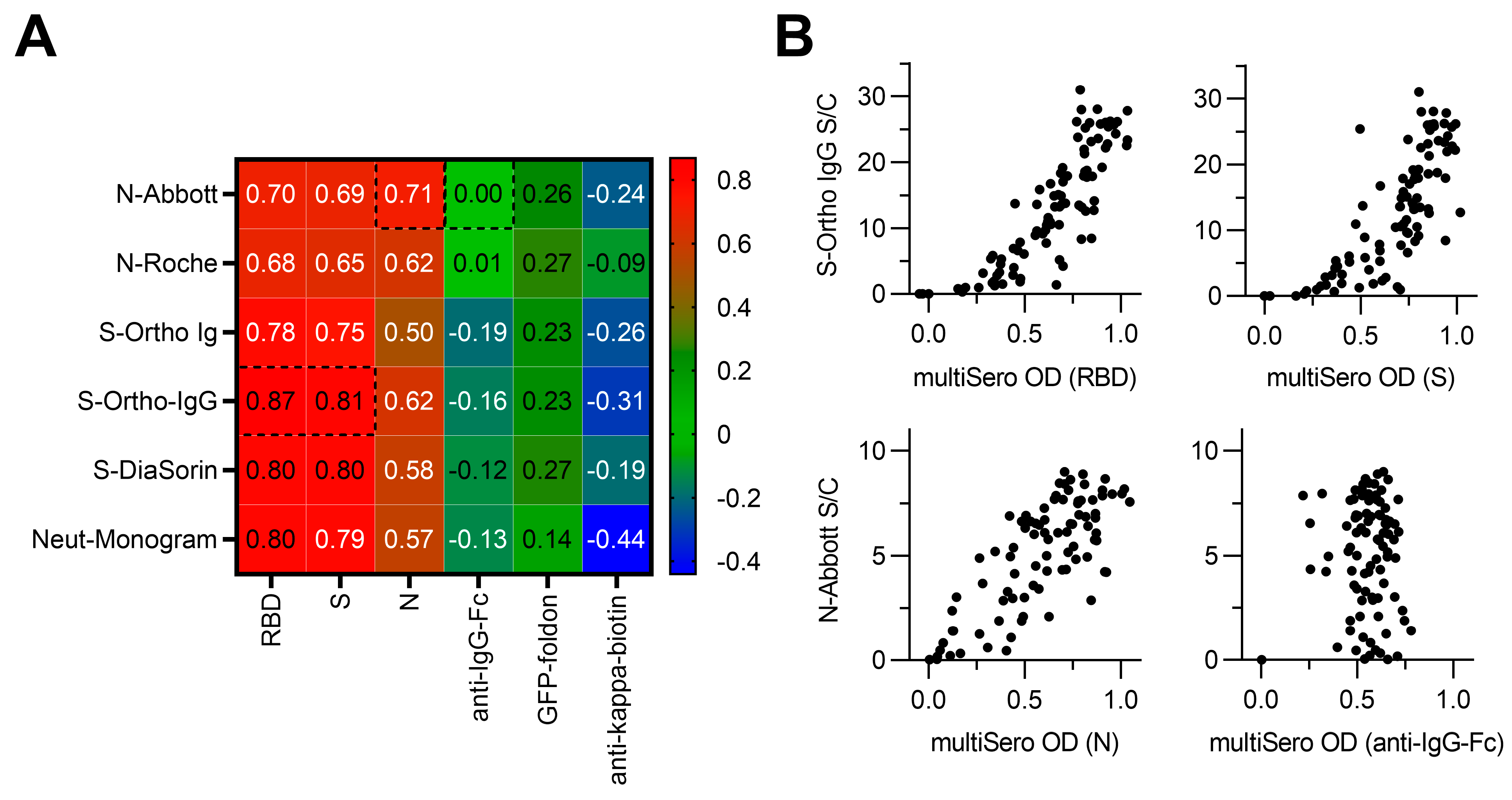
3.5. Comparison of MultiSero to Commercial SARS-CoV-2 Antibody Assays
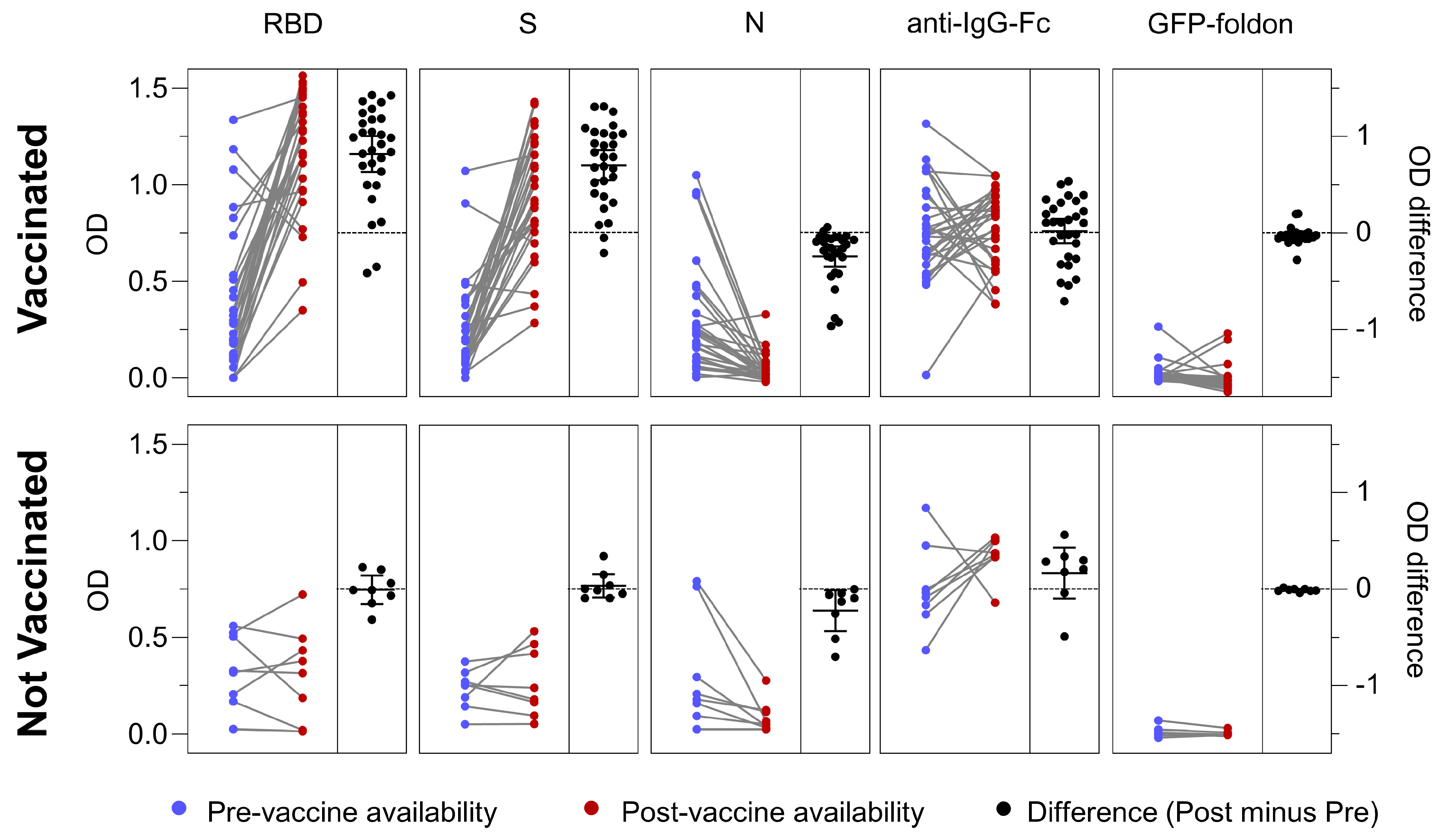
3.6. Antigen-Specific Antibody Responses in Vaccinated and Not Vaccinated Cohorts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| # | Description | Vendor | Part Number | Qty | Unit Price | Image Reference |
|---|---|---|---|---|---|---|
| Motorized Focus Assembly | ||||||
| 1 | ball bearing linear stage, stainless steel, ±6.5mm | dg-sl | LBX40-C | 1 | $61.14 | 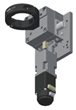 |
| 2 | adapter for mounting SM1TC to LBX40-C | XTJ-tech | 1 | $25.00 | ||
| 3 | adapter for mounting LBX40-C to C1511 | XTJ-tech | 1 | $25.00 | ||
| 4 | adapter for mounting 21H4U to LBX40-C | XTJ-tech | 1 | $55.00 | ||
| 5 | size 8 captive linear actuator, 6e-5 in/step | Haydon kerk | 21H4U-2.5-A98 v1 (based on 21H4U-2.5-907) | 1 | $130.45 | |
| 7 | Clamp for SM1 Lens Tubes | Thorlabs | SM1TC | 1 | $45.72 | |
| 8 | SM1 Lens Tube, 0.3" threaded depth | Thorlabs | SM1L03 | 1 | $12.52 | |
| 9 | SM1 adapter for Objectives with RMS thread (Olympus, BoliOptics) | Thorlabs | SM1A3 | 1 | $18.50 | |
| 10 | M3 × 15 mm (or 14 mm) socket head screws for mounting the top plate for LBX40-C (line 2) [11.5 mm + 3.5 mm/5mm] | McMaster-Carr | 91292A346 or 91292A027 | 4 | ||
| 11 | M3 × 5 mm socket head screws for mounting the LBX40-C assembly to the adapter plate for C1511 | McMaster-Carr | 91292A110 | 4 | ||
| 12 | 8-32 × 1/2" socket head screws for mounting the assembly to C1511 | McMaster-Carr | 92196A194 | 4 | ||
| 13 | 8-32 × 1/4" socket head screws for mounting SM1TC | McMaster-Carr | 92196A190 | 1 | ||
| 14 | M2.5 × 8 mm socket head screws for adapter mounting linear actuator assembly to LBX40-C | McMaster-Carr | 91292A012 | 2 | ||
| 15 | M2 × 6 mm socket head screws for mounting the linear actuator | 4 | ||||
| Total: $312.19 | ||||||
| “Microscope Body” (may be replaced by custom machined blocks) | ||||||
| 1 | Ø1.5" Post Mounting Clamp, 2.50" × 2.50" | Thorlabs | C1511 | 1 | $71.57 |  |
| 2 | Ø1.5" Mounting Post, 1/4"-20 Taps, L = 8" (other length may be used) | Thorlabs | P8 | 1 | $59.79 | |
| 3 | Aluminum Breadboard 6" × 6" × 1/2", 1/4"-20 Taps (other size may be used) | Thorlabs | MB6 | 1 | $51.48 | |
| 4 | Ø18.0 mm Sorbothane Feet, Adhesive Mounting Surface, 4 Pieces | Thorlabs | AV3 | 1 | $19.91 | |
| 5 | 1/4-20 socket head screw and washer | 1 | ||||
| Total: $202.75 | ||||||
| Imaging Lens and Camera Assembly | ||||||
| 1 | USB3 camera, Sony IMX 226, 1.85 um, 12 MP, 32 fps | Daheng | MER-1220-32U3M | 1 | $260.00 |  |
| 2 | f = 50 mm machine vision lens (1/1.8", f2.4, 10 MP rated) | HIKROBOT | MVL-HF5024M-10MP | 1 | $136.00 | |
| 3 | M27 ext - SM1 int adapter for MVL-HF5024M-10MP | Thorlabs | SM1A35 | 1 | $21.86 | |
| Total: $417.86 | ||||||
| Alternative 140 mm × (up to) 140 mm stage | ||||||
| 1 | motorized translation stage, 80 mm travel | Kgg-robot | SSMD20-R02-080L (or Squid 130 mm travel cross roller bearing stage) | 1 | $150–800 |  |
| 2 | motorized translation stage, 140 mm travel | Kgg-robot | MVD30-RE18-200L (or Squid 130 mm travel cross roller bearing stage) | 1 | $150–800 | |
| 3 | well plate holder (MIC-6 Al) | XTJ-tech | 1 | $80.00 | ||
| (Total: $400-$1000, depending on options) | ||||||
| LED matrix illuminator | ||||||
| 1 | APA102-2020 8 × 8 RGB LED Grid | Adafruit | 3444 (replaced with custom PCBA) | 1 | $24.95 |  |
| 2 | Cage Plate, for mounting the LED matrix | Thorlabs | CP37 | 1 | $19.91 | |
| 3 | Cage Plate, for condenser | Thorlabs | CP33 | 1 | $16.89 | |
| 4 | Aspheric Condenser Lens w/ Diffuser, Ø25 mm, f = 20.1 mm, NA = 0.60, 600 Grit, ARC: 350 nm–700 nm | Thorlabs | ACL2520U-DG6-A | 1 | $30.84 | |
| 5 | Cage Rods 1" (pack of 4), for connecting the assembly | Thorlabs | ER1-P4 | 1 | $19.77 | |
| Total: $112.36 | ||||||
| Objective | ||||||
| 1 | 4×/0.13 (0.17 coverslip correction) Plan Fluor WD 16.3mm | Boli Optics | FM13013231 | 1 | $88.98 | |
| Total: $88.98 | ||||||
| Parts in gray are commonly used hardware | ||||||
References
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Pérez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; de Larrea, N.F.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A Nationwide, Population-Based Seroepidemiological Study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Routledge, I.; Epstein, A.; Takahashi, S.; Janson, O.; Hakim, J.; Duarte, E.; Turcios, K.; Vinden, J.; Sujishi, K.; Rangel, J.; et al. Citywide serosurveillance of the initial SARS-CoV-2 outbreak in San Francisco using electronic health records. Nat. Commun. 2021, 12, 3566. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Stone, M.; Sulaeman, H.; Fink, R.V.; Dave, H.; Levy, M.E.; Di Germanio, C.; Green, V.; Notari, E.; Saa, P.; et al. Estimated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Based on Blood Donations, July 2020–May 2021. JAMA 2021, 326, 1400–1409. [Google Scholar] [CrossRef]
- Mercado-Reyes, M.; Malagón-Rojas, J.; Rodríguez-Barraquer, I.; Zapata-Bedoya, S.; Wiesner, M.; Cucunubá, Z.; Toloza-Pérez, Y.G.; Hernández-Ortiz, J.P.; Acosta-Reyes, J.; Parra-Barrera, E.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies in Colombia, 2020: A population-based study. Lancet Reg. Health—Am. 2022, 9, 100195. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.; Takahashi, S.; Nayebare, P.; Cuu, G.; Rek, J.; Zedi, M.; Kizza, T.; Arinaitwe, E.; Nankabirwa, J.I.; Kamya, M.; et al. Seroprevalence of Antibodies to SARS-CoV-2 in Rural Households in Eastern Uganda, 2020–2022. JAMA Netw. Open 2023, 6, e2255978. [Google Scholar] [CrossRef]
- Bergeri, I.; Whelan, M.G.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022, 19, e1004107. [Google Scholar] [CrossRef]
- Waltari, E.; Carabajal, E.; Sanyal, M.; Friedland, N.; McCutcheon, K.M. Adaption of a Conventional ELISA to a 96-Well ELISA-Array for Measuring the Antibody Responses to Influenza Virus Proteins and Vaccines. J. Immunol. Methods 2020, 481–482, 112789. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Y.; Kang, X.; Zhang, X.; Hao, H.; Chen, W.; Liu, L.; Li, X.; Li, L.; Yuan, Q.; et al. A Multiplex ELISA-Based Protein Array for Screening Diagnostic Antigens and Diagnosis of Flaviviridae Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1327–1336. [Google Scholar] [CrossRef]
- Aznar, I.; Frankena, K.; More, S.J.; Whelan, C.; Martin, W.; Gormley, E.; Corner, L.A.L.; Murphy, D.; De Jong, M.C.M. Optimising and Evaluating the Characteristics of a Multiple Antigen ELISA for Detection of Mycobacterium Bovis Infection in a Badger Vaccine Field Trial. PLoS ONE 2014, 9, e100139. [Google Scholar] [CrossRef] [PubMed]
- Zamecnik, C.R.; Rajan, J.V.; Yamauchi, K.A.; Mann, S.A.; Loudermilk, R.P.; Sowa, G.M.; Zorn, K.C.; Alvarenga, B.D.; Gaebler, C.; Caskey, M.; et al. ReScan, a Multiplex Diagnostic Pipeline, Pans Human Sera for SARS-CoV-2 Antigens. Cell Rep. Med. 2020, 1, 100123. [Google Scholar] [CrossRef]
- Kang, X.; Li, Y.; Fan, L.; Lin, F.; Wei, J.; Zhu, X.; Hu, Y.; Li, J.; Chang, G.; Zhu, Q.; et al. Development of an ELISA-Array for Simultaneous Detection of Five Encephalitis Viruses. Virol. J. 2012, 9, 56. [Google Scholar] [CrossRef]
- Tian, L.; Elsheikh, E.B.; Patrone, P.N.; Kearsley, A.J.; Gaigalas, A.K.; Inwood, S.; Lin-Gibson, S.; Esposito, D.; Wang, L. Towards Quantitative and Standardized Serological and Neutralization Assays for COVID-19. Int. J. Mol. Sci. 2021, 22, 2723. [Google Scholar] [CrossRef] [PubMed]
- Lyashchenko, K.P.; Singh, M.; Colangeli, R.; Gennaro, M.L. A Multi-Antigen Print Immunoassay for the Development of Serological Diagnosis of Infectious Diseases. J. Immunol. Methods 2000, 242, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-L.; Zhao, J.-W.; Sun, Z.-Q.; Yang, E.-Z.; Yan, J.-H.; Zhao, Q.; Zhang, G.-L.; Zhang, H.-M.; Qi, Y.-M.; Wang, H.-H.; et al. Development and Evaluation of a Novel Multiple-Antigen ELISA for Serodiagnosis of Tuberculosis. Tuberculosis 2009, 89, 278–284. [Google Scholar] [CrossRef]
- Shete, P.B.; Ravindran, R.; Chang, E.; Worodria, W.; Chaisson, L.H.; Andama, A.; Davis, J.L.; Luciw, P.A.; Huang, L.; Khan, I.H.; et al. Evaluation of Antibody Responses to Panels of M. Tuberculosis Antigens as a Screening Tool for Active Tuberculosis in Uganda. PLoS ONE 2017, 12, e0180122. [Google Scholar] [CrossRef]
- Khaliq, A.; Ravindran, R.; Hussainy, S.F.; Krishnan, V.V.; Ambreen, A.; Yusuf, N.W.; Irum, S.; Rashid, A.; Jamil, M.; Zaffar, F.; et al. Field Evaluation of a Blood Based Test for Active Tuberculosis in Endemic Settings. PLoS ONE 2017, 12, e0173359. [Google Scholar] [CrossRef]
- Menon, S.; Stansfield, S.H.; Logan, B.; Hocking, J.S.; Timms, P.; Rombauts, L.; Allan, J.A.; Huston, W.M. Development and Evaluation of a Multi-Antigen Peptide ELISA for the Diagnosis of Chlamydia Trachomatis-Related Infertility in Women. J. Med. Microbiol. 2016, 65, 915–922. [Google Scholar] [CrossRef]
- Hara, Y.; Chin, C.-Y.; Mohamed, R.; Puthucheary, S.D.; Nathan, S. Multiple-Antigen ELISA for Melioidosis—A Novel Approach to the Improved Serodiagnosis of Melioidosis. BMC Infect. Dis. 2013, 13, 165. [Google Scholar] [CrossRef]
- den Hartog, G.; Schepp, R.M.; Kuijer, M.; GeurtsvanKessel, C.; van Beek, J.; Rots, N.; Koopmans, M.P.G.; van der Klis, F.R.M.; van Binnendijk, R.S. SARS-CoV-2–Specific Antibody Detection for Seroepidemiology: A Multiplex Analysis Approach Accounting for Accurate Seroprevalence. J. Infect. Dis. 2020, 222, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Fotis, C.; Meimetis, N.; Tsolakos, N.; Politou, M.; Akinosoglou, K.; Pliaka, V.; Minia, A.; Terpos, E.; Trougakos, I.P.; Mentis, A.; et al. Accurate SARS-CoV-2 Seroprevalence Surveys Require Robust Multi-Antigen Assays. Sci. Rep. 2021, 11, 6614. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent Antibody Responses to SARS-CoV-2 in Convalescent Individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Waltari, E.; Nafees, S.; McCutcheon, K.M.; Wong, J.; Pak, J.E. AIRRscape: An interactive tool for exploring B-cell receptor repertoires and antibody responses. PLoS Comput. Biol. 2022, 18, e1010052. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody Responses to SARS-CoV-2 in Patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Tighe, P.J.; Ryder, R.R.; Todd, I.; Fairclough, L.C. ELISA in the multiplex era: Potentials and pitfalls. Proteom. Clin. Appl. 2015, 9, 406–422. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A Serological Assay to Detect SARS-CoV-2 Seroconversion in Humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef]
- Pilarowski, G.; Lebel, P.; Sunshine, S.; Liu, J.; Crawford, E.; Marquez, C.; Rubio, L.; Chamie, G.; Martinez, J.; Peng, J.; et al. Performance Characteristics of a Rapid Severe Acute Respiratory Syndrome Coronavirus 2 Antigen Detection Assay at a Public Plaza Testing Site in San Francisco. J. Infect. Dis. 2021, 223, 1139–1144. [Google Scholar] [CrossRef]
- Powell, A.E.; Zhang, K.; Sanyal, M.; Tang, S.; Weidenbacher, P.A.; Li, S.; Pham, T.D.; Pak, J.E.; Chiu, W.; Kim, P.S. A Single Immunization with Spike-Functionalized Ferritin Vaccines Elicits Neutralizing Antibody Responses against SARS-CoV-2 in Mice. ACS Cent. Sci. 2021, 7, 183–199. [Google Scholar] [CrossRef]
- Li, H.; Krishnamurthy, D.; Li, E.; Vyas, P.; Akireddy, N.; Chai, C.; Prakash, M. Squid: Simplifying Quantitative Imaging Platform Development and Deployment. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bradski, J. The OpenCV Library. Dr. Dobb’s J. Software Tools. 2000, 120, 122–125. [Google Scholar]
- Doucet, A.; Johansen, A.M. A Tutorial on Particle Filtering and Smoothing: Fifteen Years Later. Handb. Nonlinear Filter. 2009, 12, 3. [Google Scholar]
- de Bruijne, M.; Nielsen, M. Shape Particle Filtering for Image Segmentation. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2004; Barillot, C., Haynor, D.R., Hellier, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 168–175. [Google Scholar]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.-C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.P.; Wilson, I.A. A Highly Conserved Cryptic Epitope in the Receptor-Binding Domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Takahashi, S.; Hakim, J.; Kelly, J.D.; Torres, L.; Iyer, N.S.; Turcios, K.; Janson, O.; Munter, S.E.; Thanh, C.; et al. SARS-CoV-2 Antibody Magnitude and Detectability Are Driven by Disease Severity, Timing, and Assay. Sci. Adv. 2021, 7, eabh3409. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Donina, S.; Zabrodskaya, Y.; Rudenko, L.; Isakova-Sivak, I. Cross-Reactivity of SARS-CoV-2 Nucleocapsid-Binding Antibodies and Its Implication for COVID-19 Serology Tests. Viruses 2022, 14, 2041. [Google Scholar] [CrossRef]
- Becker, M.; Strengert, M.; Junker, D.; Kaiser, P.D.; Kerrinnes, T.; Traenkle, B.; Dinter, H.; Häring, J.; Ghozzi, S.; Zeck, A.; et al. Exploring beyond Clinical Routine SARS-CoV-2 Serology Using MultiCoV-Ab to Evaluate Endemic Coronavirus Cross-Reactivity. Nat. Commun. 2021, 12, 1152. [Google Scholar] [CrossRef]
- Klumpp-Thomas, C.; Kalish, H.; Drew, M.; Hunsberger, S.; Snead, K.; Fay, M.P.; Mehalko, J.; Shunmugavel, A.; Wall, V.; Frank, P.; et al. Standardization of ELISA Protocols for Serosurveys of the SARS-CoV-2 Pandemic Using Clinical and at-Home Blood Sampling. Nat. Commun. 2021, 12, 113. [Google Scholar] [CrossRef]
- Azman, A.S.; Lessler, J.; Luquero, F.J.; Bhuiyan, T.R.; Khan, A.I.; Chowdhury, F.; Kabir, A.; Gurwith, M.; Weil, A.A.; Harris, J.B.; et al. Estimating Cholera Incidence with Cross-Sectional Serology. Sci. Transl. Med. 2019, 11, eaau6242. [Google Scholar] [CrossRef]
- Cervia, C.; Zurbuchen, Y.; Taeschler, P.; Ballouz, T.; Menges, D.; Hasler, S.; Adamo, S.; Raeber, M.E.; Bächli, E.; Rudiger, A.; et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat. Commun. 2022, 13, 446. [Google Scholar] [CrossRef]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020, 5, eabe0367. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Swets, M.C.; Russell, C.D.; Harrison, E.M.; Docherty, A.B.; Lone, N.; Girvan, M.; Hardwick, H.E.; Visser, L.G.; Openshaw, P.J.M.; Groeneveld, G.H.; et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet 2022, 399, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Muecksch, F.; Weisblum, Y.; Da Silva, J.; Bednarski, E.; Cho, A.; Wang, Z.; Gaebler, C.; Caskey, M.; Nussenzweig, M.C.; et al. Plasma Neutralization of the SARS-CoV-2 Omicron Variant. N. Engl. J. Med. 2022, 386, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Haselbeck, A.H.; Im, J.; Prifti, K.; Marks, F.; Holm, M.; Zellweger, R.M. Serology as a Tool to Assess Infectious Disease Landscapes and Guide Public Health Policy. Pathogens 2022, 11, 732. [Google Scholar] [CrossRef]
- Wiens, K.E.; Jauregui, B.; Arnold, B.F.; Banke, K.; Wade, D.; Hayford, K.; Costero-Saint Denis, A.; Hall, R.H.; Salje, H.; Rodriguez-Barraquer, I.; et al. Building an integrated serosurveillance platform to inform public health interventions: Insights from an experts’ meeting on serum biomarkers. PLoS Negl. Trop. Dis. 2022, 16, e0010657. [Google Scholar] [CrossRef]
- Butt, J.; Murugan, R.; Hippchen, T.; Olberg, S.; van Straaten, M.; Wardemann, H.; Stebbins, E.; Kräusslich, H.-G.; Bartenschlager, R.; Brenner, H.; et al. From Multiplex Serology to Serolomics-A Novel Approach to the Antibody Response against the SARS-CoV-2 Proteome. Viruses 2021, 13, 749. [Google Scholar] [CrossRef]
- Mariën, J.; Ceulemans, A.; Michiels, J.; Heyndrickx, L.; Kerkhof, K.; Foque, N.; Widdowson, M.-A.; Mortgat, L.; Duysburgh, E.; Desombere, I.; et al. Evaluating SARS-CoV-2 spike and nucleocapsid proteins as targets for antibody detection in severe and mild COVID-19 cases using a Luminex bead-based assay. J. Virol. Methods 2021, 288, 114025. [Google Scholar] [CrossRef]
- Prados de la Torre, E.; Obando, I.; Vidal, M.; de Felipe, B.; Aguilar, R.; Izquierdo, L.; Carolis, C.; Olbrich, P.; Capilla-Miranda, A.; Serra, P.; et al. SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays. Viruses 2022, 14, 2039. [Google Scholar] [CrossRef]
- Liu, H.; Crooks, R.M. Three-Dimensional Paper Microfluidic Devices Assembled Using the Principles of Origami. J. Am. Chem. Soc. 2011, 133, 17564–17566. [Google Scholar] [CrossRef]
- Sanjay, S.T.; Dou, M.; Sun, J.; Li, X. A Paper/Polymer Hybrid Microfluidic Microplate for Rapid Quantitative Detection of Multiple Disease Biomarkers. Sci. Rep. 2016, 6, 30474. [Google Scholar] [CrossRef]
- Carrilho, E.; Phillips, S.T.; Vella, S.J.; Martinez, A.W.; Whitesides, G.M. Paper Microzone Plates. Anal. Chem. 2009, 81, 5990–5998. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrum, J.R.; Waltari, E.; Janson, O.; Guo, S.-M.; Folkesson, J.; Chhun, B.B.; Vinden, J.; Ivanov, I.E.; Forst, M.L.; Li, H.; et al. MultiSero: An Open-Source Multiplex-ELISA Platform for Measuring Antibody Responses to Infection. Pathogens 2023, 12, 671. https://doi.org/10.3390/pathogens12050671
Byrum JR, Waltari E, Janson O, Guo S-M, Folkesson J, Chhun BB, Vinden J, Ivanov IE, Forst ML, Li H, et al. MultiSero: An Open-Source Multiplex-ELISA Platform for Measuring Antibody Responses to Infection. Pathogens. 2023; 12(5):671. https://doi.org/10.3390/pathogens12050671
Chicago/Turabian StyleByrum, Janie R., Eric Waltari, Owen Janson, Syuan-Ming Guo, Jenny Folkesson, Bryant B. Chhun, Joanna Vinden, Ivan E. Ivanov, Marcus L. Forst, Hongquan Li, and et al. 2023. "MultiSero: An Open-Source Multiplex-ELISA Platform for Measuring Antibody Responses to Infection" Pathogens 12, no. 5: 671. https://doi.org/10.3390/pathogens12050671







