Production of Escovopsis weberi (Ascomycota: Hypocreales) Mycelial Pellets and Their Effects on Leaf-Cutting Ant Fungal Gardens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Collection of Colonies, Maintenance, and Mini-Colony Establishment
2.2. Escovopsis Isolation, Identification, and Conidial Production
2.3. Mycelial Pellet Production in Liquid Culture
2.4. Biomass Production
2.5. Bait Production
2.6. Mini-Colony Bioassays
2.7. Statistical Analyses
3. Results
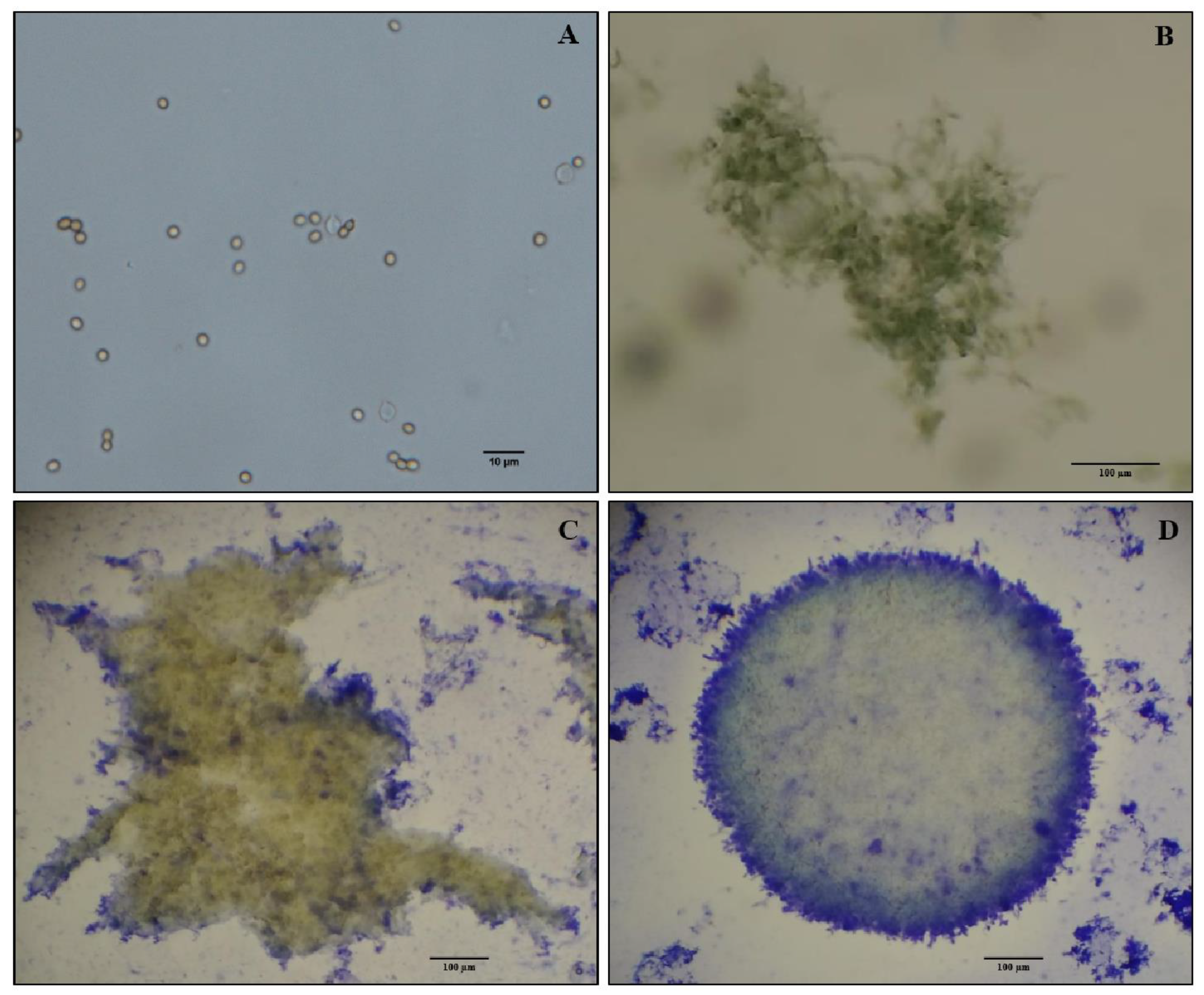
3.1. MP Production in Liquid Culture
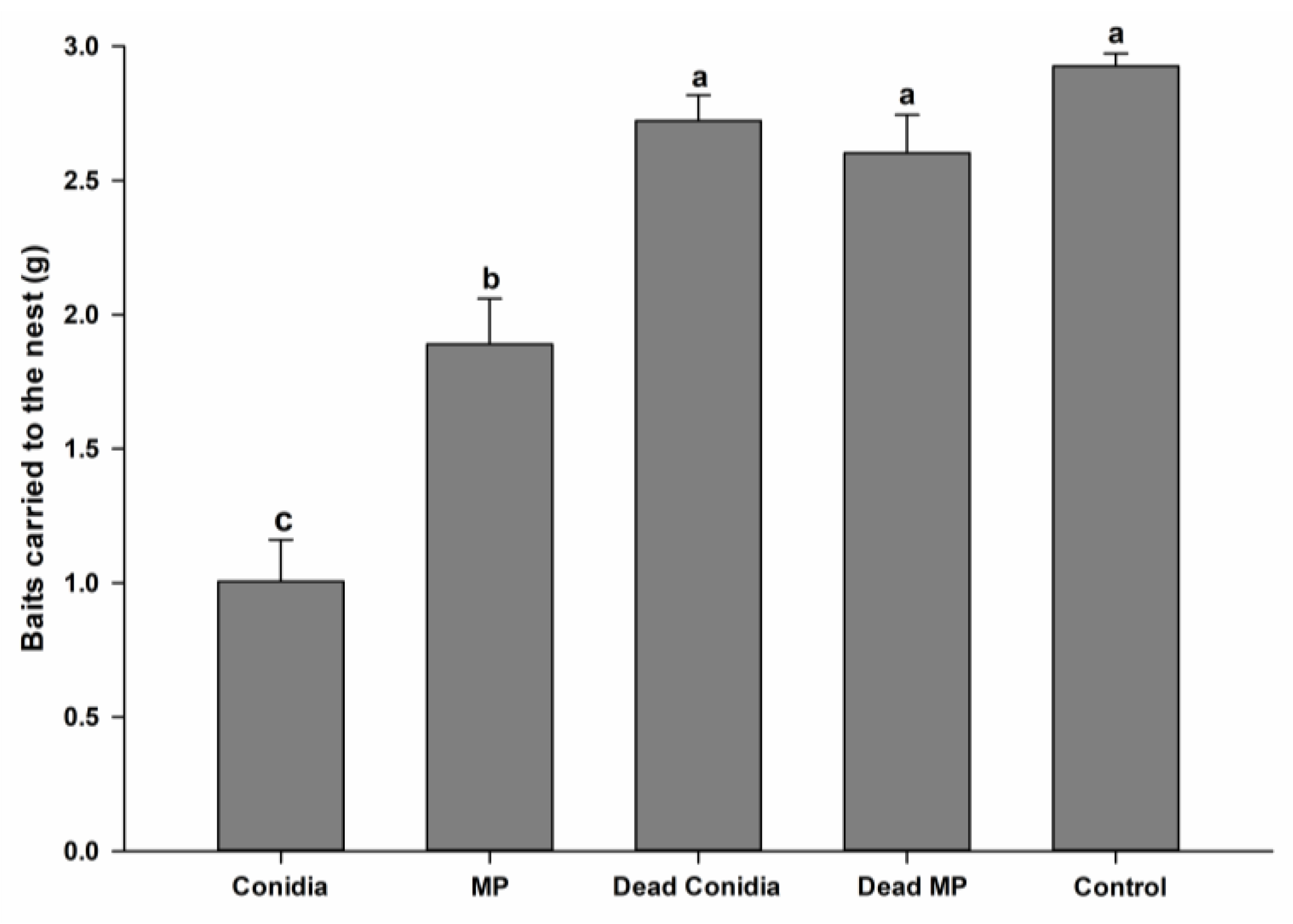
3.2. Mini-Colony Bioassays
3.3. Leaf Area Cut following Bait Exposure
3.4. Waste Disposal
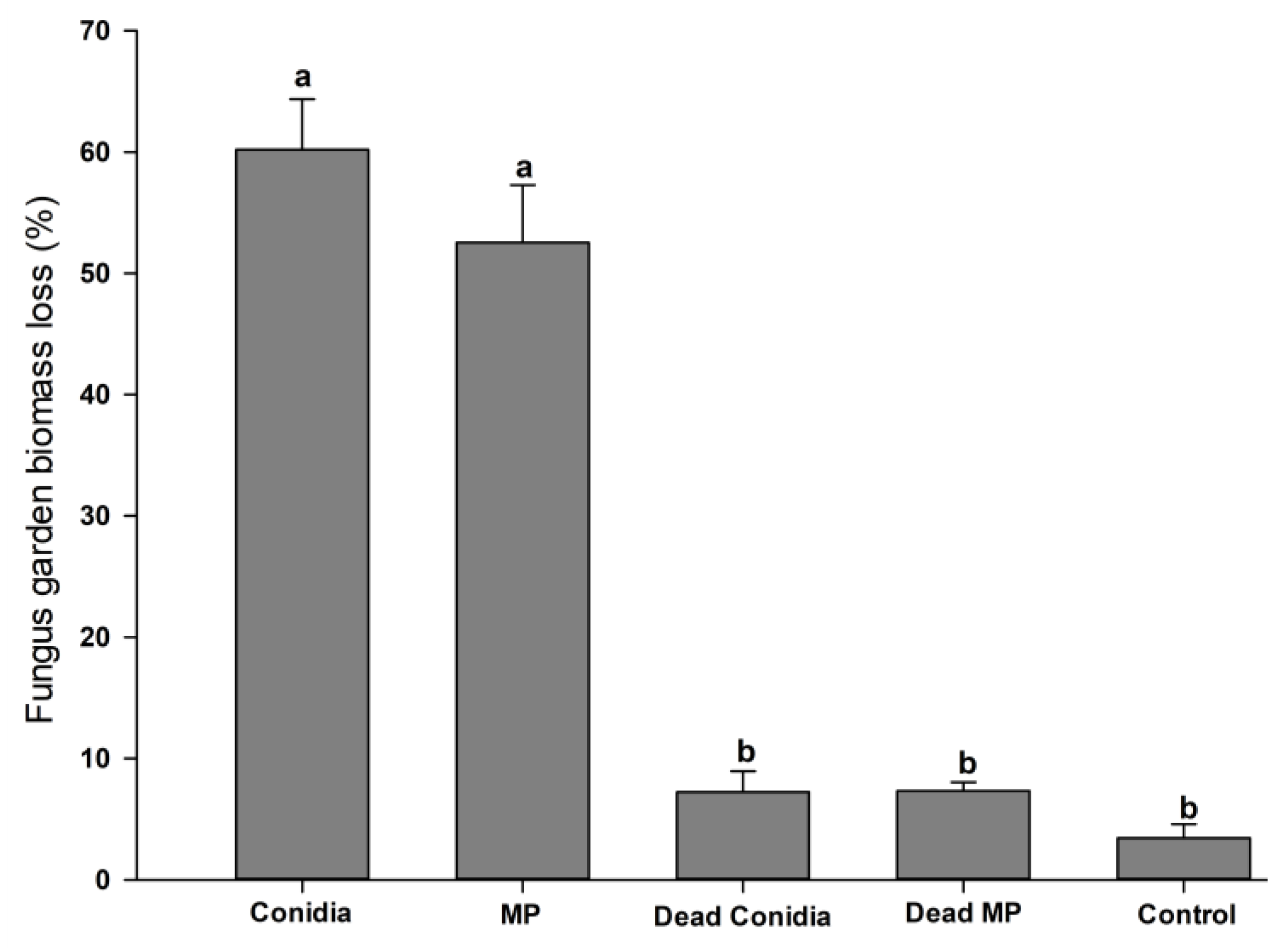
3.5. Fungal Garden Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montoya-Lerma, J.; Giraldo-Echeverri, C.; Armbrecht, I.; Farji-Brener, A.; Calle, Z. Leaf-cutting ants revisited: Towards rational management and control. Int. J. Pest Manag. 2012, 58, 225–247. [Google Scholar] [CrossRef]
- Murguia-Gonzalez, J.; Presa-Parra, E.; Serna-Lagunes, R.; Andres-Meza, P.; Rosas-Mejia, M.; Garcia-Martinez, M.A. Low Concentration of Azadirachtin Has the Same Toxic Effect as Imidacloprid + Lambda-Cyhalothrin in Workers of Two Species of Leaf-Cutter Ants. Southwest. Entomol. 2022, 47, 313–324. [Google Scholar] [CrossRef]
- Della Lucia, T.M.C.; Gandra, L.C.; Guedes, R.N.C. Managing leaf-cutting ants: Peculiarities, trends and challenges. Pest Manag. Sci. 2013, 70, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinoza, C.; Zavala, I.I.; Couttolenc, A.; Landa-Cadena, G.; Valenzuela, J.; Trigos, Á. In vitro isolation and identification of Leucoagaricus gongylophorus from Atta mexicana (Hymenoptera: Formicidae) fungal garden. Rev. Mex. Micol. 2017, 46, 3–8. [Google Scholar]
- Bustamante, S.; Amarillo-Suárez, A. Foraging plasticity of Atta cephalotes (Hymenoptera: Formicidae) in response to temperature differences between forest and pasture. Rev. Biol. Trop. 2019, 67, 963–974. [Google Scholar] [CrossRef]
- Quinlan, R.J.; Cherrett, J.M. The role of the fungus in the diet of the leafcutting ant Atta cephalotes. Ecol. Entomol. 1979, 4, 151–160. [Google Scholar] [CrossRef]
- Shik, J.Z.; Rytter, W.; Arnan, X.; Michelsen, A. Disentangling nutritional pathways linking leafcutter ants and their co-evolved fungal symbionts using stable isotopes. Ecology 2018, 99, 1999–2009. [Google Scholar] [CrossRef]
- Vigueras, G.; Paredes-Hernandez, D.; Revah, S.; Valenzuela, J.; Olivares-Hernandez, R.; Le Borgne, S. Growth and enzymatic activity of Leucoagaricus gongylophorus, a mutualistic fungus isolated from the leaf-cutting ant Atta mexicana, on cellulose and lignocellulosic biomass. Lett. Appl. Microbiol. 2017, 65, 173–181. [Google Scholar] [CrossRef]
- Della Lucia, T.M.C.; Amaral, K.D. Past and Current Strategies for the Control of Leaf-Cutting Ants in Brazil. In Forest Pest and Disease Management in Latin America; Estay, S.E., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 31–43. [Google Scholar] [CrossRef]
- Afonso, R.; Miller, D.C. Forest plantations and local economic development: Evidence from Minas Gerais, Brazil. For. Policy Econ. 2021, 133, 102618. [Google Scholar] [CrossRef]
- Souza, A.; Zanetti, R.; Calegario, N. Economic damage level for leaf-cutting ants in function of the productivity index of eucalyptus plantations in an Atlantic Forest region. Neotrop. Entomol. 2011, 40, 483–488. [Google Scholar]
- Giesel, A.; Boff, P.; Boff, M.I.C.; Fernandes, P. Ocorrência de formigas cortadeiras em campos de altitude no sul do Brasil. Res. Soc. Dev. 2020, 9, e839986365. [Google Scholar] [CrossRef]
- Vinha, G.L.; De La Cruz, R.A.; Della Lucia, T.M.C.; Wilcken, C.F.; Silva, E.D.; Lemes, P.G.; Zanuncio, J.C. Leaf-cutting ants in commercial forest plantations of Brazil: Biological aspects and control methods. South. For. 2020, 82, 95–103. [Google Scholar] [CrossRef]
- Hudson, T.M.; Turner, B.L.; Herz, H.; Robinson, J.S. Temporal patterns of nutrients availability around nests of leaf-cutting ants (Atta colombica) in secondary moist tropical forest. Soil Biol. Biochem. 2009, 41, 1088–1093. [Google Scholar] [CrossRef]
- Pikart, T.G.; Souza, G.K.; Zanuncio, T.V.; Zanetti, R.; Polanczyk, R.A.; Serrão, J.E.; Zanuncio, J.C. Dispersion of seeds of tree species by the leaf-cutting ant Acromyrmex subterraneus molestans (Hymenoptera: Formicidae) in Viçosa, Minas Gerais State, Brazil. Sociobiology 2010, 52, 645–652. [Google Scholar]
- Farji-Brener, A.; Tadey, M. Consequences of leaf-cutting ants on plant fitness: Integrating negative effects of herbivory and positive effects from soil improvement. Insectes Soc. 2016, 64, 45–54. [Google Scholar] [CrossRef]
- García-Cárdenas, D.R.; Armbrecht, I.; Torres, W.; Baena, M.L.; Montoya-Lerma, J. Functional significance of the leaf-cutting ant Atta cephalotes (Formicidae) in coffee plantations: An enemy or an ally? Pedobiologia 2022, 93, 150825. [Google Scholar] [CrossRef]
- Cherrett, J.M. The economic importance and control of leaf-cutting ants. In Economic Impact and Control of Social Insects, 2nd ed.; Vinson, S.B., Ed.; Praeger: New York, NY, USA, 1986; pp. 165–192. [Google Scholar]
- Della Lucia, T.M.C. Hormigas de importancia económica en la region neotropical. In Introducción a las Hormigas de la Region Neotropical, 1st ed.; Fernandez, F., Ed.; Instituto de Investigación de Recursos Biologicos Alexander Von Humboldt: Bogotá, Colombia, 2003; pp. 337–349. [Google Scholar]
- Perri, D.V.; Gorosito, N.B.; Schilman, P.E.; Casaubón, E.A.; Dávila, C.; Fernández, P.C. Push-pull to manage leaf-cutting ants: An effective strategy in forestry plantations. Pest Manag. Sci. 2021, 77, 432–439. [Google Scholar] [CrossRef]
- Soares, W.L.; Porto, M.F.S. Estimating the social cost of pesticide use: An assessment from acute poisoning in Brazil. Ecol. Econ. 2009, 68, 2721–2728. [Google Scholar] [CrossRef]
- Britto, J.S.; Forti, L.C.; Oliveira, M.A.; Zanetti, R.; Wilcken, C.F.; Zanuncio, J.C.; Loeck, A.E.; Caldato, N.; Nagamoto, N.S.; Lemes, P.G.; et al. Use of alternatives to PFOS, its salts and PFOSF for the control of leaf-cutting ants Atta and Acromyrmex. Int. J. Res. Environ. Stud. 2016, 3, 11–92. [Google Scholar]
- Nascimento, M.M.; Rocha, G.O.; Andrade, J.B. Pesticides in the atmospheric environment: An overview on their determination methodologies. Anal. Methods 2018, 10, 4484–4504. [Google Scholar] [CrossRef]
- Flury, M. Experimental evidence of transport of pesticides through field soils—A review. J. Environ. Qual. 1996, 25, 25–45. [Google Scholar] [CrossRef]
- Cocco, P. On the rumors about the silent spring: Review of the scientific evidence linking occupacitional and enviromental pesticide exposure to endocrine disruption health effects. Cad. Saude Publica 2002, 18, 379–402. [Google Scholar] [CrossRef]
- Laabs, V.; Amelung, W.; Pinto, A.; Zech, W. Fate of pesticides in tropical soils of Brazil under field conditions. J. Environ. Qual. 2002, 31, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Jones, C.M. Editorial overview: Pests and resistance: Resistance to pesticides in arthropod crop pests and disease vectors: Mechanisms, models and tools. Curr. Opin. Insect Sci. 2018, 27, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Travaglini, R.V.; Vieira, A.S.; Arnosti, A.A.; Camargo, R.S.; Stefanelli, L.E.P.; Forti, L.C.; Camargo-Mathias, M.I. Leaf-cutter ants and microbial control. In The Complex World of Ants, 2nd ed.; Shields, V., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Currie, C.R.; Mueller, U.G.; Malloch, D. The agricultural pathology of ant fungus gardens. Proc. Natl. Acad. Sci. USA 1999, 96, 7998–8002. [Google Scholar] [CrossRef] [Green Version]
- Folgarait, P.; Gorosito, N.; Poulsen, M.; Currie, C.R. Preliminary in vitro insights into the use of natural fungal pathogens of leaf-cutting ants as biocontrol agents. Curr. Microbiol. 2011, 63, 250–258. [Google Scholar] [CrossRef]
- Varanda-Haifig, S.S.; Albarici, T.R.; Nunes, P.H.; Haifig, I.; Vieira, P.C.; Rodrigues, A. Nature of the interactions between hypocrealean fungi and the mutualistic fungus of leaf-cutter ants. Antonie Leeuwenhoek 2017, 110, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.; Rodrigues, A.; Pagnocca, F.C.; Bueno, O.C. Susceptibility of the ant-cultivated fungus Leucoagaricus gongylophorus (Agaricales: Basidiomycota) towards microfungi. Mycopathologia 2006, 162, 115–119. [Google Scholar] [CrossRef]
- Taerum, S.J.; Cafaro, M.J.; Little, A.E.; Schultz, T.R.; Currie, C.R. Low host-pathogen specificity in the leaf-cutting ants microbe symbiosis. Proc. R. Soc. 2007, 274, 1971–1978. [Google Scholar] [CrossRef] [Green Version]
- Marfetan, J.R.; Romero, A.I.; Folgarait, P.J. Pathogenic interaction between Escovopsis weberi and Leucoagaricus sp.: Mechanisms involved and virulence levels. Fungal Ecol. 2015, 17, 52–61. [Google Scholar] [CrossRef]
- Bot, A.N.M.; Ortius-Lechner, D.; Finster, K.; Maile, R.; Boomsma, J.J. Variable sensitivity of fungi and bacteria to compounds produced by the metapleural glands of leaf-cutting ants. Insectes Soc. 2002, 49, 363–370. [Google Scholar] [CrossRef]
- Fernandez-Marın, H.; Zimmerman, J.K.; Rehner, S.A.; Wcislo, W.T. Active use of the metapleural glands by ants in controlling fungal infection. Proc. R. Soc. B 2006, 273, 1689–1695. [Google Scholar] [CrossRef] [Green Version]
- Bot, A.N.M.; Currie, C.R.; Hart, A.G.; Boomsma, J.J. Waste management in leaf-cutting ants. Ethol. Ecol. Evol. 2001, 13, 225–237. [Google Scholar] [CrossRef]
- Currie, C.R.; Stuart, A.E. Weeding and grooming of pathogen in agriculture by ants. Proc. R. Soc. B 2001, 268, 1033–1039. [Google Scholar] [CrossRef] [Green Version]
- Mattoso, T.C.; Moreira, D.D.O.; Samuels, R.I. Symbiotic bacteria on the cuticle of the leaf-cutting ant Acromyrmex subterraneus subterraneus protect workers from attack by entomopathogenic fungi. Biol. Lett. 2011, 8, 461–464. [Google Scholar] [CrossRef] [Green Version]
- Samuels, R.I.; Mattoso, T.C.; Moreira, D.D.O. Chemical warfare: Leaf-cutting ants defend themselves and their gardens against parasite attack by deploying antibiotic secreting bacteria. Commun. Integr. Biol. 2013, 6, e23095. [Google Scholar] [CrossRef] [Green Version]
- Currie, C.R. Prevalence and impact of a virulent parasite on a tripartite mutualism. Oecologia 2001, 128, 99–106. [Google Scholar] [CrossRef]
- Smith, M.E.; Henkel, T.W.; Rollins, J.A. How many fungi make sclerotia? Fungal Ecol. 2014, 13, 211–220. [Google Scholar] [CrossRef]
- Coley-Smith, J.R.; Cooke, R.C. Survival and germination of fungal sclerotia. Annu. Rev. Phytopathol. 1971, 9, 65–92. [Google Scholar] [CrossRef]
- Song, Z. Fungal microsclerotia development: Essential prerequisites, influencing factors and molecular mechanism. Appl. Microbiol. Biotechnol. 2018, 102, 9873–9880. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jaronski, S.T. Production of microsclerotia of the fungal entomopathogen Metarhizium anisopliae and their potential for use as a biocontrol agent for soil-inhabiting insects. Mycol. Res. 2009, 113, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Kobori, N.N.; Mascarin, G.M.; Jackson, M.A.; Schisler, D.A. Liquid culture production of microsclerotia and submerged conidia by Trichoderma harzianum active against damping-off disease caused by Rhizoctonia solani. Fungal Biol. 2015, 119, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huarte-Bonnet, C.; Paixão, F.R.S.; Mascarin, G.M.; Santana, M.; Fernandes, E.K.K.; Pedrini, N. The entomopathogenic fungus Beauveria bassiana produces microsclerotia-like pellets mediated by oxidative stress and peroxisome biogenesis. Environ. Microbiol. Rep. 2019, 11, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Quintana, K. Revisión de la biología y métodos de control de la hormiga arriera Atta cephalotes (Hymenoptera: Myrmicinae). In Manejo Integrado de Insectos Herbívoros en Sistemas Ganaderos Sostenibles; Castaño-Quintana, K., Chará-Orozco, J., Giraldo, C., Calle, Z., Eds.; Fundación Centro para la Investigación en Sistemas Sostenibles de Producción Agropecuaria: Cali, Colombia, 2019; pp. 108–149. [Google Scholar]
- Song, Z.Y.; Yin, Y.P.; Jiang, S.S.; Liu, J.J.; Wang, Z.K. Optimization of culture medium for microsclerotia production by Nomuraea rileyi and analysis of their viability for use as a mycoinsecticide. BioControl 2014, 59, 597–605. [Google Scholar] [CrossRef]
- Fowler, H.G.; Della Lucia, T.M.C.; Moreira, D.D.O. Posição taxonomica das formigas cortadeiras. In As Formigas Cortadeiras, 1st ed.; Della Lucia, T.M.C., Ed.; Folha de Viçosa: Viçosa, Brazil, 1993; pp. 4–25. [Google Scholar]
- Choi, Y.W.; Hyde, K.D.; Ho, W.H. Single spore isolation of fungi. Fungal Divers. 1999, 3, 29–38. [Google Scholar]
- Carolino, A.T.; Teodoro, T.B.P.; Gomes, S.A.; Samuels, R.I. Using Chick Pea flour for the production of Metarhizium anisopliae blastospores for control of Aedes albopictus larvae. 2023; manuscript in preparation. [Google Scholar]
- Jackson, M.A.; Schisler, D.A. Liquid culture production of microsclerotia of Colletotrichum truncatum for use as bioherbicidal propagules. Mycol. Res. 1995, 99, 879–884. [Google Scholar] [CrossRef]
- Shearer, J.F.; Jackson, M.A. Liquid culturing of microsclerotia of Mycoleptodiscus terrestris, a potential biological control agent for the management of Hydrilla. Biol. Control 2006, 38, 298–306. [Google Scholar] [CrossRef]
- Boyette, C.D.; Abbas, H.K.; Johnson, B.; Hoagland, R.E.; Weaver, M.A. Biological control of the weed Sesbania exaltata using a microsclerotia formulation of the bioherbicide Colletotrichum truncatum. Am. J. Plant Sci. 2014, 5, 2672–2685. [Google Scholar] [CrossRef] [Green Version]
- Villamizar, L.F.; Nelson, T.L.; Jones, S.A.; Jackson, T.A.; Hurst, M.R.H.; Marshall, S.D.G. Formation of microsclerotia in three species of Beauveria and storage stability of a prototype granular formulation. Biocontrol. Sci. Technol. 2018, 28, 1097–1113. [Google Scholar] [CrossRef]
- Chet, I.; Henis, Y.; Kislev, N. Ultrastructure of sclerotia and hyphae of Sclerotium rolfsii Sacc. J. Gen. Microbiol. 1969, 57, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.Y.; Shen, L.; Zhong, Q.; Yin, Y.P.; Wang, Z.K. Liquid culture production of microsclerotia of Purpureocillium lilacinum for use as bionematicide. Nematology 2016, 18, 719–726. [Google Scholar] [CrossRef]
- Currie, C.R. A community of ants, fungi, and bacteria: A multilateral approach to studying symbiosis. Annu. Rev. Microbiol. 2001, 55, 357–380. [Google Scholar] [CrossRef] [Green Version]
- Augustin, J.O.; Simões, T.G.; Dijksterhuis, J.; Elliot, S.L.; Evans, H.C. Putting the waste out: A proposed mechanism for transmission of the mycoparasite Escovopsis between leafcutter ant colonies. R. Soc. Open Sci. 2017, 4, 161013. [Google Scholar] [CrossRef] [Green Version]
- Boaretto, M.A.C.; Forti, L.C. Perspectivas no controle de formigas cortadeiras. Sér. Téc. IPEF 1997, 11, 31–46. [Google Scholar]
- Beauvais, A.; Latgé, J.P. Special issue: Fungal cell wall. J. Fungi 2018, 4, 91. [Google Scholar] [CrossRef] [Green Version]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U.; et al. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 2018, 79, 243–262. [Google Scholar] [CrossRef] [Green Version]
- Zanuncio, J.C.; Lemes, P.G.; Antunes, L.R.; Maia, J.L.S.; Mendes, J.E.P.; Tanganelli, K.M.; Salva, J.F.; Serrão, J.E. The impact of the Forest Stewardship Council (FSC) pesticide policy in the management of leaf-cutting ants and termites in certified forests in Brazil. Ann. For. Sci. 2016, 73, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Goes, A.C.; Barcoto, M.O.; Kooij, P.W.; Bueno, O.C.; Rodrigues, A. How do leaf-cutting ants recognize antagonistic microbes in their fungal crops? Front. Ecol. Evol. 2020, 8, 95. [Google Scholar] [CrossRef]
- Kandasamy, D.; Gershenzon, J.; Andersson, M.N.; Hammerbacher, A. Volatile organic compounds influence the interaction of the Eurasian spruce bark beetle (Ips typographus) with its fungal symbionts. ISME J. 2019, 13, 1788–1800. [Google Scholar] [CrossRef] [Green Version]
- Moisan, K.; Cordovez, V.; Zande, E.M.V.; Raaijmakers, J.M.; Dicke, M.; Barbosa, D.L. Volatiles of pathogenic and non-pathogenic soil-borne fungi affect plant development and resistance to insects. Oecologia 2019, 190, 589–604. [Google Scholar] [CrossRef] [Green Version]
- Roncal, T.; Cordobés, S.; Sterner, O.; Ugalde, U. Conidiation in Penicillium cyclopium is induced by conidiogenone, an endogenous diterpene. Eukaryot. Cell 2002, 1, 823–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, A.G.; Bot, A.N.M.; Brown, M.J.F. A colony-level response to disease control in a leaf-cutting ant. Sci. Nat. 2002, 89, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Little, A.E.F.; Murakami, T.; Mueller, U.G.; Currie, C.R. Defending against parasites: Fungus-growing ants combine specialized behaviours and microbial symbionts to protect their fungus gardens. Biol. Lett. 2006, 2, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonadies, E.; Wcislo, W.T.; Gálvez, D.; Hughes, W.H.O.; Fernández-Marín, H. Hygiene defense behaviors used by a fungus-growing ant depend on the fungal pathogen stages. Insects 2019, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Heine, D.; Holmes, N.A.; Worsley, S.F.; Santos, A.C.A.; Innocent, T.M.; Scherlach, K.; Patrick, E.H.; Yu, D.W.; Murrell, J.C.; Vieria, P.C.; et al. Chemical warfare between leafcutter ant symbionts and a co-evolved pathogen. Nat. Commun. 2018, 9, 2208. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Kobori, N.N.; Vital, R.C.J.; Jackson, M.A.; Quintela, E.D. Production of microsclerotia by brazilian strains of Metarhizium spp. using submerged liquid culture fermentation. World J. Microbiol. Biotechnol. 2013, 30, 1583–1590. [Google Scholar] [CrossRef]
- Goble, T.A.; Hajek, A.E.; Jackson, M.A.; Gardescu, S. Microsclerotia of Metarhizium brunneum F52 applied in hydromulch for control of asian longhorned beetles (Coleoptera: Cerambycidae). Econ. Entomol. 2015, 108, 433–443. [Google Scholar] [CrossRef]

| Evaluation Time (Months) | Median (×108) |
|---|---|
| 1 | 11.68 a |
| 2 | 12.40 a |
| 3 | 11.72 a |
| 4 | 14.54 a |
| 5 | 12.64 a |
| 6 | 9.67 abc |
| 7 | 10.23 ab |
| 8 | 9.44 abc |
| 9 | 10.05 abc |
| 10 | 1.3 d |
| 11 | 3.35 cd |
| 12 | 4.68 bcd |
| Treatments | Times | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Conidia | 0.20 ± 0.05 Ba | 0.58 ± 0.05 Aa | 0.51 ± 0.03 Aa |
| MP | 0.21 ± 0.05 Ba | 0.56 ± 0.05 Aa | 0.47 ± 0.02 Aa |
| Dead Conidia | 0.15 ± 0.16 Aa | 0.17 ± 0.02 Ab | 0.16 ± 0.01 Ab |
| Dead MP | 0.21 ± 0.02 Aa | 0.20 ± 0.01 Ab | 0.20 ± 0.02 Ab |
| Control | 0.16 ± 0.02 Aa | 0.19 ± 0.02 Ab | 0.17 ± 0.02 Ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teodoro, T.B.P.; Carolino, A.T.; Queiroz, R.R.d.S.; Oliveira, P.B.d.; Moreira, D.D.O.; Silva, G.A.; Samuels, R.I. Production of Escovopsis weberi (Ascomycota: Hypocreales) Mycelial Pellets and Their Effects on Leaf-Cutting Ant Fungal Gardens. Pathogens 2023, 12, 330. https://doi.org/10.3390/pathogens12020330
Teodoro TBP, Carolino AT, Queiroz RRdS, Oliveira PBd, Moreira DDO, Silva GA, Samuels RI. Production of Escovopsis weberi (Ascomycota: Hypocreales) Mycelial Pellets and Their Effects on Leaf-Cutting Ant Fungal Gardens. Pathogens. 2023; 12(2):330. https://doi.org/10.3390/pathogens12020330
Chicago/Turabian StyleTeodoro, Thais Berçot Pontes, Aline Teixeira Carolino, Raymyson Rhuryo de Sousa Queiroz, Patrícia Batista de Oliveira, Denise Dolores Oliveira Moreira, Gerson Adriano Silva, and Richard Ian Samuels. 2023. "Production of Escovopsis weberi (Ascomycota: Hypocreales) Mycelial Pellets and Their Effects on Leaf-Cutting Ant Fungal Gardens" Pathogens 12, no. 2: 330. https://doi.org/10.3390/pathogens12020330









