Tumor Cell Plasticity in Equine Papillomavirus-Positive Versus-Negative Squamous Cell Carcinoma of the Head and Neck
Abstract
:1. Introduction
2. Results
2.1. Twenty-Two Percent of HNSCC Samples Harbor EcPV2 DNA
2.2. Histopathological Findings
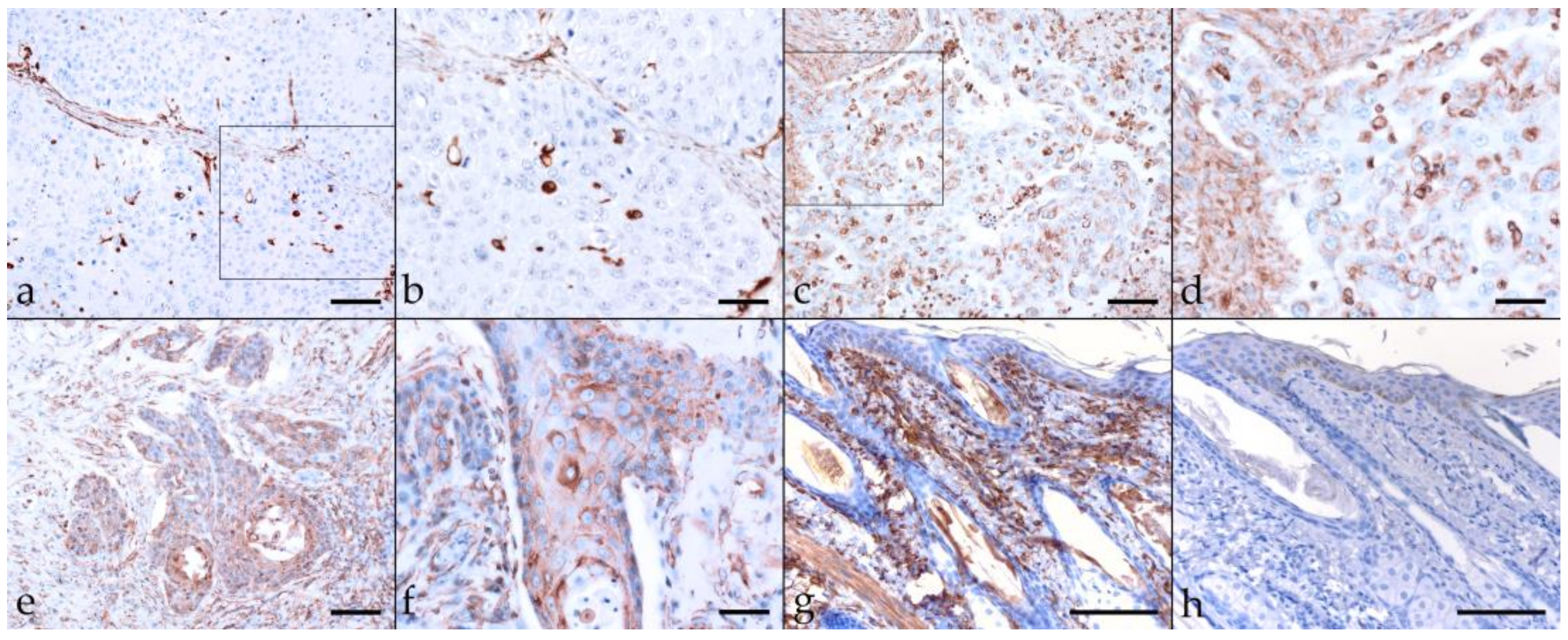
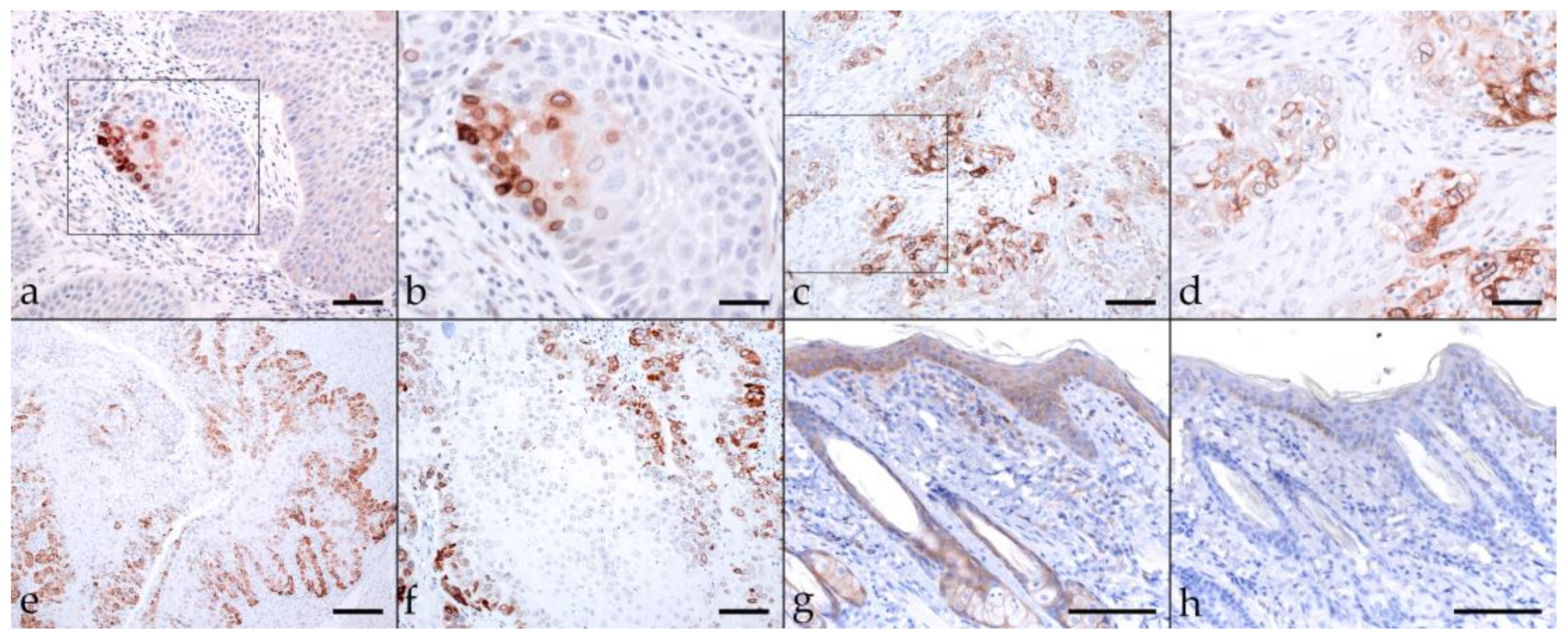
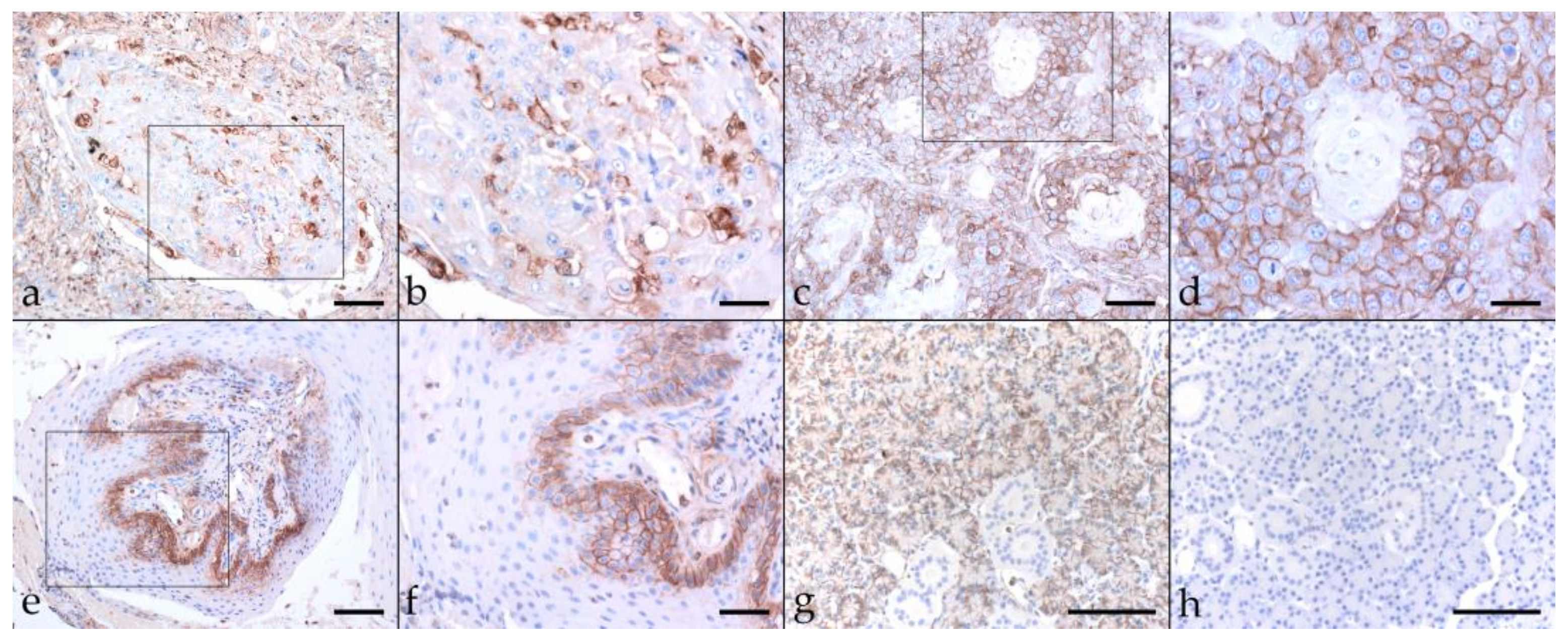
2.3. Immunohistochemical Staining Reveals Tumor Cell Plasticity in Equine HNSCC
2.3.1. Keratin (KRT)
2.3.2. β-Catenin
2.3.3. Vimentin
2.3.4. Cyclooxygenase-2 (COX-2)
2.3.5. CD44
2.3.6. CD271 (p75NTR)
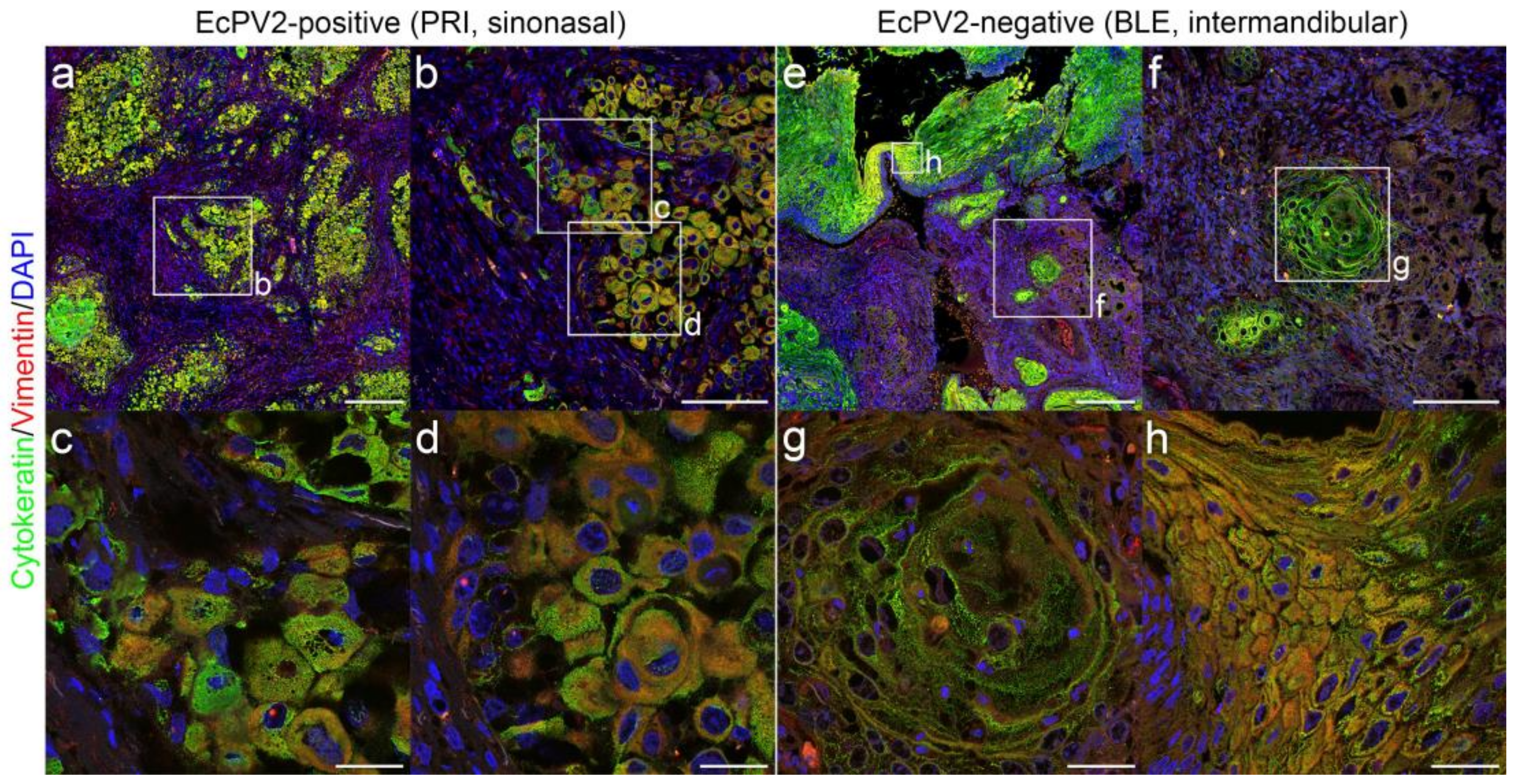
2.4. Immunofluorescent Double-Staining of HNSCCs for Keratins and Vimentin Reveals pEMT
2.5. Immunofluorescent Double-Staining of HNSCC Sections Reveals CD44+ CD271+ Tumor Cells
3. Discussion
4. Method
4.1. Sample Material
4.2. DNA Extraction
4.3. EcPV PCR
4.4. Histopathological Examination
4.5. Immunohistochemical Staining (IHC)
4.6. Immunofluorescent Staining (IF)
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dayyani, F.; Etzel, C.J.; Liu, M.; Ho, C.H.; Lippman, S.M.; Tsao, A.S. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Gissmann, L. Linking human papillomaviruses to cervical cancer: A long and winding road. In Papillomavirus Research: From Natural History to Vaccines and Beyond; Campo, M.S., Ed.; Caister Academic Press: Wymondham, UK, 2006; pp. 3–9. [Google Scholar]
- Ashrafi, G.H.; Brown, D.R.; Fife, K.H.; Campo, M.S. Down-regulation of MHC class I is a property common to papillomavirus E5 proteins. Virus Res. 2006, 120, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Pullos, A.N.; Castilho, R.M.; Squarize, C.H. HPV Infection of the Head and Neck Region and Its Stem Cells. J. Dent. Res. 2015, 94, 1532–1543. [Google Scholar] [CrossRef]
- Secretan, B.; Straif, K.; Baan, R.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009, 10, 1033–1034. [Google Scholar] [CrossRef]
- Kikuchi, K.; Inoue, H.; Miyazaki, Y.; Ide, F.; Kojima, M.; Kusama, K. Epstein-Barr virus (EBV)-associated epithelial and non-epithelial lesions of the oral cavity. Jpn. Dent. Sci. Rev. 2017, 53, 95–109. [Google Scholar] [CrossRef]
- Fleming, J.C.; Woo, J.; Moutasim, K.; Mellone, M.; Frampton, S.J.; Mead, A.; Ahmed, W.; Wood, O.; Robinson, H.; Ward, M.; et al. HPV, tumour metabolism and novel target identification in head and neck squamous cell carcinoma. Br. J. Cancer 2019, 120, 356–367. [Google Scholar] [CrossRef]
- Scott, D.W.; Miller, W.H., Jr. Squamous cell carcinoma. In Equine Dermatology, 2nd ed.; Scott, D.W., Miller, W.H., Jr., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2011; pp. 473–476. [Google Scholar]
- Sykora, S.; Brandt, S. Papillomavirus infection and squamous cell carcinoma in horses. Vet. J. 2017, 223, 48–54. [Google Scholar] [CrossRef]
- Scase, T.; Brandt, S.; Kainzbauer, C.; Sykora, S.; Bijmholt, S.; Hughes, K.; Sharpe, S.; Foote, A. Equus caballus papillomavirus-2 (EcPV-2): An infectious cause for equine genital cancer? Equine Vet. J. 2010, 42, 738–745. [Google Scholar] [CrossRef]
- Lassaline, M.; Cranford, T.L.; Latimer, C.A.; Bellone, R.R. Limbal squamous cell carcinoma in Haflinger horses. Vet. Ophthalmol. 2015, 18, 404–408. [Google Scholar] [CrossRef]
- Knight, C.G.; Dunowska, M.; Munday, J.S.; Peters-Kennedy, J.; Rosa, B.V. Comparison of the levels of Equus caballus papillomavirus type 2 (EcPV-2) DNA in equine squamous cell carcinomas and non-cancerous tissues using quantitative PCR. Vet. Microbiol. 2013, 166, 257–262. [Google Scholar] [CrossRef]
- Sykora, S.; Jindra, C.; Hofer, M.; Steinborn, R.; Brandt, S. Equine papillomavirus type 2: An equine equivalent to human papillomavirus 16? Vet. J. 2017, 225, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Norgard, R.J.; Stanger, B.Z. Cellular Plasticity in Cancer. Cancer Discov. 2019, 9, 837–851. [Google Scholar] [CrossRef] [Green Version]
- Baum, B.; Settleman, J.; Quinlan, M.P. Transitions between epithelial and mesenchymal states in development and disease. Semin. Cell Dev. Biol. 2008, 19, 294–308. [Google Scholar] [CrossRef]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef] [Green Version]
- Greenburg, G.; Hay, E.D. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J. Cell Biol. 1982, 95, 333–339. [Google Scholar] [CrossRef]
- Greenburg, G.; Hay, E.D. Cytodifferentiation and tissue phenotype change during transformation of embryonic lens epithelium to mesenchyme-like cells in vitro. Dev. Biol. 1986, 115, 363–379. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Y.; Hummel, M.; Hoffmann, T.K.; Gross, M.; Kaufmann, A.M.; Albers, A.E. Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS ONE 2011, 6, e16466. [Google Scholar] [CrossRef]
- Chen, C.; Zimmermann, M.; Tinhofer, I.; Kaufmann, A.M.; Albers, A.E. Epithelial-to-mesenchymal transition and cancer stem(-like) cells in head and neck squamous cell carcinoma. Cancer Lett. 2013, 338, 47–56. [Google Scholar] [CrossRef]
- Mandal, M.; Myers, J.N.; Lippman, S.M.; Johnson, F.M.; Williams, M.D.; Rayala, S.; Ohshiro, K.; Rosenthal, D.I.; Weber, R.S.; Gallick, G.E.; et al. Epithelial to mesenchymal transition in head and neck squamous carcinoma: Association of Src activation with E-cadherin down-regulation, vimentin expression, and aggressive tumor features. Cancer 2008, 112, 2088–2100. [Google Scholar] [CrossRef]
- Nijkamp, M.M.; Span, P.N.; Hoogsteen, I.J.; van der Kogel, A.J.; Kaanders, J.H.; Bussink, J. Expression of E-cadherin and vimentin correlates with metastasis formation in head and neck squamous cell carcinoma patients. Radiother. Oncol. 2011, 99, 344–348. [Google Scholar] [CrossRef]
- Saitoh, M. Involvement of partial EMT in cancer progression. J. Biochem. 2018, 164, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Wang, Q.; An, J.; Long, Q.; Wang, H.; Xiang, M.; Xiang, M.; Zhao, Y.; Liu, Y.; Liu, J.; et al. Partial EMT in Squamous Cell Carcinoma: A Snapshot. Int. J. Biol. Sci. 2021, 17, 3036–3047. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Porath, I.; Thomson, M.W.; Carey, V.J.; Ge, R.; Bell, G.W.; Regev, A.; Weinberg, R.A. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008, 40, 499–507. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; De Maria, R.; Kroemer, G.; Galluzzi, L. DNA Damage in Stem Cells. Mol. Cell 2017, 66, 306–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Keith, B.; Simon, M.C. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Oshimori, N. Cancer stem cells and their niche in the progression of squamous cell carcinoma. Cancer Sci. 2020, 111, 3985–3992. [Google Scholar] [CrossRef]
- Vidal, A.; Redmer, T. Decoding the Role of CD271 in Melanoma. Cancers 2020, 12, 2460. [Google Scholar] [CrossRef]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.; Earle, C.; Wong, G.; Spevak, C.C.; Krueger, K. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene 2012, 31, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourguignon, L.Y.; Wong, G.; Earle, C.; Chen, L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J. Biol. Chem. 2012, 287, 32800–32824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, K.E.; Wu, F.; Keysar, S.B.; Morton, J.J.; Miller, B.; Chimed, T.S.; Le, P.N.; Nieto, C.; Chowdhury, F.N.; Tyagi, A.; et al. Cancer Cell CD44 Mediates Macrophage/Monocyte-Driven Regulation of Head and Neck Cancer Stem Cells. Cancer Res. 2020, 80, 4185–4198. [Google Scholar] [CrossRef]
- Keysar, S.B.; Le, P.N.; Miller, B.; Jackson, B.C.; Eagles, J.R.; Nieto, C.; Kim, J.; Tang, B.; Glogowska, M.J.; Morton, J.J.; et al. Regulation of Head and Neck Squamous Cancer Stem Cells by PI3K and SOX2. J. Natl. Cancer Inst. 2017, 109, djw189. [Google Scholar] [CrossRef]
- Suarez-Bonnet, A.; Willis, C.; Pittaway, R.; Smith, K.; Mair, T.; Priestnall, S.L. Molecular carcinogenesis in equine penile cancer: A potential animal model for human penile cancer. Urol Oncol. Semin. Orig. Investig. 2018, 36, 532.e9–532.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armando, F.; Mecocci, S.; Orlandi, V.; Porcellato, I.; Cappelli, K.; Mechelli, L.; Brachelente, C.; Pepe, M.; Gialletti, R.; Ghelardi, A.; et al. Investigation of the Epithelial to Mesenchymal Transition (EMT) Process in Equine Papillomavirus-2 (EcPV-2)-Positive Penile Squamous Cell Carcinomas. Int. J. Mol. Sci. 2021, 22, 10588. [Google Scholar] [CrossRef]
- Armando, F.; Godizzi, F.; Razzuoli, E.; Leonardi, F.; Angelone, M.; Corradi, A.; Meloni, D.; Ferrari, L.; Passeri, B. Epithelial to Mesenchymal Transition (EMT) in a Laryngeal Squamous Cell Carcinoma of a Horse: Future Perspectives. Animals 2020, 10, 2318. [Google Scholar] [CrossRef]
- Lange, C.E.; Tobler, K.; Ackermann, M.; Favrot, C. Identification of two novel equine papillomavirus sequences suggests three genera in one cluster. Vet. Microbiol. 2011, 149, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Lange, C.E.; Vetsch, E.; Ackermann, M.; Favrot, C.; Tobler, K. Four novel papillomavirus sequences support a broad diversity among equine papillomaviruses. J. Gen. Virol. 2013, 94, 1365–1372. [Google Scholar] [CrossRef] [Green Version]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moll, R.; Franke, W.W.; Schiller, D.L.; Geiger, B.; Krepler, R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31, 11–24. [Google Scholar] [CrossRef]
- Makarova, G.; Bette, M.; Schmidt, A.; Jacob, R.; Cai, C.; Rodepeter, F.; Betz, T.; Sitterberg, J.; Bakowsky, U.; Moll, R.; et al. Epidermal growth factor-induced modulation of cytokeratin expression levels influences the morphological phenotype of head and neck squamous cell carcinoma cells. Cell Tissue Res. 2013, 351, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Kitajima, S.; Ogawa, I.; Hiraoka, M.; Sargolzaei, S.; Keikhaee, M.R.; Sato, S.; Miyauchi, M.; Takata, T. Invasion and metastasis of oral cancer cells require methylation of E-cadherin and/or degradation of membranous beta-catenin. Clin. Cancer Res. 2004, 10, 5455–5463. [Google Scholar] [CrossRef] [Green Version]
- Stenner, M.; Yosef, B.; Huebbers, C.U.; Preuss, S.F.; Dienes, H.P.; Speel, E.J.; Odenthal, M.; Klussmann, J.P. Nuclear translocation of beta-catenin and decreased expression of epithelial cadherin in human papillomavirus-positive tonsillar cancer: An early event in human papillomavirus-related tumour progression? Histopathology 2011, 58, 1117–1126. [Google Scholar] [CrossRef]
- Wang, W.; Wen, Q.; Luo, J.; Chu, S.; Chen, L.; Xu, L.; Zang, H.; Alnemah, M.M.; Li, J.; Zhou, J.; et al. Suppression of beta-catenin Nuclear Translocation By CGP57380 Decelerates Poor Progression and Potentiates Radiation-Induced Apoptosis in Nasopharyngeal Carcinoma. Theranostics 2017, 7, 2134–2149. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Watanabe, Y.; Imanishi, Y.; Ozawa, H.; Sakamoto, K.; Fujii, R.; Shigetomi, S.; Habu, N.; Otsuka, K.; Sato, Y.; Sekimizu, M.; et al. Selective EP2 and Cox-2 inhibition suppresses cell migration by reversing epithelial-to-mesenchymal transition and Cox-2 overexpression and E-cadherin downregulation are implicated in neck metastasis of hypopharyngeal cancer. Am. J. Transl. Res. 2020, 12, 1096–1113. [Google Scholar]
- Sadasivam, S.; Subramanian, R. A perspective on challenges and opportunities in characterizing oral cancer stem cells. Front. Biosci. 2020, 25, 1011–1021. [Google Scholar] [CrossRef]
- Murillo-Sauca, O.; Chung, M.K.; Shin, J.H.; Karamboulas, C.; Kwok, S.; Jung, Y.H.; Oakley, R.; Tysome, J.R.; Farnebo, L.O.; Kaplan, M.J.; et al. CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma. Oncotarget 2014, 5, 6854–6866. [Google Scholar] [CrossRef] [Green Version]
- Gale, N.; Poljak, M.; Zidar, N. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: What is New in the 2017 WHO Blue Book for Tumours of the Hypopharynx, Larynx, Trachea and Parapharyngeal Space. Head Neck Pathol. 2017, 11, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, D.W.; Miller, W.H., Jr. Squamous cell carcinoma. In Equine Dermatology, 1st ed.; Scott, D.W., Miller, W.H., Jr., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2003; pp. 707–712. [Google Scholar]
- Knottenbelt, D.C. Squamous cell carcinoma. In Pascoe’s Principles and Practice of Equine Dermatology, 2nd ed.; Knottenbelt, D.C., Ed.; Saunders Elsevier: London, UK, 2009; pp. 427–433. [Google Scholar]
- Pascoe, R.R.; Knottenbelt, D.C. Squamous cell carcinoma. In Manual of Equine Dermatology; Saunders, W.B., Ed.; Harcourt Publishers: London, UK, 1999; pp. 261–266. [Google Scholar]
- Budras, K.-D.; Sack, W.O.; Röck, S. Head. In Anatomy of the Horse, 6th ed.; Schlütersche Verlagsgesellschaft: Hannover, Germany, 2011. [Google Scholar]
- Hibi, H.; Hatama, S.; Obata, A.; Shibahara, T.; Kadota, K. Laryngeal squamous cell carcinoma and papilloma associated with Equus caballus papillomavirus 2 in a horse. J. Vet. Med. Sci. 2019, 81, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kainzbauer, C.; Rushton, J.; Tober, R.; Scase, T.; Nell, B.; Sykora, S.; Brandt, S. Bovine papillomavirus type 1 and Equus caballus papillomavirus 2 in equine squamous cell carcinoma of the head and neck in a Connemara mare. Equine Vet. J. 2012, 44, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, L.; Willemsen, A.; Vanderstraeten, E.; Bracho, M.A.; De Baere, C.; Bravo, I.G.; Martens, A. EcPV2 DNA in equine genital squamous cell carcinomas and normal genital mucosa. Vet. Microbiol. 2012, 158, 33–41. [Google Scholar] [CrossRef]
- Sykora, S.; Samek, L.; Schonthaler, K.; Palm, F.; Borzacchiello, G.; Aurich, C.; Brandt, S. EcPV-2 is transcriptionally active in equine SCC but only rarely detectable in swabs and semen from healthy horses. Vet. Microbiol. 2012, 158, 194–198. [Google Scholar] [CrossRef]
- Ramsauer, A.S.; Wachoski-Dark, G.L.; Fraefel, C.; Ackermann, M.; Brandt, S.; Grest, P.; Knight, C.; Favrot, C.; Tobler, K. Establishment of a Three-Dimensional In Vitro Model of Equine Papillomavirus Type 2 Infection. Viruses 2021, 13, 1404. [Google Scholar] [CrossRef]
- Van der Velden, L.A. Expression of cytokeratin subtypes and vimentin in squamous cell carcinoma of the floor of the mouth and the mobile tongue. Otorhinolaryngol. Nova 2001, 11, 186–192. [Google Scholar] [CrossRef]
- Jung, A.; Schrauder, M.; Oswald, U.; Knoll, C.; Sellberg, P.; Palmqvist, R.; Niedobitek, G.; Brabletz, T.; Kirchner, T. The invasion front of human colorectal adenocarcinomas shows co-localization of nuclear beta-catenin, cyclin D1, and p16INK4A and is a region of low proliferation. Am. J. Pathol. 2001, 159, 1613–1617. [Google Scholar] [CrossRef]
- Gomez-Valenzuela, F.; Escobar, E.; Perez-Tomas, R.; Montecinos, V.P. The Inflammatory Profile of the Tumor Microenvironment, Orchestrated by Cyclooxygenase-2, Promotes Epithelial-Mesenchymal Transition. Front. Oncol. 2021, 11, 686792. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Dannenberg, A.J. Cyclooxygenase-2 transcription is regulated by human papillomavirus 16 E6 and E7 oncoproteins: Evidence of a corepressor/coactivator exchange. Cancer Res. 2007, 67, 3976–3985. [Google Scholar] [CrossRef] [Green Version]
- Dawood, S.; Austin, L.; Cristofanilli, M. Cancer stem cells: Implications for cancer therapy. Oncology 2014, 28, 1101–1107. [Google Scholar] [PubMed]
- Chung, M.K.; Jung, Y.H.; Lee, J.K.; Cho, S.Y.; Murillo-Sauca, O.; Uppaluri, R.; Shin, J.H.; Sunwoo, J.B. CD271 Confers an Invasive and Metastatic Phenotype of Head and Neck Squamous Cell Carcinoma through the Upregulation of Slug. Clin. Cancer Res. 2018, 24, 674–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkashty, O.A.; Abu Elghanam, G.; Su, X.; Liu, Y.; Chauvin, P.J.; Tran, S.D.; Elkashty, O. Cancer stem cells enrichment with surface markers CD271 and CD44 in human head and neck squamous cell carcinomas. Carcinogenesis 2020, 41, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Boiko, A.D.; Razorenova, O.V.; van de Rijn, M.; Swetter, S.M.; Johnson, D.L.; Ly, D.P.; Butler, P.D.; Yang, G.P.; Joshua, B.; Kaplan, M.J.; et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 2010, 466, 133–137. [Google Scholar] [CrossRef]
- Huang, S.D.; Yuan, Y.; Liu, X.H.; Gong, D.J.; Bai, C.G.; Wang, F.; Luo, J.-H.; Xu, Z.-Y. Self-renewal and chemotherapy resistance of p75NTR positive cells in esophageal squamous cell carcinomas. BMC Cancer 2009, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Imai, T.; Tamai, K.; Oizumi, S.; Oyama, K.; Yamaguchi, K.; Sato, I.; Satoh, K.; Matsuura, K.; Saijo, S.; Sugamura, K.; et al. CD271 defines a stem cell-like population in hypopharyngeal cancer. PLoS ONE 2013, 8, e62002. [Google Scholar]
- Okumura, T.; Shimada, Y.; Imamura, M.; Yasumoto, S. Neurotrophin receptor p75 characterizes human esophageal keratinocyte stem cells in vitro. Oncogene 2003, 22, 4017–4026. [Google Scholar] [CrossRef] [Green Version]
- Weissenbacher-Lang, C.; Kristen, T.; Mendel, V.; Brunthaler, R.; Schwarz, L.; Weissenbock, H. Porcine circovirus type 2 (PCV2) genotyping in Austrian pigs in the years 2002 to 2017. BMC Vet. Res. 2020, 16, 198. [Google Scholar] [CrossRef]
- Brandt, S.; Haralambus, R.; Schoster, A.; Kirnbauer, R.; Stanek, C. Peripheral blood mononuclear cells represent a reservoir of bovine papillomavirus DNA in sarcoid-affected equines. J. Gen. Virol. 2008, 89, 1390–1395. [Google Scholar] [CrossRef]
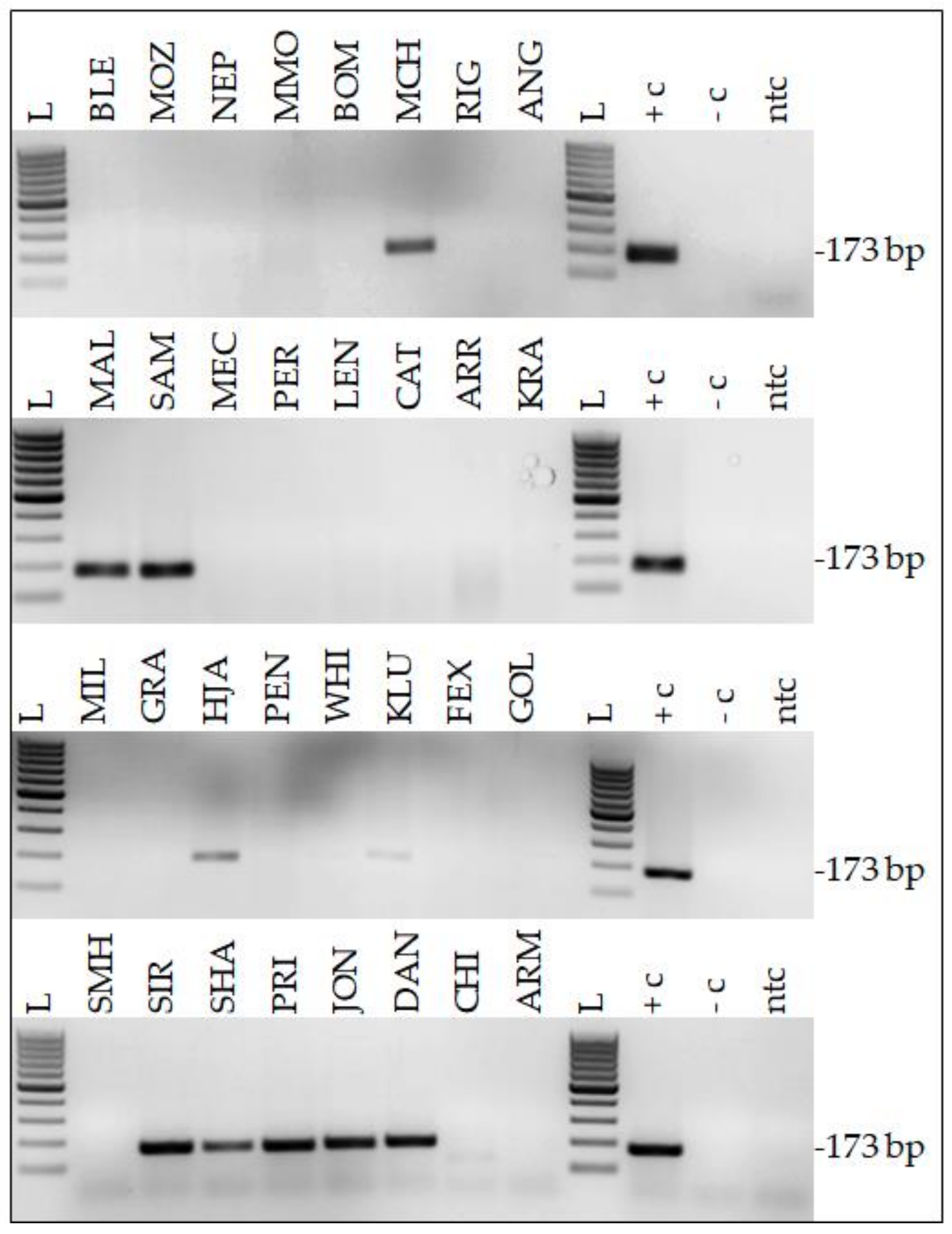




| Code | Breed | Age | Color | Sex | Sample Origin/Histopathological Diagnosis | Cornification | Native, FFPE | EcPV2 DNA |
|---|---|---|---|---|---|---|---|---|
| EQUINE HNSCC PATIENTS AND TUMOR SAMPLES THEREFROM SELECTED FOR IHC ANALYSIS (n = 22) | ||||||||
| DAN | WB | 22 | Chestnut | G | Nasal and oral SCC, retropharyngeal LN metastases | + | N, F | YES |
| MAL | Arabian TB | 19 | Grey | M | Nasal SCC with orbital infiltration | + | F | YES |
| VAL | Icelandic horse | 18 | Black | M | SCC of dorsal nasal concha, osteolysis | ++ | N, F | No |
| DIA | Trotter | 17 | Black | M | SCC of the left nasal cavity and paranasal sinus, osteolysis | + | N, F | No |
| MMO | Haflinger mix | 13 | Bay | G | Sinus SCC | No | F | No |
| PRI | Shetland pony | 26 | Black | G | Nasal SCC | ++ | N, F | YES |
| HJA | Icelandic horse | 16 | Bay | G | Maxillary sinus SCC, oral and retropharyngeal LN metastases | + | F | YES |
| KLU | Noriker horse | 17 | Piebald | G | Maxillary sinus SCC invading lymphatics and blood vessels | + | F | YES |
| FIL | WB | 26 | Chestnut | G | Maxillary sinus SCC | No | N, F | No |
| PER | Trotter | 20 | Bay | G | Maxillary sinus SCC, mandibular and retropharyngeal LN metastases | + | F | No |
| SHM | Cob | 23 | Piebald | G | Sinonasal SCC | ++ | F | No |
| MEC | Shetland pony | 25 | Black | M | Oral SCC right mandibula, maxilla | ++ | F | No |
| BLE | WB | 11 | Piebald | M | Intermandibular SCC (recurrent lesion) | + | F | No |
| SHA | Pinto | 17 | Skewbald | M | Lingual and intermandibular SCC | + | N, F | YES |
| BOM | WB | 24 | Bay | M | Lingual SCC, retropharyngeal and tracheal LNs metastases | No | F | No |
| JON | Pony | 25 | Chestnut | G | Gingival/palatal SCC | No | N, F | YES |
| LUK | WB | 17 | Grey | G | Palatal SCC invading maxillary sinus | ++ | F | No |
| SIR | WB | 11 | Fuchs | M | Mandibular SCC | ++ | F | YES |
| KRA | Icelandic horse | 21 | Fuchs | G | Oral SCC, regional LNs metastases | No | F | No |
| VLU | Icelandic horse | 30 | Chestnut | G | Oral SCC | ++ | N, F | YES |
| SAM | Haflinger-WB | 13 | Sorrel | G | Pharyngeal SCC, retropharyngeal LN metastases | + | N, F | YES |
| MCH | Connemara | 15 | Grey | M | Periocular SCC with metastases (parotis, larynx, jugular notch) | + | N, F | YES |
| EQUINE HNSCC PATIENTS AND TUMOR SAMPLES THEREFROM (NO IHC ANALYSIS) (n = 27) | ||||||||
| MIL | WB | 22 | Chestnut | M | Maxillary sinus SCC | + | N, F | No |
| GER | Trakehner | 18 | Black | G | Sinonasal SCC with mandibular LN metastases | No | N, F | No |
| STA | Haflinger | 7 | Sorrel | G | Nasal SCC, mandibular LN metastases | No | N, F | No |
| ATH | Haflinger | 22 | Sorrel | G | Oral vestibule SCC | + | N, F | No |
| BEL | Haflinger | 22 | Sorrel | M | Paranasal sinus SCC with pronounced osteolysis | No | N, F | No |
| JAN | Hungarian WB | 17 | Bay | M | Maxillary sinus SCC, retropharyngeal LN metastases | ++ | F | No |
| CAT | Trotter | 7 | Bay | M | Sinonasal SCC, osteolysis right maxilla | + | F | No |
| CHI | Trotter | 23 | Bay | M | Maxillary SCC invading oral cavity and brain | No | F | No |
| ANG | Haflinger | 16 | Chestnut | M | Maxillary SCC, suspected metastatic activity | No | F | No |
| SNI | Connemara | 16 | Grey | M | Mandibular SCC invading lymphatics, LN metastases | No | N, F | No |
| PEN | Shetland pony | 26 | Piebald | M | Mandibular SCC, osteolysis | ++ | F | No |
| ARR | TB | 21 | Bay | M | Mandibular SCC left, osteolysis | + | N, F | No |
| FEX | Pony | 27 | Sorrel | G | Mandibular SCC invading tongue, trachea, esophagus | No | F | No |
| GAJ | Trotter | 13 | Bay | G | Palatal/gingival SCC, bone arrosion | + | N, F | No |
| WHI | WB | 20 | Skewbald | M | Ulcerative, lingual SCC, invading left stylohyoid | ++ | F | No |
| IAS | Arabian TB | 22 | Grey | M | Lingual SCC, retropharyngeal LN metastases | ++ | F | No |
| NEP | Lusitano | 21 | Grey | G | Lingual base SCC | + | F | No |
| MOZ | WB | 27 | Grey | G | Lingual base SCC, retropharyngeal LNs metastases | + | F | No |
| KAR | Haflinger | 23 | Sorrel | M | Lingual SCC, retropharyngeal, mandibular, cranial LN metastases | + | F | No |
| GOL | Haflinger | 23 | Sorrel | G | Labial SCC (+conjunctival and cutaneous CIS) | ++ | F | No |
| NAV | Haflinger | 17 | Sorrel | G | Labial SCC (+right eye: CIS; left eye: SCC) | ++ | N, F | No |
| JOY | Haflinger | 20 | Sorrel | M | Labial SCC (recurrent lesion), mandibular LN metastases | + | F | No |
| LEN | WB | 23 | Chestnut | M | Maxillary SCC, mandibular LN metastases | + | F | No |
| RIG | Haflinger | 9 | Sorrel | G | Mandibular and labial SCC, mandibular LN metastases | + | F | No |
| GRA | Arabohaflinger | 20 | Sorrel | G | Nasal CIS | ++ | F | No |
| ARM | Haflinger | 12 | Sorrel | M | Cutaneous perinasal CIS (+conjunctival SCC) | No | F | No |
| DAL | Trotter | 12 | Bay | G | Paranasal sinus and oral SCC | + | F | No |
| HNSCC (n = 22) | Keratin | β-Catenin | Vimentin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Tumor Analyzed | I | D | % +Cells | P | I | D | % +Cells | I | D | % +Cells | P |
| DAN | nasal | 1–3 | diffuse | ≤100 | central+ | 3 | diffuse | ≤100 | 3 | patchy | <50 | |
| MAL | nasal | 2–3 | diffuse | ≤100 | 3 | diffuse | ≤100 | 3 | patchy | <10 | ||
| VAL | sinonasal | 3 | diffuse | ≤100 | 1–2 | diffuse | ≤100 | 3 | patchy | <10 | ||
| DIA | sinonasal | 1–2 | diffuse | ≤100 | central+ | 1 | patchy | >50 | 3 | patchy | <10 | |
| MMO | sinonasal | 2–3 | diffuse | ≤100 | 2 | diffuse | ≤100 | 1–3 | patchy | <50 | ||
| PRI | sinonasal | 3 | diffuse | ≤100 | 3 | diffuse | ≤100 | 2–3 | patchy | <50 | marginal | |
| HJA | sinonasal | 1–3 | diffuse | ≤100 | 1–3 | diffuse | ≤100 | 3 | patchy | <10 | ||
| KLU | maxillary sinus | 1–2 | diffuse | ≤100 | 1–3 | patchy | ≤100 | 1–3 | patchy | >50 | ||
| FIL | maxillary sinus | 3 | diffuse | ≤100 | 1 | diffuse | ≤100 | 3 | patchy | >50 | ||
| PER | maxillary sinus | 2–3 | diffuse | ≤100 | central+ | 2–3 | diffuse | ≤100 | 2–3 | patchy | <50 | |
| SHM | sinus | 2–3 | diffuse | ≤100 | central+ | 3 | patchy | ≤100 | 3 | patchy | <50 | |
| MEC | mandibular | 2–3 | diffuse | ≤100 | central+ | 1–2 | patchy | >50 | 2–3 | patchy | <50 | |
| BLE | intermandibular | 2–3 | diffuse | ≤100 | central+ | 3 | diffuse | ≤100 | 3 | patchy | <10 | |
| SHA | linguomandibular | 3 | diffuse | ≤100 | 3 | diffuse | ≤100 | 3 | patchy | <10 | ||
| BOM | lingual | 3 | diffuse | ≤100 | 1 | diffuse | ≤100 | 3 | patchy | <10 | ||
| JON | gingivopalatal | 1–3 | diffuse | ≤100 | central+ | 1 | diffuse | ≤100 | 3 | patchy | <10 | |
| LUK | palatal | 3 | diffuse | ≤100 | 2 | diffuse | ≤100 | 3 | patchy | <50 | ||
| SIR | labiopalatal | 1–2 | diffuse | ≤100 | central+ | 2 | diffuse | ≤100 | 3 | patchy | <10 | |
| KRA | oral | 2–3 | diffuse | ≤100 | 1–2 | patchy | >50 | 3 | patchy | <10 | ||
| VLU | oral | 1–2 | diffuse | ≤100 | central+ | 1 | patchy | >50 | 1 | patchy | <10 | |
| SAM | laryngeal | 3 | diffuse | ≤100 | 3 | diffuse | ≤100 | 3 | patchy | <10 | ||
| MCH | periocular | 3 | diffuse | ≤100 | 1 | diffuse | ≤100 | 3 | patchy | <10 | ||
| HNSCC (n = 22) | COX-2 | CD44 | CD271 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Tumor Analyzed | I | % +Cells | P | I | D | % +Cells | I | D | % +Cells | P |
| DAN | nasal | 0–3 | >50 | 0–2 | patchy | >50 | 1–2 | diffuse | ≤100 | ||
| MAL | nasal | 0–3 | <50 | 0–1 | patchy | <50 | 1–3 | diffuse | ≤100 | ||
| VAL | sinonasal | 0–3 | <10 | marginal | 0–2 | patchy | <50 | 1 | diffuse | ≤100 | |
| DIA | sinonasal | 0–1 | <10 | 0–2 | patchy | <50 | 0–1 | diffuse | >50 | ||
| MMO | sinonasal | 0–3 | >50 | 0–3 | patchy | >50 | 1 | diffuse | ≤100 | ||
| PRI | sinonasal | 2–3 | >50 | 1–2 | diffuse | na | 0–2 | diffuse | >50 | central+ | |
| HJA | sinonasal | 0–3 | >50 | 0–1 | patchy | <50 | 1–2 | diffuse | ≤100 | ||
| KLU | maxillary sinus | 0–3 | <50 | marginal | 0–3 | patchy | >50 | 1–2 | patchy | ≤100 | |
| FIL | maxillary sinus | 0–3 | <50 | 0–2 | patchy | <50 | 1–2 | diffuse | ≤100 | ||
| PER | maxillary sinus | 0–3 | <50 | marginal | 0–2 | patchy | >50 | 1–2 | diffuse | ≤100 | |
| SHM | sinus | 0–3 | >50 | 1–3 | patchy | >50 | 1–3 | diffuse | ≤100 | ||
| MEC | mandibular | 0–3 | <50 | 0–3 | patchy | >50 | 0–1 | patchy | >50 | ||
| BLE | intermandibular | 0–3 | <50 | 1–2 | patchy | <50 | 1–2 | diffuse | ≤100 | ||
| SHA | linguomandibular | 0–2 | <50 | 0–2 | patchy | <50 | 1–2 | diffuse | ≤100 | ||
| BOM | lingual | 0–1 | <10 | 0–2 | patchy | >50 | 0–1 | patchy | >50 | ||
| JON | gingivopalatal | 0–3 | <50 | 0–3 | patchy | >50 | 1–2 | diffuse | ≤100 | ||
| LUK | palatal | 0–3 | >50 | 0–2 | patchy | >50 | 1–2 | diffuse | ≤100 | ||
| SIR | labiopalatal | 0–3 | <50 | 0–2 | patchy | >50 | 2–3 | diffuse | ≤100 | ||
| KRA | oral | 0–3 | <50 | 0–1 | patchy | <50 | 1 | diffuse | ≤100 | ||
| VLU | oral | 0–3 | <10 | marginal | 0–2 | patchy | >50 | 1–2 | diffuse | ≤100 | central+ |
| SAM | laryngeal | 0–3 | >50 | 1–3 | patchy | <50 | 1–2 | patchy | >50 | ||
| MCH | periocular | 0–3 | <50 | 0–2 | patchy | <10 | 1 | diffuse | 100 | ||
| Host | Type | Clone | Target Protein | Provider | Dilution | HIER |
|---|---|---|---|---|---|---|
| IHC (single staining) | ||||||
| Primary Abs | ||||||
| Mouse | Monoclonal | AE1 | LMW keratins | Cell Marque, Sigma-Aldrich, Vienna, Austria | 1:650 | pH 9 |
| Mouse | Monoclonal | AE3 | HMW keratins | Cell Marque, Sigma-Aldrich, Vienna, Austria | 1:650 | pH 9 |
| Mouse | Monoclonal | 9G2 | Beta-catenin | Acris Antibodies, Herford, Germany | 1:500 | pH 9 |
| Mouse | Monoclonal | V9 | Vimentin | Dako, Hamburg, Germany | 1:500 | pH 6 |
| Rabbit | Recombinant | EPR3208 | CD146 | Abcam, Cambridge, UK | 1:500 | pH 6 |
| Rabbit | Monoclonal | SP21 | COX2 | Thermo Fisher Scientific, Vienna, Austria | 1:400 | pH 6 |
| Rat | Monoclonal | IM7 | CD44 | Santa Cruz Biotechnology, Dallas, Texas, USA | 1:200 | pH 6 |
| Rabbit | Monoclonal | D4B3 | CD271 (p75NTR) | Cell Signaling Technology, Frankfurt, Germany | 1:1000 | pH 9 |
| Secondary Abs | ||||||
| BrightVision Poly-HRP anti-mouse Ab | ImmunoLogic, Duiven, The Netherlands | |||||
| BrightVision Poly-HRP anti-rabbit Ab | ImmunoLogic, Duiven, The Netherlands | |||||
| Goat anti-rat HRP conjugated | Abcam, Cambridge, UK | |||||
| IF (double staining) | ||||||
| Primary Abs | ||||||
| Mouse | Monoclonal | AE1 | LMW keratins | Cell Marque, Sigma-Aldrich, Vienna, Austria | 1:400 | pH 9 |
| Mouse | Monoclonal | AE3 | HMW keratins | Cell Marque, Sigma-Aldrich, Vienna, Austria | 1:400 | pH 9 |
| Rabbit | Polyclonal | Vimentin | Sigma Prestige, Merck, Vienna, Austria | 1:500 | pH 9 | |
| Rat | Monoclonal | IM7 | CD44 | Santa Cruz Biotechnology, Dallas, Texas, USA | 1:500 | pH 9 |
| Rabbit | Monoclonal | D4B3 | CD271 (p75NTR) | Cell Signaling Technology, Frankfurt, Germany | 1:250 | pH 9 |
| Secondary Abs | ||||||
| Donkey anti-mouse Ab A488 1:800 | Jackson ImmunoResearch Europe, LTD, Ely, UK | |||||
| Donkey anti-rabbit Ab A568 1:400 | Invitrogen, Thermo Fisher Scientific, Vienna, Austria | |||||
| Goat anti-rat Ab A488 1:800 | Invitrogen, Thermo Fisher Scientific, Vienna, Austria | |||||
| Goat anti-rabbit Ab A568 1:1500 | Invitrogen, Thermo Fisher Scientific, Vienna, Austria | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strohmayer, C.; Klang, A.; Kummer, S.; Walter, I.; Jindra, C.; Weissenbacher-Lang, C.; Redmer, T.; Kneissl, S.; Brandt, S. Tumor Cell Plasticity in Equine Papillomavirus-Positive Versus-Negative Squamous Cell Carcinoma of the Head and Neck. Pathogens 2022, 11, 266. https://doi.org/10.3390/pathogens11020266
Strohmayer C, Klang A, Kummer S, Walter I, Jindra C, Weissenbacher-Lang C, Redmer T, Kneissl S, Brandt S. Tumor Cell Plasticity in Equine Papillomavirus-Positive Versus-Negative Squamous Cell Carcinoma of the Head and Neck. Pathogens. 2022; 11(2):266. https://doi.org/10.3390/pathogens11020266
Chicago/Turabian StyleStrohmayer, Carina, Andrea Klang, Stefan Kummer, Ingrid Walter, Christoph Jindra, Christiane Weissenbacher-Lang, Torben Redmer, Sibylle Kneissl, and Sabine Brandt. 2022. "Tumor Cell Plasticity in Equine Papillomavirus-Positive Versus-Negative Squamous Cell Carcinoma of the Head and Neck" Pathogens 11, no. 2: 266. https://doi.org/10.3390/pathogens11020266
APA StyleStrohmayer, C., Klang, A., Kummer, S., Walter, I., Jindra, C., Weissenbacher-Lang, C., Redmer, T., Kneissl, S., & Brandt, S. (2022). Tumor Cell Plasticity in Equine Papillomavirus-Positive Versus-Negative Squamous Cell Carcinoma of the Head and Neck. Pathogens, 11(2), 266. https://doi.org/10.3390/pathogens11020266








