Ceragenins and Ceragenin-Based Core-Shell Nanosystems as New Antibacterial Agents against Gram-Negative Rods Causing Nosocomial Infections
Abstract
:1. Introduction
2. HAIs Caused by Multidrug-Resistant Gram-Negative Rods: Where We Are and Where We Could Go with New Treatment Strategies
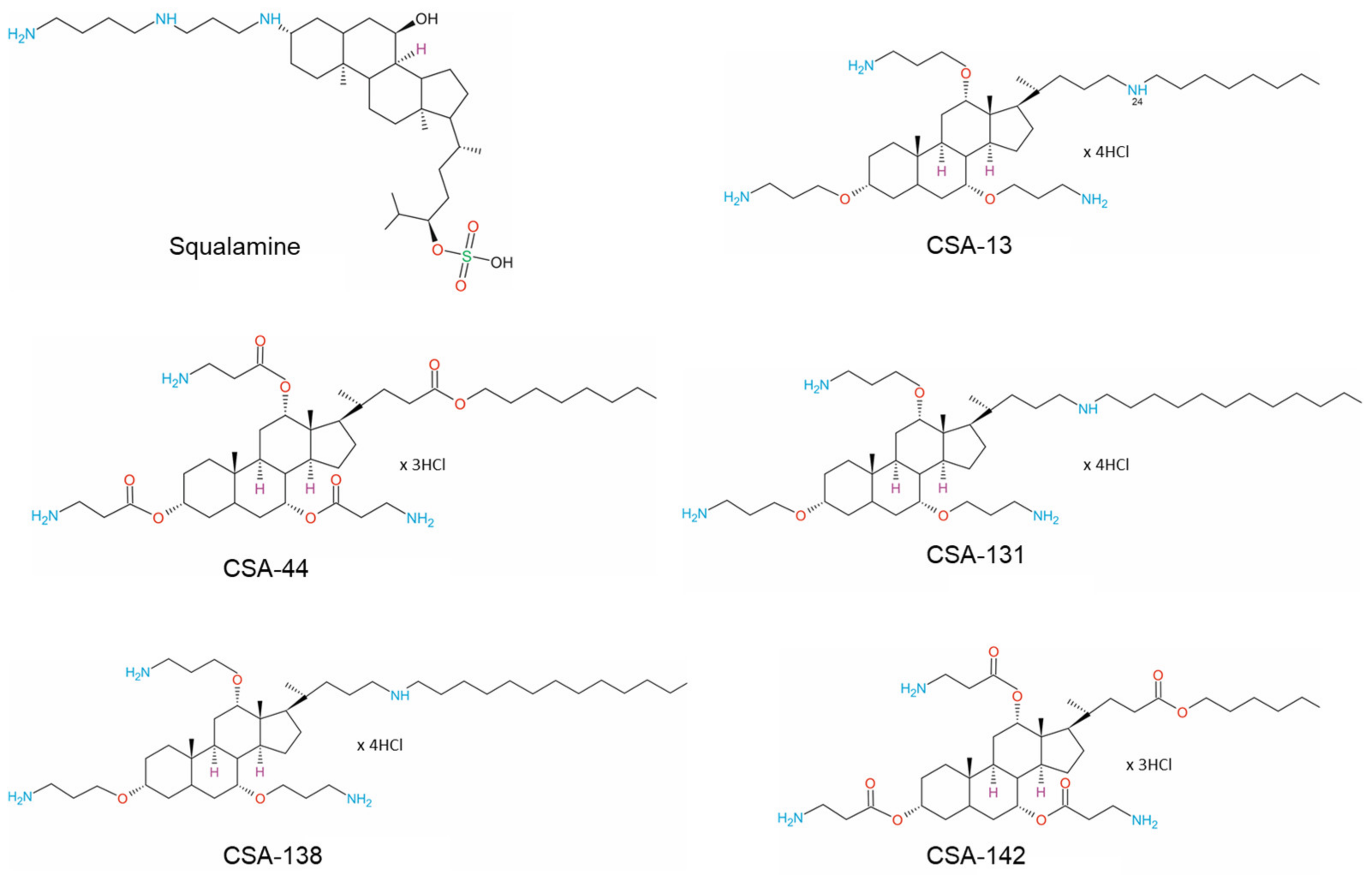
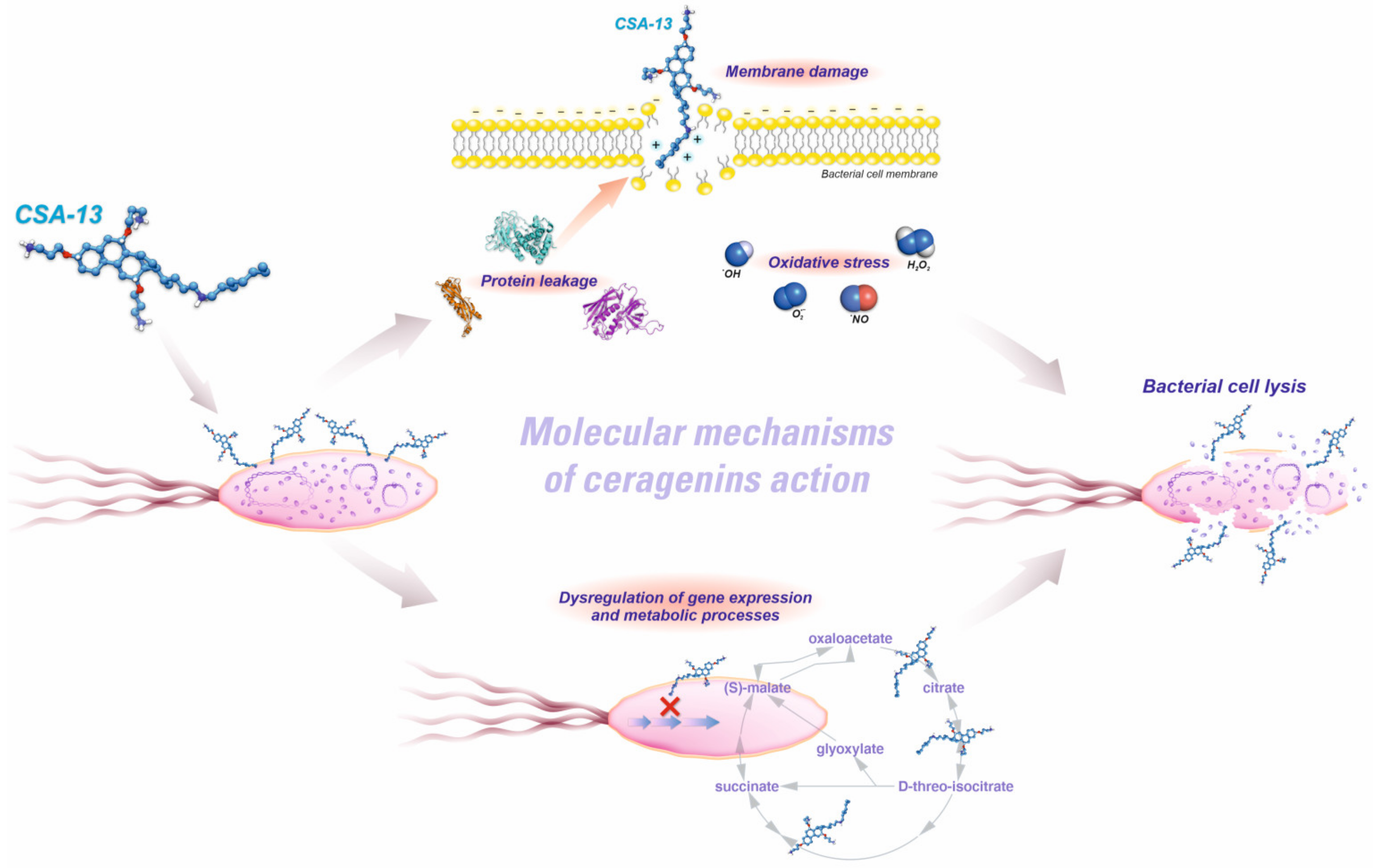
3. Physicochemical Properties of Ceragenins and Ceragenin-Based Nanosystems That Defined Their Antibacterial Properties and Specified Bacterial Targeting
4. Activity of Ceragenins and Ceragenin-Based Nanosystems against Multi-Drug Resistance Gram-Negative Rods
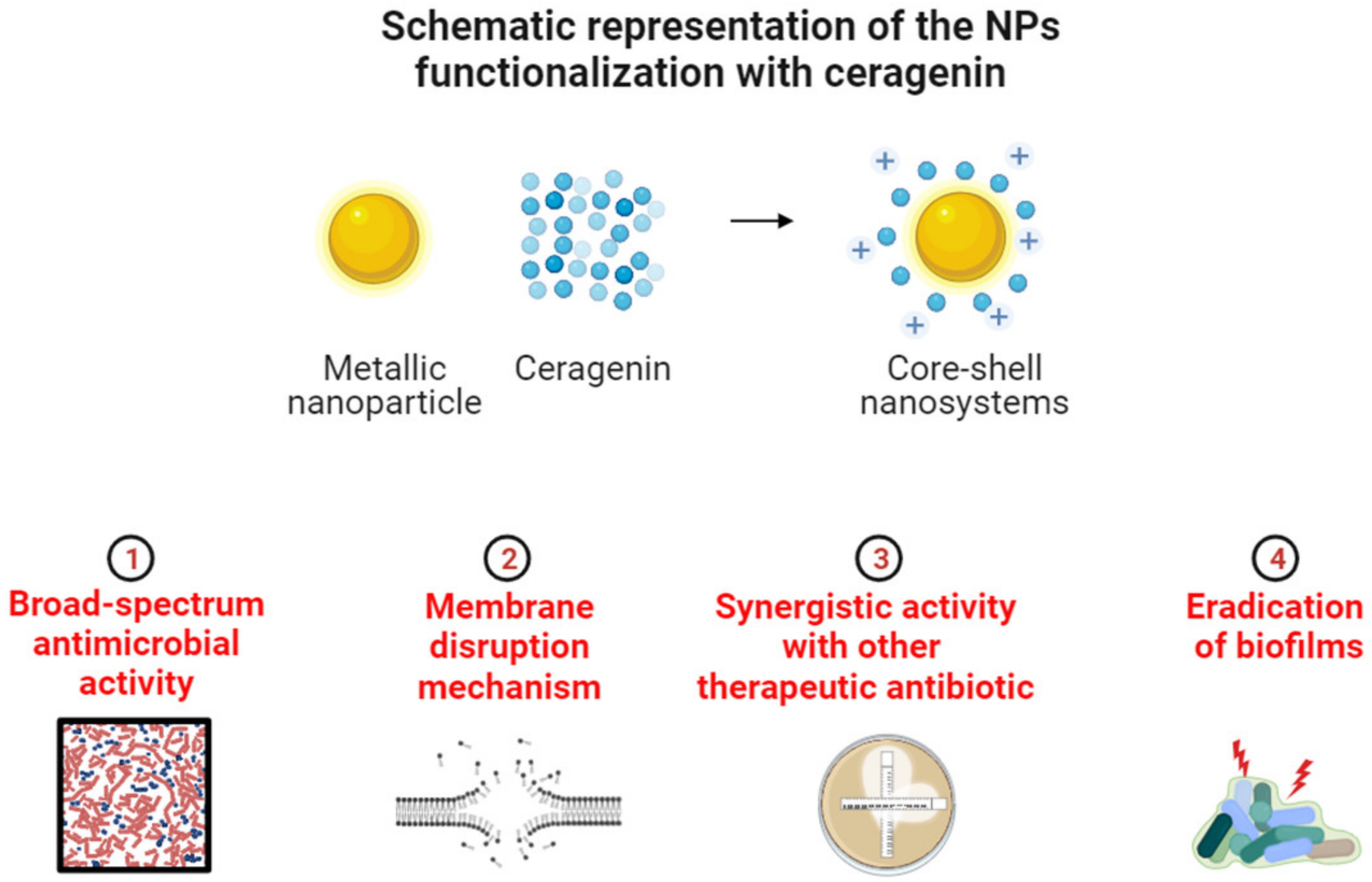
4.1. Ceragenins as Potent Antimicrobials with a Broad-Spectrum of Activity
4.2. Enhancing Antibiotic Potency: Unveiling the Synergistic Activity of Ceragenins with Conventional Antibiotics
4.3. Demonstrating the Proven Anti-Biofilm Efficacy of Ceragenin for Clinical Application
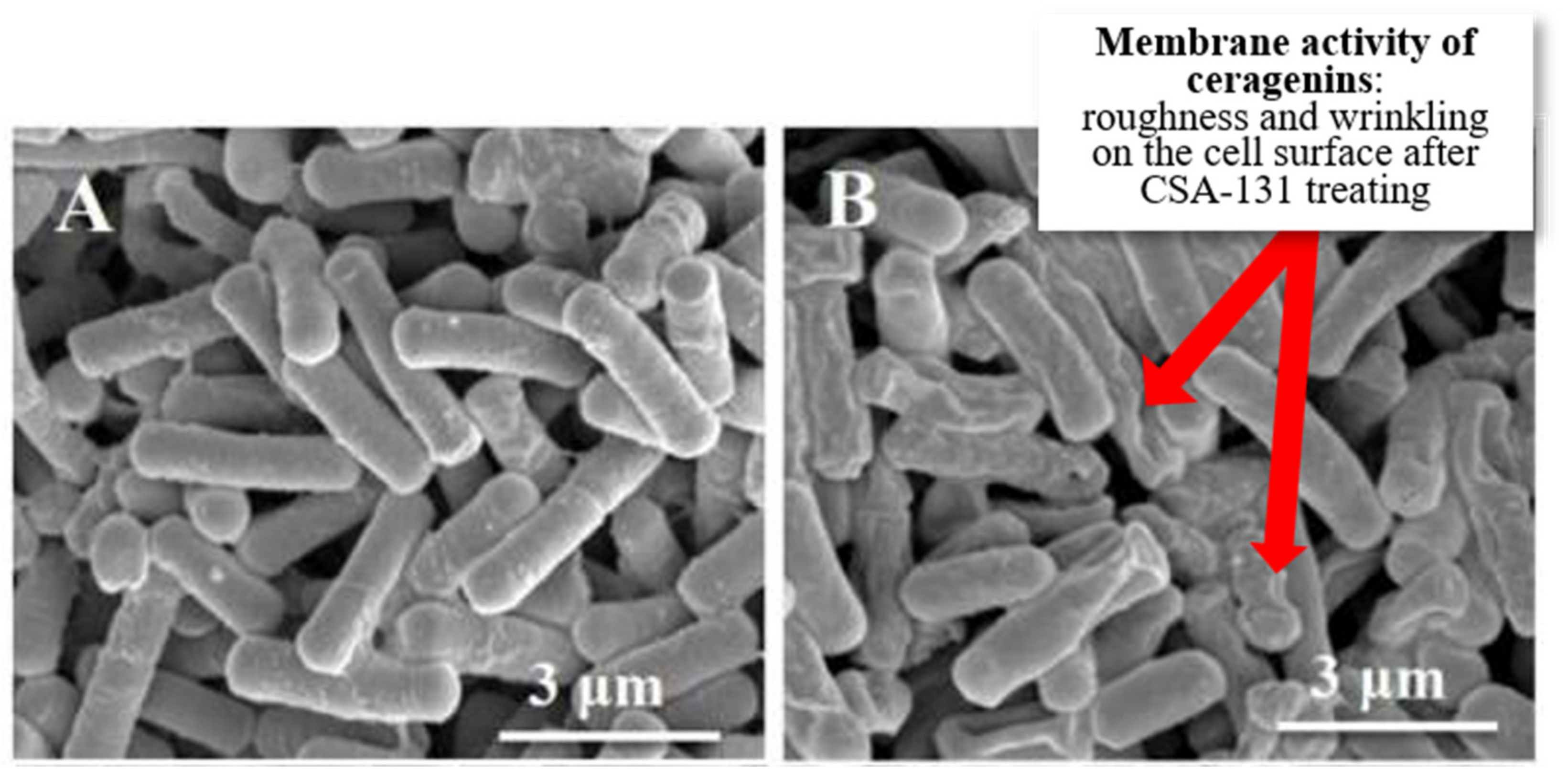
4.4. Investigating Morphological Alterations in Bacterial Cells following Exposure to Ceragenins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- World Health Organization. Health Care Associated Infections FactSheet; World Health Organization: Geneva, Switzerland, 2015; Volume 4. [Google Scholar]
- European Centre for Disease Prevention and Control; Suetens, C.; Hopkins, S.; Kolman, J.; Högberg, L.D. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals: 2011–2012; ECDC: Stockholm, Sweden, 2013. [Google Scholar]
- Koch, A.M.; Nilsen, R.M.; Eriksen, H.M.; Cox, R.J.; Harthug, S. Mortality related to hospital-associated infections in a tertiary hospital; repeated cross-sectional studies between 2004–2011. Antimicrob. Resist. Infect. Control 2015, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, M.; Ollgren, J.; Virtanen, M.J.; Lyytikäinen, O. Risk factors for death in a cohort of patients with and without healthcare-associated infections in Finnish acute care hospitals. J. Hosp. Infect. 2008, 70, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Sikora, A.; Zahra, F. Nosocomial Infections; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Baier, C.; Linke, L.; Eder, M.; Schwab, F.; Chaberny, I.F.; Vonberg, R.P.; Ebadi, E. Incidence, risk factors and healthcare costs of central line-associated nosocomial bloodstream infections in hematologic and oncologic patients. PLoS ONE 2020, 15, e0227772. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Bell, T.; O’Grady, N.P. Prevention of Central Line-Associated Bloodstream Infections. Infect. Dis. Clin. N. Am. 2017, 31, 551–559. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018, 23, 1800516. [Google Scholar] [CrossRef]
- Letica-Kriegel, A.S.; Salmasian, H.; Vawdrey, D.K.; Youngerman, B.E.; Green, R.A.; Furuya, E.Y.; Calfee, D.P.; Perotte, R. Identifying the risk factors for catheter-associated urinary tract infections: A large cross-sectional study of six hospitals. BMJ Open 2019, 9, e022137. [Google Scholar] [CrossRef]
- Isikgoz Tasbakan, M.; Durusoy, R.; Pullukcu, H.; Sipahi, O.R.; Ulusoy, S. Hospital-acquired urinary tract infection point prevalence in Turkey: Differences in risk factors among patient groups. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 31. [Google Scholar] [CrossRef]
- Mioton, L.M.; Jordan, S.W.; Hanwright, P.J.; Bilimoria, K.Y.; Kim, J.Y. The Relationship between Preoperative Wound Classification and Postoperative Infection: A Multi-Institutional Analysis of 15,289 Patients. Arch. Plast. Surg. 2013, 40, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Bruce, J.; Carpenter, J.; Wilson, A.P.; Wilson, J.; Pearson, A.; Lamping, D.L.; Krukowski, Z.H.; Reeves, B.C. Identification of risk factors by systematic review and development of risk-adjusted models for surgical site infection. Health Technol. Assess. 2011, 15, 1–156. [Google Scholar] [CrossRef]
- Klompas, M.; Branson, R.; Eichenwald, E.C.; Greene, L.R.; Howell, M.D.; Lee, G.; Magill, S.S.; Maragakis, L.L.; Priebe, G.P.; Speck, K.; et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 2014, 35 (Suppl. 2), S133–S154. [Google Scholar] [CrossRef]
- Komiya, K.; Ishii, H.; Kadota, J. Healthcare-associated Pneumonia and Aspiration Pneumonia. Aging Dis. 2015, 6, 27–37. [Google Scholar] [CrossRef]
- Kózka, M.; Sega, A.; Wojnar-Gruszka, K.; Tarnawska, A.; Gniadek, A. Risk Factors of Pneumonia Associated with Mechanical Ventilation. Int. J. Environ. Res. Public Health 2020, 17, 656. [Google Scholar] [CrossRef]
- Kumar, S.T.; Yassin, A.; Bhowmick, T.; Dixit, D. Recommendations From the 2016 Guidelines for the Management of Adults with Hospital-Acquired or Ventilator-Associated Pneumonia. Pharm. Ther. Peer-Rev. J. Formul. Manag. 2017, 42, 767–772. [Google Scholar]
- Modi, A.R.; Kovacs, C.S. Hospital-acquired and ventilator-associated pneumonia: Diagnosis, management, and prevention. Clevel. Clin. J. Med. 2020, 87, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Jaruratanasirikul, S.; Nitchot, W.; Wongpoowarak, W.; Samaeng, M.; Nawakitrangsan, M. Population pharmacokinetics and Monte Carlo simulations of sulbactam to optimize dosage regimens in patients with ventilator-associated pneumonia caused by Acinetobacter baumannii. Eur. J. Pharm. Sci. 2019, 136, 104940. [Google Scholar] [CrossRef]
- Roberts, K.; Smith, C.F.; Snelling, A.M.; Kerr, K.G.; Banfield, K.R.; Sleigh, P.A.; Beggs, C.B. Aerial dissemination of Clostridium difficile spores. BMC Infect. Dis. 2008, 8, 7. [Google Scholar] [CrossRef]
- Loo, V.G.; Bourgault, A.M.; Poirier, L.; Lamothe, F.; Michaud, S.; Turgeon, N.; Toye, B.; Beaudoin, A.; Frost, E.H.; Gilca, R.; et al. Host and pathogen factors for Clostridium difficile infection and colonization. N. Engl. J. Med. 2011, 365, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Mehrad, B.; Clark, N.M.; Zhanel, G.G.; Lynch, J.P., 3rd. Antimicrobial resistance in hospital-acquired gram-negative bacterial infections. Chest 2015, 147, 1413–1421. [Google Scholar] [CrossRef]
- Zhao, S.; Adamiak, J.W.; Bonifay, V.; Mehla, J.; Zgurskaya, H.I.; Tan, D.S. Defining new chemical space for drug penetration into Gram-negative bacteria. Nat. Chem. Biol. 2020, 16, 1293–1302. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance; Welcome Collection: London, UK, 2014. [Google Scholar]
- World Health Organization. Global action plan on antimicrobial resistance. In Antimicrobial Resistance Division, National Action Plans and Monitoring and Evaluation; World Health Organization: Geneva, Switzerland, 2015; ISBN 9789241509763. [Google Scholar]
- Eichenberger, E.M.; Thaden, J.T. Epidemiology and mechanisms of resistance of extensively drug resistant Gram-negative bacteria. Antibiotics 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Musser, J.M.; Beres, S.B.; Zhu, L.; Olsen, R.J.; Vuopio, J.; Hyyryläinen, H.-L.; Gröndahl-Yli-Hannuksela, K.; Kristinsson, K.G.; Darenberg, J.; Henriques-Normark, B. Reduced in vitro susceptibility of Streptococcus pyogenes to β-lactam antibiotics associated with mutations in the pbp2x gene is geographically widespread. J. Clin. Microbiol. 2020, 58, e01993-19. [Google Scholar] [CrossRef]
- Lopatkin, A.J.; Bening, S.C.; Manson, A.L.; Stokes, J.M.; Kohanski, M.A.; Badran, A.H.; Earl, A.M.; Cheney, N.J.; Yang, J.H.; Collins, J.J. Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science 2021, 371, eaba0862. [Google Scholar] [CrossRef]
- Von Wintersdorff, C.J.; Penders, J.; Van Niekerk, J.M.; Mills, N.D.; Majumder, S.; Van Alphen, L.B.; Savelkoul, P.H.; Wolffs, P.F. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Horizontal gene transfer mediated bacterial antibiotic resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef]
- Ma, Y.X.; Wang, C.Y.; Li, Y.Y.; Li, J.; Wan, Q.Q.; Chen, J.H.; Tay, F.R.; Niu, L.N. Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv. Sci. 2020, 7, 1901872. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control. Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.K. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control. Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Wesseling, C.M.J.; Martin, N.I. Synergy by Perturbing the Gram-Negative Outer Membrane: Opening the Door for Gram-Positive Specific Antibiotics. ACS Infect. Dis. 2022, 8, 1731–1757. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.K.; Seiple, I.B.; Cirz, R.T.; Rosenberg, O.S. Leaks in the Pipeline: A Failure Analysis of Gram-Negative Antibiotic Development from 2010 to 2020. Antimicrob. Agents Chemother. 2022, 66, e0005422. [Google Scholar] [CrossRef]
- Butler, M.S.; Henderson, I.R.; Capon, R.J.; Blaskovich, M.A.T. Antibiotics in the clinical pipeline as of December 2022. J. Antibiot. 2023, 76, 431–473. [Google Scholar] [CrossRef]
- Yusuf, E.; Bax, H.I.; Verkaik, N.J.; van Westreenen, M. An Update on Eight “New” Antibiotics against Multidrug-Resistant Gram-Negative Bacteria. J. Clin. Med. 2021, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Ontong, J.C.; Ozioma, N.F.; Voravuthikunchai, S.P.; Chusri, S. Synergistic antibacterial effects of colistin in combination with aminoglycoside, carbapenems, cephalosporins, fluoroquinolones, tetracyclines, fosfomycin, and piperacillin on multidrug resistant Klebsiella pneumoniae isolates. PLoS ONE 2021, 16, e0244673. [Google Scholar] [CrossRef]
- Jones, F.; Hu, Y.; Coates, A. The Efficacy of Using Combination Therapy against Multi-Drug and Extensively Drug-Resistant Pseudomonas aeruginosa in Clinical Settings. Antibiotics 2022, 11, 323. [Google Scholar] [CrossRef]
- Ling, H.; Lou, X.; Luo, Q.; He, Z.; Sun, M.; Sun, J. Recent advances in bacteriophage-based therapeutics: Insight into the post-antibiotic era. Acta Pharm. Sin. B 2022, 12, 4348–4364. [Google Scholar] [CrossRef]
- Shlaes, D.M. Innovation, nontraditional antibacterial drugs, and clinical utility. ACS Infect. Dis. 2021, 7, 2027–2028. [Google Scholar] [CrossRef]
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E. Analysis of the clinical pipeline of treatments for drug-resistant bacterial infections: Despite progress, more action is needed. Antimicrob. Agents Chemother. 2022, 66, e01991-21. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Villedieu, A.; Bagdasarian, N.; Karah, N.; Teare, L.; Elamin, W.F. Control and management of multidrug resistant Acinetobacter baumannii: A review of the evidence and proposal of novel approaches. Infect. Prev. Pract. 2020, 2, 100077. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Spencer, J.J.; Pitts, R.E.; Pearson, R.A.; King, L.B. The effects of antimicrobial peptides WAM-1 and LL-37 on multidrug-resistant Acinetobacter baumannii. Pathog. Dis. 2018, 76, fty007. [Google Scholar] [CrossRef]
- Kazakova, O.; Giniyatullina, G.; Babkov, D.; Wimmer, Z. From Marine Metabolites to the Drugs of the Future: Squalamine, Trodusquemine, Their Steroid and Triterpene Analogues. Int. J. Mol. Sci. 2022, 23, 1075. [Google Scholar] [CrossRef] [PubMed]
- Dao, A.; Mills, R.J.; Kamble, S.; Savage, P.B.; Little, D.G.; Schindeler, A. The application of ceragenins to orthopedic surgery and medicine. J. Orthop. Res. 2020, 38, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt-Guzel, C.; Hacioglu, M.; Savage, P.B. Investigation of the in vitro antifungal and antibiofilm activities of ceragenins CSA-8, CSA-13, CSA-44, CSA-131, and CSA-138 against Candida species. Diagn. Microbiol. Infect. Dis. 2018, 91, 324–330. [Google Scholar] [CrossRef]
- Hacioglu, M.; Haciosmanoglu, E.; Birteksoz-Tan, A.S.; Bozkurt-Guzel, C.; Savage, P.B. Effects of ceragenins and conventional antimicrobials on Candida albicans and Staphylococcus aureus mono and multispecies biofilms. Diagn. Microbiol. Infect. Dis. 2019, 95, 114863. [Google Scholar] [CrossRef] [PubMed]
- Durnaś, B.; Fiedoruk, K.; Cieśluk, M.; Deptuła, P.; Król, G.; Piktel, E.; Savage, P.B.; Bucki, R. Lysozyme increases bactericidal activity of ceragenin CSA-13 against Bacillus subtilis. Med. Stud./Stud. Med. 2019, 35, 1–9. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Holden, B.S.; Savage, P.B. Ceragenins as non-peptide mimics of endogenous antimicrobial peptides. Fight. Antimicrob. Resist. 2018, 1, 139–169. [Google Scholar]
- Mitchell, G.; Silvis, M.R.; Talkington, K.C.; Budzik, J.M.; Dodd, C.E.; Paluba, J.M.; Oki, E.A.; Trotta, K.L.; Licht, D.J.; Jimenez-Morales, D. Ceragenins and antimicrobial peptides kill bacteria through distinct mechanisms. Mbio 2022, 13, e02726-21. [Google Scholar] [CrossRef]
- Durnaś, B.; Wnorowska, U.; Pogoda, K.; Deptuła, P.; Wątek, M.; Piktel, E.; Głuszek, S.; Gu, X.; Savage, P.B.; Niemirowicz, K.; et al. Candidacidal Activity of Selected Ceragenins and Human Cathelicidin LL-37 in Experimental Settings Mimicking Infection Sites. PLoS ONE 2016, 11, e0157242. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska, S.J.; Depciuch, J.; Deptuła, P.; Piktel, E.; Daniluk, T.; Zakrzewska, M.; Czarnowski, M.; Cieśluk, M.; Durnaś, B.; et al. Ceragenin-Coated Non-Spherical Gold Nanoparticles as Novel Candidacidal Agents. Pharmaceutics 2021, 13, 1940. [Google Scholar] [CrossRef]
- Li, Y. Design and Synthesis of Ceragenins-Cationic Steroid Antimicrobial Compounds, Structural Improvement and Synthesis of Cyclopentenone Prostaglandins and Modification and Synthesis of Derivatives of Ribityllumazines: Potential Antigens for Activation of MAIT Cells; Brigham Young University: Provo, UT, USA, 2019. [Google Scholar]
- Hashemi, M.; Holden, B.; Durnas, B.; Bucki, R.; Savage, P. Ceragenins as mimics of endogenous antimicrobial peptides. J. Antimicrob. Agents 2017, 3, 1000141. [Google Scholar] [CrossRef]
- Leszczynska, K.; Namiot, D.; Byfield, F.J.; Cruz, K.; Zendzian-Piotrowska, M.; Fein, D.E.; Savage, P.B.; Diamond, S.; McCulloch, C.A.; Janmey, P.A.; et al. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J. Antimicrob. Chemother. 2013, 68, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Sostarecz, A.G.; Byfield, F.J.; Savage, P.B.; Janmey, P.A. Resistance of the antibacterial agent ceragenin CSA-13 to inactivation by DNA or F-actin and its activity in cystic fibrosis sputum. J. Antimicrob. Chemother. 2007, 60, 535–545. [Google Scholar] [CrossRef]
- Li, C.; Budge, L.P.; Driscoll, C.D.; Willardson, B.M.; Allman, G.W.; Savage, P.B. Incremental conversion of outer-membrane permeabilizers into potent antibiotics for Gram-negative bacteria. J. Am. Chem. Soc. 1999, 121, 931–940. [Google Scholar] [CrossRef]
- Ozbek-Celik, B.; Damar-Celik, D.; Mataraci-Kara, E.; Bozkurt-Guzel, C.; Savage, P.B. Comparative In Vitro Activities of First and Second-Generation Ceragenins Alone and in Combination with Antibiotics Against Multidrug-Resistant Klebsiella pneumoniae Strains. Antibiotics 2019, 8, 130. [Google Scholar] [CrossRef]
- Chmielewska, S.J.; Skłodowski, K.; Piktel, E.; Suprewicz, Ł.; Fiedoruk, K.; Daniluk, T.; Wolak, P.; Savage, P.B.; Bucki, R. NDM-1 Carbapenemase-Producing Enterobacteriaceae are Highly Susceptible to Ceragenins CSA-13, CSA-44, and CSA-131. Infect. Drug Resist. 2020, 13, 3277–3294. [Google Scholar] [CrossRef]
- Paprocka, P.; Mańkowska, A.; Skłodowski, K.; Król, G.; Wollny, T.; Lesiak, A.; Głuszek, K.; Savage, P.B.; Durnaś, B.; Bucki, R. Bactericidal activity of ceragenin in combination with ceftazidime, levofloxacin, co-trimoxazole, and colistin against the opportunistic pathogen Stenotrophomonas maltophilia. Pathogens 2022, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt Güzel, Ç.; Avci, N.M.; Savage, P. In Vitro Activities of the Cationic Steroid Antibiotics CSA-13, CSA-131, CSA-138, CSA-142, and CSA-192 Against Carbapenem-resistant Pseudomonas aeruginosa. Turk. J. Pharm. Sci. 2020, 17, 63–67. [Google Scholar] [CrossRef]
- Wnorowska, U.; Piktel, E.; Deptuła, P.; Wollny, T.; Król, G.; Głuszek, K.; Durnaś, B.; Pogoda, K.; Savage, P.B.; Bucki, R. Ceragenin CSA-13 displays high antibacterial efficiency in a mouse model of urinary tract infection. Sci. Rep. 2022, 12, 19164. [Google Scholar] [CrossRef]
- Bucki, R.; Niemirowicz, K.; Wnorowska, U.; Byfield, F.J.; Piktel, E.; Wątek, M.; Janmey, P.A.; Savage, P.B. Bactericidal Activity of Ceragenin CSA-13 in Cell Culture and in an Animal Model of Peritoneal Infection. Antimicrob. Agents Chemother. 2015, 59, 6274–6282. [Google Scholar] [CrossRef]
- Piktel, E.; Oscilowska, I.; Suprewicz, Ł.; Depciuch, J.; Marcińczyk, N.; Chabielska, E.; Wolak, P.; Głuszek, K.; Klimek, J.; Zieliński, P.M.; et al. Peanut-Shaped Gold Nanoparticles with Shells of Ceragenin CSA-131 Display the Ability to Inhibit Ovarian Cancer Growth In Vitro and in a Tumor Xenograft Model. Cancers 2021, 13, 5424. [Google Scholar] [CrossRef]
- Fraimow, H.S.; Tsigrelis, C. Antimicrobial resistance in the intensive care unit: Mechanisms, epidemiology, and management of specific resistant pathogens. Crit. Care Clin. 2011, 27, 163–205. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Rovig, J.; Weber, S.; Hilton, B.; Forouzan, M.M.; Savage, P.B. Susceptibility of colistin-resistant, Gram-negative bacteria to antimicrobial peptides and ceragenins. Antimicrob. Agents Chemother. 2017, 61, e00292-17. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Komarow, L.; Chen, L.; Ge, L.; Hanson, B.M.; Cober, E.; Herc, E.; Alenazi, T.; Kaye, K.S.; Garcia-Diaz, J. Global epidemiology and clinical outcomes of carbapenem-resistant Pseudomonas aeruginosa and associated carbapenemases (POP): A prospective cohort study. Lancet Microbe 2023, 4, e159–e170. [Google Scholar] [CrossRef]
- Yacouba, A.; Olowo-Okere, A. Global trends and current status in colistin resistance research: A bibliometric analysis (1973–2019). F1000Research 2020, 9, 856. [Google Scholar] [CrossRef]
- Giani, T.; Arena, F.; Pollini, S.; Di Pilato, V.; D’Andrea, M.M.; Henrici De Angelis, L.; Bassetti, M.; Rossolini, G.M. Italian nationwide survey on Pseudomonas aeruginosa from invasive infections: Activity of ceftolozane/tazobactam and comparators, and molecular epidemiology of carbapenemase producers. J. Antimicrob. Chemother. 2018, 73, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, P.; Durnaś, B.; Mańkowska, A.; Skłodowski, K.; Król, G.; Zakrzewska, M.; Czarnowski, M.; Kot, P.; Fortunka, K.; Góźdź, S.; et al. New β-Lactam Antibiotics and Ceragenins—A Study to Assess Their Potential in Treatment of Infections Caused by Multidrug-Resistant Strains of Pseudomonas aeruginosa. Infect. Drug Resist. 2021, 14, 5681–5698. [Google Scholar] [CrossRef] [PubMed]
- Vila-Farrés, X.; Callarisa, A.E.; Gu, X.; Savage, P.B.; Giralt, E.; Vila, J. CSA-131, a ceragenin active against colistin-resistant Acinetobacter baumannii and Pseudomonas aeruginosa clinical isolates. Int. J. Antimicrob. Agents 2015, 46, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt-Guzel, C.; Inci, G.; Oyardi, O.; Savage, P.B. Synergistic Activity of Ceragenins Against Carbapenem-Resistant Acinetobacter baumannii Strains in Both Checkerboard and Dynamic Time-Kill Assays. Curr. Microbiol. 2020, 77, 1419–1428. [Google Scholar] [CrossRef]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Y.; Yang, J.; Liu, Y.; Hu, F.; Zhu, K.; Jiang, X. Gold Nanoclusters for targeting methicillin-resistant staphylococcus aureus in vivo. Angew. Chem. Int. Ed. 2018, 57, 3958–3962. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, W.; Qin, Z.; Chen, Y.; Jiang, H.; Wang, X. Mercaptopyrimidine-conjugated gold nanoclusters as nanoantibiotics for combating multidrug-resistant superbugs. Bioconjug. Chem. 2018, 29, 3094–3103. [Google Scholar] [CrossRef]
- Chmielewska, S.J.; Skłodowski, K.; Depciuch, J.; Deptuła, P.; Piktel, E.; Fiedoruk, K.; Kot, P.; Paprocka, P.; Fortunka, K.; Wollny, T.; et al. Bactericidal Properties of Rod-, Peanut-, and Star-Shaped Gold Nanoparticles Coated with Ceragenin CSA-131 against Multidrug-Resistant Bacterial Strains. Pharmaceutics 2021, 13, 425. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Cieśluk, M.; Chmielewska, S.; Durnaś, B.; Król, G.; Wollny, T.; Deptuła, P.; Kochanowicz, J. Rod-shaped gold nanoparticles exert potent candidacidal activity and decrease the adhesion of fungal cells. Nanomedicine 2020, 15, 2733–2752. [Google Scholar] [CrossRef]
- Piktel, E.; Ościłowska, I.; Suprewicz, Ł.; Depciuch, J.; Marcińczyk, N.; Chabielska, E.; Wolak, P.; Wollny, T.; Janion, M.; Parlinska-Wojtan, M. ROS-mediated apoptosis and autophagy in ovarian cancer cells treated with peanut-shaped gold nanoparticles. Int. J. Nanomed. 2021, 16, 1993–2011. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Surel, U.; Wilczewska, A.Z.; Mystkowska, J.; Piktel, E.; Gu, X.; Namiot, Z.; Kułakowska, A.; Savage, P.B.; Bucki, R. Bactericidal activity and biocompatibility of ceragenin-coated magnetic nanoparticles. J. Nanobiotechnol. 2015, 13, 32. [Google Scholar] [CrossRef]
- Piktel, E.; Prokop, I.; Wnorowska, U.; Król, G.; Cieśluk, M.; Niemirowicz, K.; Savage, P.B.; Bucki, R. Ceragenin CSA-13 as free molecules and attached to magnetic nanoparticle surfaces induce caspase-dependent apoptosis in human breast cancer cells via disruption of cell oxidative balance. Oncotarget 2018, 9, 21904. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Prokop, I.; Wilczewska, A.Z.; Wnorowska, U.; Piktel, E.; Wątek, M.; Savage, P.B.; Bucki, R. Magnetic nanoparticles enhance the anticancer activity of cathelicidin LL-37 peptide against colon cancer cells. Int. J. Nanomed. 2015, 10, 3843–3853. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.N.; Jones, R.N.; Sader, H.S.; Savage, P.B.; Rybak, M.J. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J. Antimicrob. Chemother. 2008, 61, 365–370. [Google Scholar] [CrossRef]
- Bozkurt-Guzel, C.; Savage, P.B.; Gerceker, A.A. In vitro Activities of the Novel Ceragenin CSA-13, Alone or in Combination with Colistin, Tobramycin, and Ciprofloxacin, against Pseudomonas aeruginosa Strains Isolated from Cystic Fibrosis Patients. Chemotherapy 2012, 57, 505–510. [Google Scholar] [CrossRef]
- Bozkurt-Guzel, C.; Savage, P.B.; Akcali, A.; Ozbek-Celik, B. Potential Synergy Activity of the Novel Ceragenin, CSA-13, against Carbapenem-Resistant Acinetobacter baumannii Strains Isolated from Bacteremia Patients. BioMed Res. Int. 2014, 2014, 710273. [Google Scholar] [CrossRef] [PubMed]
- Bamford, N.C.; MacPhee, C.E.; Stanley-Wall, N.R. Microbial Primer: An introduction to biofilms–what they are, why they form and their impact on built and natural environments. Microbiology 2023, 169, 001338. [Google Scholar] [CrossRef]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019, 8, 76. [Google Scholar] [CrossRef]
- Vuotto, C.; Longo, F.; Balice, M.P.; Donelli, G.; Varaldo, P.E. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens 2014, 3, 743–758. [Google Scholar] [CrossRef]
- Shadkam, S.; Goli, H.R.; Mirzaei, B.; Gholami, M.; Ahanjan, M. Correlation between antimicrobial resistance and biofilm formation capability among Klebsiella pneumoniae strains isolated from hospitalized patients in Iran. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 13. [Google Scholar] [CrossRef]
- Dutt, Y.; Dhiman, R.; Singh, T.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Raj, V.S.; Chang, C.M.; Priyadarshini, A. The Association between Biofilm Formation and Antimicrobial Resistance with Possible Ingenious Bio-Remedial Approaches. Antibiotics 2022, 11, 930. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, D.; Singh, A.; Sunita, K. Colistin Resistance and Management of Drug Resistant Infections. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 4315030. [Google Scholar] [CrossRef]
- Hacioglu, M.; Oyardi, O.; Bozkurt-Guzel, C.; Savage, P.B. Antibiofilm activities of ceragenins and antimicrobial peptides against fungal-bacterial mono and multispecies biofilms. J. Antibiot. 2020, 73, 455–462. [Google Scholar] [CrossRef]
- Nagant, C.; Pitts, B.; Stewart, P.S.; Feng, Y.; Savage, P.B.; Dehaye, J.P. Study of the effect of antimicrobial peptide mimic, CSA-13, on an established biofilm formed by P seudomonas aeruginosa. Microbiologyopen 2013, 2, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Jennings, J.D.; Snarr, J.; Chaudhary, V.; Pollard, J.E.; Savage, P.B. Optimization of ceragenins for prevention of bacterial colonization of hydrogel contact lenses. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6217–6223. [Google Scholar] [CrossRef] [PubMed]
- Nagant, C.; Feng, Y.; Lucas, B.; Braeckmans, K.; Savage, P.; Dehaye, J.-P. Effect of a low concentration of a cationic steroid antibiotic (CSA-13) on the formation of a biofilm by Pseudomonas aeruginosa. J. Appl. Microbiol. 2011, 111, 763–772. [Google Scholar] [CrossRef]
- Whiteley, M.; Bangera, M.G.; Bumgarner, R.E.; Parsek, M.R.; Teitzel, G.M.; Lory, S.; Greenberg, E. Gene expression in Pseudomonas aeruginosa biofilms. Nature 2001, 413, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Wnorowska, U.; Niemirowicz, K.; Myint, M.; Diamond, S.L.; Wróblewska, M.; Savage, P.B.; Janmey, P.A.; Bucki, R. Bactericidal activities of cathelicidin LL-37 and select cationic lipids against the hypervirulent Pseudomonas aeruginosa strain LESB58. Antimicrob. Agents Chemother. 2015, 59, 3808–3815. [Google Scholar] [CrossRef]
- Wnorowska, U.; Fiedoruk, K.; Piktel, E.; Prasad, S.V.; Sulik, M.; Janion, M.; Daniluk, T.; Savage, P.B.; Bucki, R. Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: Current status and potential future applications. J. Nanobiotechnol. 2020, 18, 3. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Piktel, E.; Wilczewska, A.Z.; Markiewicz, K.H.; Durnaś, B.; Wątek, M.; Puszkarz, I.; Wróblewska, M.; Niklińska, W.; Savage, P.B. Core–shell magnetic nanoparticles display synergistic antibacterial effects against Pseudomonas aeruginosa and Staphylococcus aureus when combined with cathelicidin LL-37 or selected ceragenins. Int. J. Nanomed. 2016, 11, 5443–5455. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, A.; Kaczorek, E. Nanomechanical changes in probiotic bacteria under antibiotics exposure: Implications on Lactobacillus biofilm formation. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2023, 1870, 119533. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Mmuoegbulam, A.O.; Holden, B.S.; Coburn, J.; Wilson, J.; Taylor, M.F.; Reiley, J.; Baradaran, D.; Stenquist, T.; Deng, S.; et al. Susceptibility of Multidrug-Resistant Bacteria, Isolated from Water and Plants in Nigeria, to Ceragenins. Int. J. Environ. Res. Public Health 2018, 15, 2758. [Google Scholar] [CrossRef] [PubMed]

| Type of Nosocomial Infections | Pathogens | Risk Factors | References |
|---|---|---|---|
| Central Line-Associated Blood Stream Infection (CLABSI) | Coagulase-negative Staphylococcus, Staphylococcus aureus, Enterococcus spp., Streptococcus spp., Candida spp., Acinetobacter baumannii, Escherichia coli | Chronic illness, neutropenia, malnutrition, parenteral nutrition, extremes of ages, and bone marrow transplantations, prolonged hospitalization before catheterization, prolonged time of catheterization, multi-lumen CVC, type of catheter material, multiple CVC, urgent insertion, and lack of sterile barriers or breaks in the aseptic technique | [8,9,10,11,12] |
| Catheter-Associated Urinary Tract Infection (CAUTI) | Escherichia coli, Klebsiella spp., Acinetobacter baumannii, Pseudomonas aeruginosa, Enterococcus spp., and Candida spp. | Duration of catheterization, female sex, paraplegia, cerebrovascular disease, older age, diabetes mellitus, history of UTI in the preceding year, and recent antibiotic use within 90 days | [8,10,12,13,14,15] |
| Skin and Soft Tissue Infection (SSI) | Staphylococcus aureus, Escherichia coli, Klebsiella spp., Enterobacter spp., Pseudomonas aeruginosa, Acinetobacter baumannii, Enterococcus spp. | Duration of surgery, wound class, hypothermia and hypovolemia during surgery, hypoxemia, the urgency of surgery, more than one intervention/surgery, necessity for blood transfusion, and the type of prosthesis implanted, wound class, and duration of operation due to the time that the tissue is exposed to the environment, leading to an increased chance of contamination, immunosuppression, tobacco use, obesity, hyperglycemia, malnutrition, joint disease, and increasing age | [8,10,12,13,16,17] |
| Hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) | Klebsiella pneumoniae, Enterobacter spp., Pseudomonas aeruginosa, Acinetobacter baumannii, and Staphylococcus aureus | Prior IV antibiotic use within the last 90 days, need for ventilatory support, septic shock at the time of VAP, acute respiratory distress syndrome preceding VAP, more than five days of hospitalization before VAP onset, and need for acute renal replacement therapy | [18,19,20,21,22,23] |
| Clostridioides difficile Infection (CDI) | Clostridioides difficile | Antibiotic use and environmental contamination, both of which are modifiable risk factors. Other frequently seen risk factors include increasing age, hospitalization, multiple comorbidities, the use of gastric acid-suppressing medications, and immunosuppression | [8,24,25] |
| Tested Antimicrobial Agents | Minimal Inhibitory Concentration (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| A. baumannii Colistin-Resistant n = 1 | A. baumannii Carbapenem-Resistant n = 25 | P. aeruginosa Various Mechanisms of Resistance n = 150 | P. aeruginosa Colistin-Resistant n = 1 | K. pneumoniae Colistin-Resistant n = 5 | K. pnumoniae MDR n = 50 | K. pneumoniae NDM-1 n = 1 | K. pneumoniae NDM-1 n = 1 | |
| CSA-13 | 4 | 1–16 | 0.5–8 | <0.5 | 2–6 | 0.5–32 | 2 | 2 |
| CSA-44 | 4 | 8–16 | 0.5–8 | 1 | 1–2 | 0.5–32 | 2 | 2 |
| CSA-131 | 2 | 1–8 | 0.5–4 | <0.5 | 1–3 | 0.5–16 | 2 | 2 |
| CSA-138 | 4 | 4–32 | - | 1 | 2–8 | 1–32 | - | - |
| Colistin | - | 0.125–4 | 0.125–8 | - | 16–200 | 0.03–128 | 1 | 0.5 |
| Meropenem | - | 8–128 | - | - | - | 0.5–128 | 128 | 32 |
| Reference | [79] | [80] | [78] | [79] | [74] | [66] | [67] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karasiński, M.; Wnorowska, U.; Durnaś, B.; Król, G.; Daniluk, T.; Skłodowski, K.; Głuszek, K.; Piktel, E.; Okła, S.; Bucki, R. Ceragenins and Ceragenin-Based Core-Shell Nanosystems as New Antibacterial Agents against Gram-Negative Rods Causing Nosocomial Infections. Pathogens 2023, 12, 1346. https://doi.org/10.3390/pathogens12111346
Karasiński M, Wnorowska U, Durnaś B, Król G, Daniluk T, Skłodowski K, Głuszek K, Piktel E, Okła S, Bucki R. Ceragenins and Ceragenin-Based Core-Shell Nanosystems as New Antibacterial Agents against Gram-Negative Rods Causing Nosocomial Infections. Pathogens. 2023; 12(11):1346. https://doi.org/10.3390/pathogens12111346
Chicago/Turabian StyleKarasiński, Maciej, Urszula Wnorowska, Bonita Durnaś, Grzegorz Król, Tamara Daniluk, Karol Skłodowski, Katarzyna Głuszek, Ewelina Piktel, Sławomir Okła, and Robert Bucki. 2023. "Ceragenins and Ceragenin-Based Core-Shell Nanosystems as New Antibacterial Agents against Gram-Negative Rods Causing Nosocomial Infections" Pathogens 12, no. 11: 1346. https://doi.org/10.3390/pathogens12111346






