Molecular Detection and Genetic Diversity of Tick-Borne Pathogens in Goats from the Southern Part of Thailand
Abstract
:1. Introduction
2. Results
2.1. PCR Detection of TBPs in Goat Samples
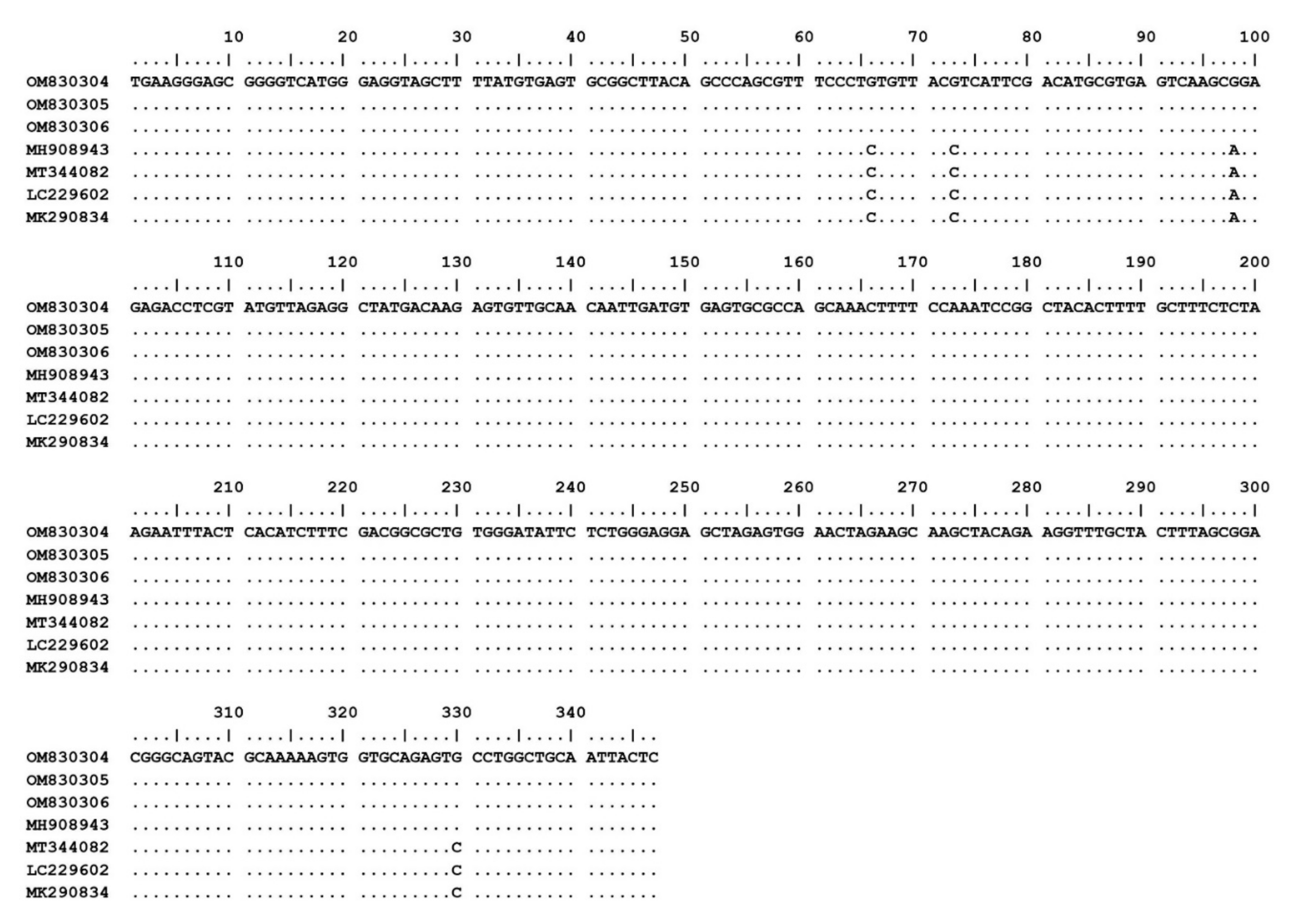
2.2. Sequencing Analysis
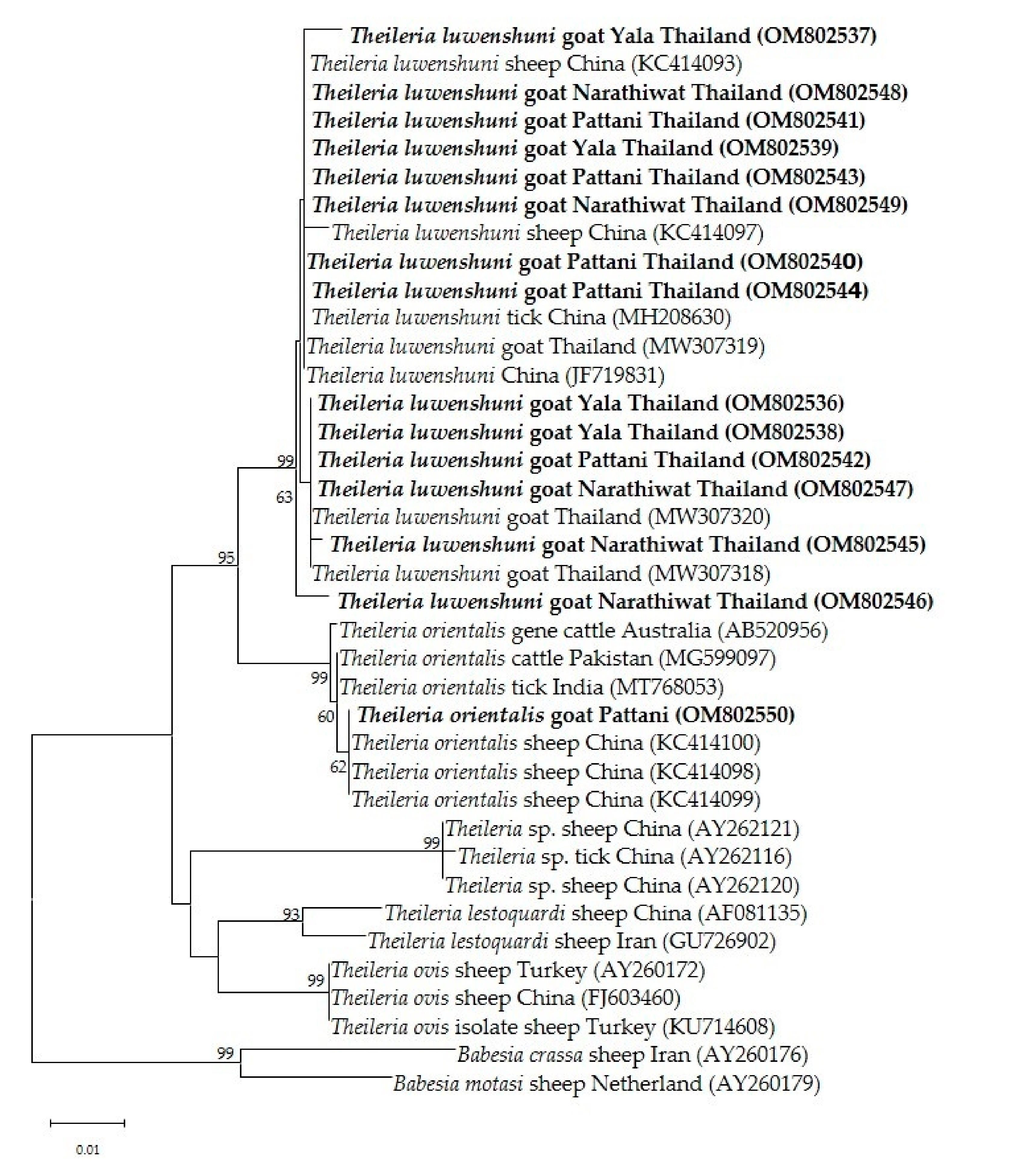
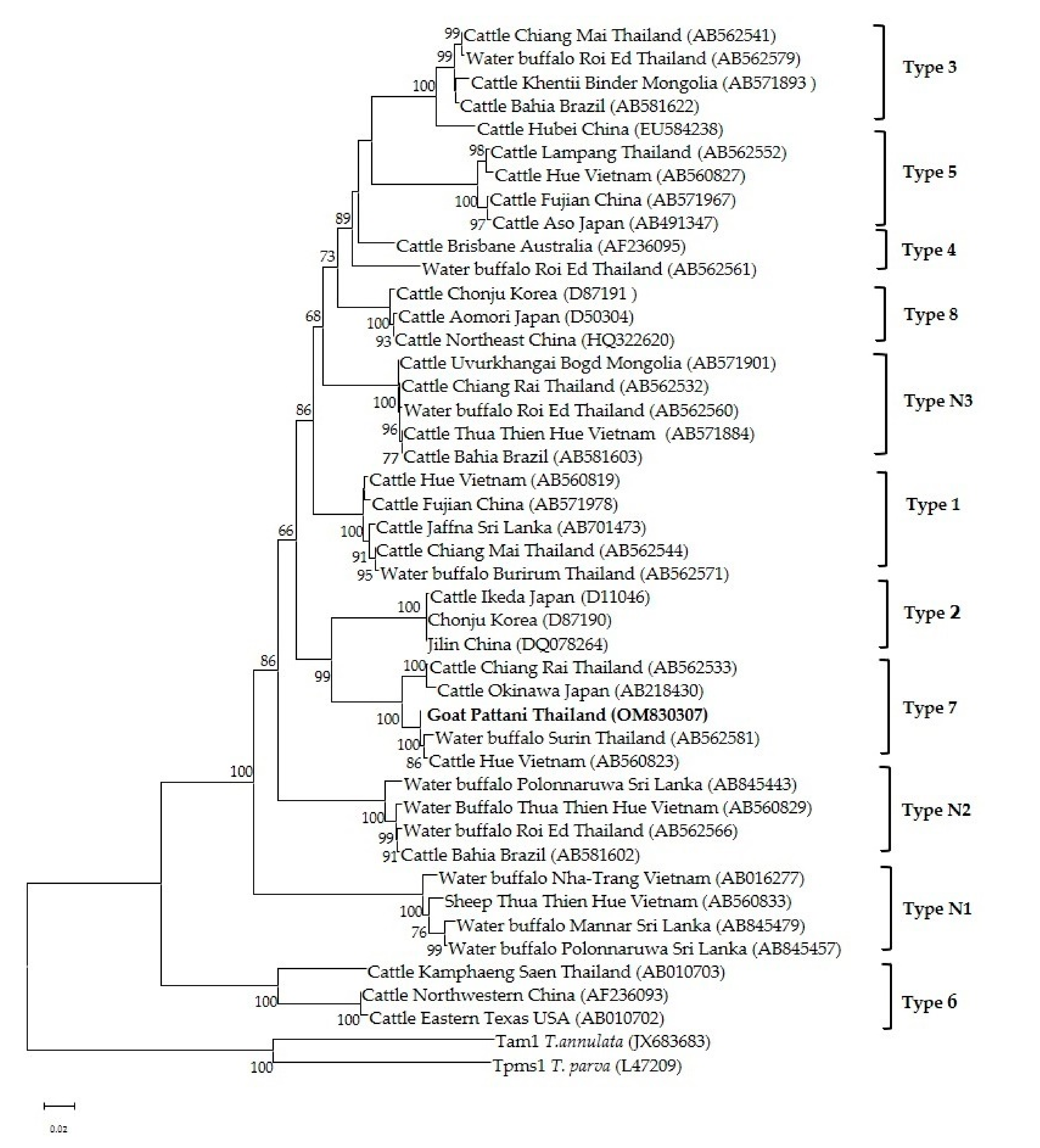
2.3. Phylogenetic Analysis
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Animal Samples and DNA Extraction
4.3. Molecular Detection of TBPs and DNA Sequencing
4.4. Phylogenetic Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dantas-Torres, F.; Chomel, D.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Alessandra, T.; Santo, C. Tick-borne diseases in sheep and goats: Clinical and diagnostic aspects. Small Rumin. Res. 2012, 106, S11–S16. [Google Scholar] [CrossRef]
- Ghafar, A.; Abbas, T.; Rehman, A.; Sandhu, Z.U.; Cabezas-Cruz, A.; Jabbar, A. Systematic review of ticks and tick-borne pathogens of small ruminants in Pakistan. Pathogens 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Luo, J.; Schnittger, L.; Lu, B.; Beyer, D.; Ma, M.; Guan, G.; Bai, Q.; Lu, C.; Ahmed, J. Phylogenetic analysis of Theileria species transmitted by Haemaphysalis qinghaiensis. Parasitol. Res. 2004, 92, 36–42. [Google Scholar] [CrossRef]
- Zaeemi, M.; Haddadzadeh, H.; Khazraiinia, P.; Kazemi, B.; Bandehpour, M. Identification of different Theileria species (Theileria lestoquardi, Theileria ovis, and Theileria annulata) in naturally infected sheep using nested PCR–RFLP. Parasitol. Res. 2010, 108, 837–843. [Google Scholar] [CrossRef]
- Bilgic, H.B.; Bakırcı, S.; Kose, O.; Unlu, A.H.; Hacılarlıoglu, S.; Eren, H.; Weir, W.; Karagenc, T. Prevalence of tick-borne haemoparasites in small ruminants in Turkey and diagnostic sensitivity of ingle-PCR and RLB. Parasites Vectors 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Altay, K.; Aktaş, M.; Dumanli, N. Theileria infections in small ruminants in the east and southeast Anatolia. Turk. Parazitol. Derg. 2007, 31, 268–271. [Google Scholar]
- Chae, J.S.; Allsopp, B.A.; Waghela, S.D.; Park, J.H.; Kakuda, T.; Sugimoto, C.; Allsopp, M.T.; Wagner, G.G.; Holman, P.J. A study of the systematics of Theileria spp. based upon small-subunit ribosomal RNA gene sequences. Parasitol. Res. 1999, 85, 877–883. [Google Scholar] [CrossRef]
- Schnittger, L.; Yin, H.; Gubbels, M.-J.; Beyer, D.; Niemann, S.; Jongejan, F.; Ahmed, J.S. Phylogeny of sheep and goat Theileria and Babesia parasites. Parasitol. Res. 2003, 91, 398–406. [Google Scholar]
- Ijaz, M.; Rehman, A.; Ali, M.M.; Umair, M.; Khalid, S.; Mehmood, K.; Hanif, A. Clinico-epidemiology and therapeutical trials on babesiosis in sheep and goats in Lahore, Pakistan. J. Anim. Plant Sci. 2013, 23, 666–669. [Google Scholar]
- Yang, J.; Li, Y.; Liu, Z.; Liu, J.; Niu, Q.; Ren, Q.; Chen, Z.; Guan, G.; Luo, J.; Yin, H. Molecular detection and characterization of Anaplasma spp. in sheep and cattle from Xinjiang, northwest China. Parasites Vectors 2015, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, A.; Rahbari, S.; Shayan, P.; Sadeghi-dehkordi, Z.; Bahonar, A. Molecular detection of Anaplasma marginale and Anaplasma ovis in sheep and goat in west highland pasture of Iran. Asian Pac. J. Trop. Biomed. 2017, 7, 455–459. [Google Scholar] [CrossRef]
- Mason, K.L.; González, M.V.; Chung, C.; Mousel, M.R.; White, S.N.; Taylor, J.B.; Scoles, G.A. Validation of an improved Anaplasma antibody competitive ELISA for detection of Anaplasma ovis antibody in domestic sheep. J. Vet. Diagn. Investig. 2017, 29, 763–766. [Google Scholar] [CrossRef] [Green Version]
- Altangerel, K.; Sivakumar, T.; Inpankaew, T.; Jittapalapong, S.; Terkawi, M.A.; Ueno, A.; Xuan, X.; Igarashi, I.; Yokoyama, N. Molecular prevalence of different genotypes of Theileria orientalis detected from cattle and water buffaloes in Thailand. J. Parasitol. 2011, 97, 1075–1079. [Google Scholar] [CrossRef]
- Jirapattharasate, C.; Moumouni, P.F.A.; Cao, S.; Iguchi, A.; Liu, M.M.; Wang, G.; Zhou, M.; Vudriko, P.; Changbunjong, T.; Sungpradit, S.; et al. Molecular epidemiology of bovine Babesia spp. and Theileria orientalis parasites in beef cattle from northern and northeastern Thailand. Parasitol. Int. 2016, 65, 62–69. [Google Scholar] [CrossRef]
- Jirapattharasate, C.; Moumouni, P.F.A.; Cao, S.; Iguchi, A.; Liu, M.M.; Wang, G.; Zhou, M.; Vudriko, P.; Efstratiou, A.; Changbunjong, T.; et al. Molecular detection and genetic diversity of bovine Babesia spp., Theileria orientalis, and Anaplasma marginale in beef cattle in Thailand. Parasitol. Res. 2017, 116, 751–762. [Google Scholar] [CrossRef]
- Saetiew, N.; Simking, P.; Inpankaew, T.; Wongpanit, K.; Kamyingkird, K.; Wongnakphet, S.; Stich, R.W.; Jittapalapong, S. Prevalence and genetic diversity of Anaplasma marginale infections in water buffaloes in Northeast Thailand. J. Trop. Med. Parasitol. 2015, 38, 9–16. [Google Scholar]
- Ros-García, A.; Barandika, J.F.; García-Pérez, A.L.; Juste, R.A.; Hurtado, A. Assessment of exposure to piroplasms in sheep grazing in communal mountain pastures by using a multiplex DNA bead-based suspension array. Parasites Vectors 2013, 6, 277. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Zhang, S.; Jia, L.; Xue, S.; Yu, L.; Kamyingkird, K.; Moumouni, P.F.A.; Moussa, A.A.; Zhou, M.; Zhang, Y.; et al. Molecular detection of Theileria species in sheep from northern China. J. Vet. Med. Sci. 2013, 75, 1227–1230. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lv, Y.; Zhang, F.; Zhang, W.; Wang, J.; Cui, Y.; Wang, R.; Jian, F.; Zhang, L.; Ning, C. Molecular and phylogenetic analysis of Anaplasma spp. in sheep and goats from six provinces of China. J. Vet. Sci. 2016, 17, 523–529. [Google Scholar] [CrossRef]
- Li, Y.; Galon, E.M.; Guo, Q.; Rizk, M.A.; Moumouni, P.F.A.; Liu, M.; Li, J.; Ji, S.; Chahan, B.; Xuan, X. Molecular detection and identification of Babesia spp., Theileria spp., and Anaplasma spp. in sheep from border regions, Northwestern China. Front. Vet. Sci. 2020, 7, 630. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-G.; Kwon, O.-D.; Kwak, D. Molecular and Phylogenetic Analysis of Tick-Borne Pathogens in Ticks Parasitizing Native Korean Goats (Capra hircus coreanae) in South Korea. Pathogens 2020, 9, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khukhuu, A.; Lan, D.T.; Long, P.T.; Ueno, A.; Li, Y.; Luo, Y.; Macedo, A.C.; Matsumoto, K.; Inokuma, H.; Kawazu, S.; et al. Molecular epidemiological survey of Theileria orientalis in Thua Thien Hue Province, Vietnam. J. Vet. Med. Sci. 2011, 73, 701–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bawm, S.; Kakisaka, K.; Thu, M.J.; Chel, H.M.; Oo, Y.M.N.; Soe, N.C.; Win, S.Y.; Htun, L.L.; Win, M.M.; Suzuki, H.; et al. First molecular detection of Theileria luwenshuni from goats in Myanmar. Parasitol. Res. 2018, 117, 3361–3364. [Google Scholar] [CrossRef]
- Yasini, S.P.; Khaki, Z.; Rahbari, S.; Kazemi, B.; Amoli, J.S.; Gharabaghi, A.; Jalali, S.M. Hematologic and clinical aspects of experimental ovine anaplasmosis caused by Anaplasma ovis in Iran. Iran. J. Parasitol. 2012, 7, 91–98. [Google Scholar]
- Kaewhom, P.; Srikijkasemwat, K. Molecular identification of Anaplasma ovis and Anaplasma marginale in sheep using PCR–RFLP method. Int. J. Agric. Technol. 2021, 17, 899–908. [Google Scholar]
- Liu, Z.; Ma, M.; Wang, Z.; Wang, J.; Peng, Y.; Li, Y.; Guan, G.; Luo, J.; Yin, H. Molecular survey and genetic identification of Anaplasma species in goats from Central and Southern China. Appl. Environ. Microbiol. 2012, 78, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Md Faruque, R.; Md Rahman, M.; Chowdhury, M.U.E. Epidemiology and molecular detection of Anaplasma spp. in goats from Chattogram district, Bangladesh. Vet. Med. Sci. 2022, 1–10. [Google Scholar] [CrossRef]
- Niaz, S.; Ur Rahman, Z.; Ali, I.; Cossío-Bayúgar, R.; Amaro-Estrada, I.; Alanazi, A.D.; Khattak, I.; Zeb, J.; Nasreen, N.; Khan, A. Molecular prevalence, characterization and associated risk factors of Anaplasma spp. and Theileria spp. in small ruminants in Northern Pakistan. Parasite 2021, 28, 3. [Google Scholar] [CrossRef]
- De la Fuente, J.; Lew, A.; Lutz, H.; Meli, M.L.; Hofmann-Lehmann, R.; Shkap, V.; Molad, T.; Mangold, A.J.; Almazán, C.; Naranjo, V.; et al. Genetic diversity of Anaplasma species major surface proteins and implications for anaplasmosis serodiagnosis and vaccine development. Anim. Health Res. Rev. 2005, 6, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ma, L.; Moumouni, P.F.A.; Jian, Y.; Wang, G.; Zhang, X.; Li, X.; Wang, G.; Lee, S.H.; Galon, E.M.; et al. Molecular survey and characterization of tick-borne pathogens in sheep from Qinghai, China. Small Rumin. Res. 2019, 175, 23–30. [Google Scholar] [CrossRef]
- Benedicto, B.; Ceylan, O.; Moumouni, P.F.A.; Lee, S.H.; Tumwebaze, M.A.; Li, J.; Galon, E.M.; Liu, M.; Li, Y.; Ji, S.; et al. Molecular detection and assessment of risk factors for tick-borne diseases in sheep and goats from Turkey. Acta Parasitol. 2020, 65, 723–732. [Google Scholar] [CrossRef]
- Pereira, A.; Parreira, R.; Cotão, A.J.; Nunes, M.; Vieira, M.L.; Azevedo, F.; Campino, L.; Maia, C. Tick-borne bacteria and protozoa detected in ticks collected from domestic animals and wildlife in central and southern Portugal. Ticks Tick Borne Dis. 2018, 9, 225–234. [Google Scholar] [CrossRef]
- De la Fuente, J.; Atkinson, M.W.; Naranjo, V.; Fernández de Mera, I.G.; Mangold, A.J.; Keating, K.A.; Kocan, K.M. Sequence analysis of the msp4 gene of Anaplasma ovis strains. Vet. Microbiol. 2007, 119, 375–381. [Google Scholar] [CrossRef]
- Han, R.; Yang, J.; Liu, Z.; Gao, S.; Niu, Q.; Hassan, M.A.; Luo, J.; Yin, H. Characterization of Anaplasma ovis strains using the major surface protein 1a repeat sequences. Parasit Vectors 2017, 10, 447. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Peasley, A.M.; Yang, J.; Li, Y.; Guan, G.; Luo, J.; Yin, H.; Brayton, K.A. The Anaplasma ovis genome reveals a high proportion of pseudogenes. BMC Genom. 2019, 20, 69. [Google Scholar] [CrossRef] [Green Version]
- Tumwebaze, M.A.; Byamukama, B.; Tayebwa, D.S.; Byaruhanga, J.; Angwe, M.K.; Galon, E.M.; Liu, M.; Lee, S.H.; Ringo, A.E.; Moumouni, P.F.A.; et al. First molecular detection of Babesia ovis, Theileria spp., Anaplasma spp., and Ehrlichia ruminantium in goats from Western Uganda. Pathogens 2020, 9, 895. [Google Scholar] [CrossRef]
- Ben, J.; Mans, R.P.; Latif, A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. 2015, 4, 104–118. [Google Scholar]
- Kaewhom, P.; Thitasarn, W. The prevalence of Theileria spp. of goat in Watthana Nakhon district, Sakaeo province. J. Mahanakorn Vet. Med. 2017, 12, 57–66. [Google Scholar]
- Yu, Z.Q.; Song, J.K.; Zhang, H.J.; Liu, T.L.; Fan, X.C.; Zhao, G.H. Molecular characterization of Theileria spp. In goats from Shaanxi province, Northwestern China. J. Parasitol. 2018, 104, 726–731. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.H.; Upadhyay, A.; Zhao, J.G.; Huang, L.Y.; Liao, C.H.; Han, Q. Identification of Theileria spp. and investigation of hematological profiles of their infections in goats in Hainan Island, China. Parasite 2022, 29, 13. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Rudra, P.G.; Singha, S.; Das, T.; Gebrekidan, H.; Uddin, B.; Chowdhury, M.Y.E. Molecular Epidemiology and Characterization of Theileria in Goats. Protist 2021, 2, 125804. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.L.C.; Nugraheni, Y.R.; Tiawsirisup, S.; Saiwichai, T.; Thiptara, A.; Kaewthamasorn, M. Development of a novel multiplex PCR assay for the detection and differentiation of Plasmodium caprae from Theileria luwenshuni and Babesia spp. in goats. Acta Trop. 2021, 220, 105957. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, K. A review of the taxonomy of Theileria sergenti/buffeli/orientalis group parasite in calltle. J. Protozool. Res. 1992, 2, 87–96. [Google Scholar]
- Ahantarig, A.; Trinachartvanit, W.; Milne, J.R. Tick-borne pathogens and diseases of animals and humans in Thailand. Southeast Asian J. Trop. Med. Public Health 2008, 39, 1015–1032. [Google Scholar]
- Hammer, J.F.; Jenkins, C.; Bogema, D.; Emery, D. Mechanical transfer of Theileria orientalis: Possible roles of biting arthropods, colostrum and husbandry practices in disease transmission. Parasite Vector. 2016, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Jirapattharasate, C.; Changbunjong, T.; Sedwisai, P.; Weluwanarak, T. Molecular detection of piroplasms in haematophagus flies in the Nakhon Pathom and Kanchanaburi Provinces, Thailand. Vet. Integr. Sci. 2018, 16, 123–133. [Google Scholar]
- Sivakumar, T.; Hayashida, K.; Sugimoto, C.; Yokoyama, N. Evolution and genetic diversity of Theileria. Infect. Genet. Evol. 2014, 27, 250–263. [Google Scholar] [CrossRef] [Green Version]
- Torina, A.; Agnone, A.; Blanda, V.; Alongi, A.; D’Agostino, R.; Caracappa, S.; Marino, A.M.; Di Marco, V.; De La Fuente, J. Development and validation of two PCR tests for the detection of and differentiation between Anaplasma ovis and Anaplasma marginale. Ticks Tick Borne Dis. 2012, 3, 283–287. [Google Scholar] [CrossRef]
- Aktaş, M.; Altay, K.; Dumanlı, N. Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet. Parasitol. 2005, 133, 277–281. [Google Scholar] [CrossRef]
- Altay, K.; Dumanli, N.; Holman, P.J.; Aktaş, M. Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet. Parasitol. 2005, 127, 99–104. [Google Scholar] [CrossRef]
- Ota, N.; Mizuno, D.; Kuboki, N.; Igarashi, I.; Nakamura, Y.; Yamashina, H.; Hanzaike, T.; Fujii, K.; Onoe, S.; Hata, H.; et al. Epidemiological survey of Theileria orientalis infection in grazing cattle in the eastern part of Hokkaido, Japan. J. Vet. Med. Sci. 2009, 71, 937–944. [Google Scholar] [CrossRef] [Green Version]
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016, 44, D7–D19. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]


| Province | No. of Tested Goats | A. ovis (%) | Theileria spp. (%) | Mixed Infections (%) | Isolated Number of T. luwenshuni | Isolated Number of T. orientalis |
|---|---|---|---|---|---|---|
| Yala | 100 | 3 (1.1) | 11 (4.2) | 2 (0.7) | 4 | 0 |
| Pattani | 100 | 1(0.4) | 10 (3.8) | 1 * (0.4) | 5 | 1 |
| Narathiwat | 62 | 0 | 6 (2.3) | 1 * (0.4) | 5 | 0 |
| Total | 262 | 4 (1.5) | 27 (10.3) | 4 (1.5) | 14 | 1 |
| Target Gene | Assay | Primer Sequences (5′→3′) | Fragment (bp) | Annealing Temp (°C) | Reference | |
|---|---|---|---|---|---|---|
| Forward | Reverse | |||||
| A. ovis (MSP4) | PCR | TGAAGGGAGCGGGGTCATGGG | GAGTAATTGCAGCCAGGCACTCT | 347 | 62 | [49] |
| A. marginale (MSP4) | PCR | CTGAAGGGGGAGTAATGGG | GGTAATAGCTGCCAGAGATTCC | 344 | 60 | [49] |
| B. ovis (18S rRNA) | PCR | TGGGCAGGACCTTGGTTCTTCT | CCGCGTAGCGCCGGCTAAATA | 549 | 62 | [50] |
| T. ovis (18S rRNA) | PCR | TCGAGACCTTCGGGT | TCCGGACATTGTAAAACAAA | 520 | 60 | [51] |
| T. orientalis (MPSP) | PCR | CTTTGCCTAGGATACTTCCT | ACGGCAAGTGGTGAGAACT | 776 | 58 | [52] |
| Theileria spp. (18S rRNA) | PCR nPCR | GAAACGGCTACCACATCT TTAAACCTCTTCCAGAGT | AGTTTCCCCGTGTTGAGT TCAGCCTTGCGACCATAC | 778 581 | 55 55 | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udonsom, R.; Mahittikorn, A.; Jirapattharasate, C. Molecular Detection and Genetic Diversity of Tick-Borne Pathogens in Goats from the Southern Part of Thailand. Pathogens 2022, 11, 477. https://doi.org/10.3390/pathogens11040477
Udonsom R, Mahittikorn A, Jirapattharasate C. Molecular Detection and Genetic Diversity of Tick-Borne Pathogens in Goats from the Southern Part of Thailand. Pathogens. 2022; 11(4):477. https://doi.org/10.3390/pathogens11040477
Chicago/Turabian StyleUdonsom, Ruenruetai, Aongart Mahittikorn, and Charoonluk Jirapattharasate. 2022. "Molecular Detection and Genetic Diversity of Tick-Borne Pathogens in Goats from the Southern Part of Thailand" Pathogens 11, no. 4: 477. https://doi.org/10.3390/pathogens11040477






