Abstract
Staphylococcus aureus is an important pathogen associated with hospital, community, and livestock-acquired infections, with the ability to develop resistance to antibiotics. Nasal carriage by hospital inpatients is a risk for opportunistic infections. Antibiotic susceptibility patterns, virulence genes and genetic population structure of S. aureus nasal isolates, from inpatients at Busia County Referral Hospital (BCRH) were analyzed. A total of 263 inpatients were randomly sampled, from May to July 2015. The majority of inpatients (85.9%) were treated empirically with antimicrobials, including ceftriaxone (65.8%) and metronidazole (49.8%). Thirty S. aureus isolates were cultured from 29 inpatients with a prevalence of 11% (10.3% methicillin-susceptible S. aureus (MSSA), 0.8% methicillin resistant S. aureus (MRSA)). Phenotypic and genotypic resistance was highest to penicillin-G (96.8%), trimethoprim (73.3%), and tetracycline (13.3%) with 20% of isolates classified as multidrug resistant. Virulence genes, Panton-Valentine leukocidin (pvl), toxic shock syndrome toxin-1 (tsst-1), and sasX gene were detected in 16.7%, 23.3% and 3.3% of isolates. Phylogenetic analysis showed 4 predominant clonal complexes CC152, CC8, CC80, and CC508. This study has identified that inpatients of BCRH were carriers of S. aureus harbouring virulence genes and resistance to a range of antibiotics. This may indicate a public health risk to other patients and the community.
1. Introduction
Staphylococcus aureus can asymptomatically colonize healthy individuals, and carriers are at higher risk of a variety of serious infections [1]. Nasal carriage has been associated with nosocomial infections with potential to cause severe morbidity and mortality [2,3]. The pathogen is a common cause of skin and soft tissue infections in humans and animals; it can cause food poisoning and more serious conditions such as pneumonia, endocarditis, osteomyelitis, sepsis, and toxic shock syndrome [4].
S. aureus has a wide range of virulence factors including toxic shock syndrome toxin-1 (TSST-1) encoded by the tsst-1 gene, which is associated with most cases of menstrual Toxic Shock Syndrome and half of non-menstrual Toxic Shock Syndrome cases [5]. A virulent cytotoxic molecule, Panton-Valentine leukocidin encoded by the pvl gene, can cause destruction of leukocytes and tissue necrosis and is often associated with skin and soft tissue infections [6,7]. Some methicillin-resistant S. aureus (MRSA) strains have been shown to harbor a virulence factor sasX, hosted on a mobile genetic element. This factor has been demonstrated in vitro and in vivo (mice) to contribute to colonization and pathogenesis, by significantly promoting nasal colonization, lung disease and abscess formation; it also supports immune system evasion [8]. Virulence factors associated with human diseases can be detected by identifying the encoding genes using molecular methods; however, there is minimal data available on virulence factors present in S. aureus from Kenya.
S. aureus is notorious for its ability to develop resistance to antibiotics, originally to penicillin and methicillin and most recently to linezolid, vancomycin, teicoplanin, and daptomycin [9], with MRSA becoming more common. Some strains have become resistant to more than one class of antibiotics, converting these strains to being multidrug-resistant (MDR) [10]. The emergence of antibiotic resistance, coupled with the shortage of newly developed antibiotics, is a major threat to the health of both humans and animals [11]. Excessive use of antimicrobial empirical therapy in hospital settings has been reported as a contributor to the emergence of bacterial resistance to antimicrobials [12,13]. Antimicrobial-resistant S. aureus infections have reached epidemic levels [14,15], necessitating the study of its global epidemiology [16,17,18].
Clonal complex 5 (CC5) (Pediatric NY Japan- ST5) and CC8 (Iberian Hanover-ST 8, 239, 247, and 250) are the most prevalent lineages of S. aureus globally; others include CC22 (EMRSA-15 Barnum-ST 22), CC30 (EMRSA-16 USA200-ST 36, 30) and CC45 (USA600 Berlin-ST 45) [19,20,21]. In addition, in the last two decades, there have been reports on the spread of livestock-associated clonal complex CC398; this is an emerging problem in many parts of the world [22]. Methicillin-susceptible S. aureus (MSSA) isolated in Africa mainly belong to CC5, CC152, CC30, and CC15, whereas CC8 (ST239 subgroup), CC5 (ST5), and CC30 (ST36) lineages are highly prevalent in Central, West and South Africa [17,23].
The purpose of this study was (1) to assess empirical antibiotic therapy use in patients at Busia County Referral Hospital (BCRH), (2) determine the prevalence of S. aureus isolated from the patients’ nasal swabs and respective antibiotic susceptibility patterns, (3) demonstrate presence of resistance and virulence genes, and (4) identify the S. aureus clones present using a whole-genome sequencing approach.
2. Materials and Methods
2.1. Study Site
The study site was BCRH, the largest health facility in Busia County. Busia County has a total of 81 health facilities, including BCRH, 6 sub-county hospitals, 12 health centers and 49 dispensaries. BCRH has a bed capacity of 160 across 7 wards: pediatrics, male medical, female medical, female surgical and gynecology, male surgical, maternity and a newborn unit. The hospital admits 40–50 patients per day and the average stay is 4 days [24].
2.2. Study Population
The study was conducted between May and July 2015. A total of 263 inpatients participated in the study; this constituted 10% of the 2674 inpatients in BCRH during the study period. This study was based on a cross-sectional random sampling design. Admitted patients who were eighteen years of age and above and able to sign the consent form were included. An assent form for children less than 18 years was signed by a parent or legal guardian. Inpatients must have been admitted for 48 h or longer, and inpatients who had recent nasal surgery or other medical conditions were excluded from the study.
2.3. Sample Collection, Handling, Bacterial Isolation and Identification
All participants were informed of the project objectives and protocol by medical and clinical officers who collected signed informed consent. Nasal samples were collected by rotating a sterile swab five times in both anterior nares, from consenting inpatients. The swabs were inoculated in tryptone soya broth/6% salt and transported in cool boxes to the lab for culturing.
The swabs were streaked on mannitol salt agar (MSA) and incubated at 37 °C overnight. Suspect S. aureus colonies (those that fermented mannitol, producing yellow colonies) were stocked in tryptone soya broth with 10% glycerol and stored at −40 °C; they were later transported on dry ice to Kenya Medical Research Institute (KEMRI) laboratories in Nairobi. The presumptive S. aureus isolates were further cultured onto MSA and repeatedly sub-cultured to get pure culture. The S. aureus isolates were identified using Gram reaction (Gram-positive cocci in clumps), catalase, coagulase (tube method using rabbit plasma) and DNase tests.
When stocking the pure S. aureus growth, a long sweep of the colonies was done to allow preservation of genetic diversity of nasal carriage of the participant. During whole genome sequencing of the sample, if sequences from multiple isolates were detected, these samples were recultured and single colonies selected for sequencing.
2.4. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility was also performed using the VITEK 2 instrument (bioMerieux, Marcy-l’Étoile, France), for benzylpenicillin, cefoxitin, oxacillin, ciprofloxacin, erythromycin, chloramphenicol, daptomycin, fusidic acid, gentamicin, linezolid, mupirocin, nitrofurantoin, rifampicin, teicoplanin, tetracycline, tigecycline, trimethoprim, vancomycin, clindamycin, and inducible resistance to clindamycin. Multi-drug resistant S. aureus were defined as isolates that were resistant to three or more antimicrobials [25,26,27].
2.5. Molecular Genotype Testing
DNA extraction from S. aureus isolates was performed using QIAGEN DNeasy® Blood & Tissue Kit (QIAGEN, Valencia, CA, USA) at KEMRI, Nairobi. Staphylococcal cassette chromosome (SCC) mec typing was performed using previously described methods [28]. Isolates were screened for pvl and tsst-1 genes by PCR using previously described oligonucleotide primers [29,30].
DNA extraction from S. aureus isolates was performed on a QIAcube, using the QIAamp 96 HT kit (QIAGEN). Genomic libraries were generated and sequenced on an Illumina HiSeq 2000 (Illumina Inc., San Diego, CA, USA) at the Wellcome Sanger Institute, Hinxton, UK. Illumina reads were analysed based on the S. aureus MLST database (https://pubmlst.org/organisms/staphylococcus-aureus, accessed on 31 March 2021) [31], analysis of virulence and antimicrobial resistance genes were conducted using the virulence finder database (https://cge.cbs.dtu.dk/services/, accessed on 31 March 2021).
2.6. Genomic Analyses
Paired-end Illumina reads were mapped to the S. aureus reference genome ST22 strain HO 5096 0412 (accession number HE681097) using Snippy v4.6.0 (https://github.com/tseemann/snippy, accessed on 31 October 2022). Whole-genome alignments were created by keeping a version of the reference genome with only substitution variants replaced (i.e., SNPs but not indels) using Snippy’s.consensus.subs.fa output files. The S. aureus species core-genome had been previously derived [32] from a collection of 800 S. aureus from multiple host species [33]. The portion of the reference genome (2.83 Mb) corresponding to the core genome (1.76 Mb) was kept from whole-genome alignments and used to generate maximum likelihood trees using IQ-TREE v1.6.10 with default settings. The resulting core-genome phylogeny was plotted with isolate metadata using ggtree v.3.0.4 [34] and ggtreeExtra v.1.2.3 on R v4.1.0 [35].
2.7. Ethical Approval
The study was approved by the Centre for Microbiology Research Centre Scientific Committee, Kenya Medical Research Institute scientific steering Committee and Ethical Review Committee (SSC No 2944, granted 13 May 2015).
3. Results
3.1. Patient Data
Sampling was conducted between 21 May and 7 July 2015. A total of 263 patients were recruited into the study, 141 females (53.6%) and 122 males (46.4%). The majority of patients were in the surgical ward (119/263, 45.2%), followed by the medical ward (91/263, 34.6%) and the pediatric ward (52/263, 19.8%) and 1 patient on the private ward. The average (mean) time spent in hospital was 8.8 days with a median stay of 5 days, with the longest stay being 103 days. The longest average inpatient stays were on the surgical ward 12.3 days, while average inpatient stays on the medical ward were 5.9 days and pediatric ward 5.8 days.
3.2. Empirical Antibiotic Therapy
A total of 226/263 (85.9%) inpatients were treated empirically using various antimicrobials. Most patients (150/263, 57.0%) received 2 antimicrobials in combination. Ceftriaxone, a third-generation cephalosporin, was the most prescribed antimicrobial in 173/263 (65.8%) inpatients; it was prescribed to 21/53 (39.6%) pediatric, 56/91 (61.5%) medical, and 96/119 (80.7%) surgical patients (Table 1). Metronidazole was the second-most prescribed antimicrobial to 131/263 (49.8%) of inpatients; 14/53 (26.4%) pediatric, 56/91 (24.2%) medical, and 95/119 (79.8%) surgical patients. The combination of ceftriaxone and metronidazole was prescribed to 111/263 (42.2%) patients; predominantly to patients in the surgical ward 84/119 (70.6%). The third-most-prescribed antimicrobial was penicillin-G (21/263, 8%); predominantly to pediatric patients (17/53, 32.1%). The next commonly used antibiotic in pediatric patients was gentamicin (10/53, 18.9%). Ciprofloxacin and erythromycin were less commonly used at 12/263 (4.6%) and 7/263 (2.7%), respectively and predominantly given to medical patients. Four patients were receiving combination therapy rifampin, isoniazid, pyrazinamide, and ethambutol (RHZE) for tuberculosis. Other antimicrobials used less frequently included: sulfamethoxazole/trimethoprim (n = 6), amoxicillin (n = 3), tinidazole (n = 3), clindamycin (n = 3), ampicillin (n = 2), doxycycline (n = 1), clarithromycin (n = 1), norfloxacin (n = 1) and flucloxacillin (n = 1).

Table 1.
Antimicrobials prescribed for inpatients in medical, surgical, and pediatric wards at Busia County Referral Hospital, May–July 2015.
3.3. Staphylococcus aureus (MRSA and MSSA) Isolated from Hospital Patients
Nasal swab samples were collected from 263 patients at BCRH; samples were obtained from inpatients who had been admitted for 48 h or longer. Of these 29 (11.0%) were colonized with S. aureus. One patient was colonized with two different S. aureus sequence types. Male patients (19/122, 15.6%) were more frequently colonized than female patients (10/141, 7.1%). Colonization rates of patients on the different wards were: pediatrics 8/53 (15.1%), medical 7/91 (7.7%) and surgical 14/119 (11.8%).
3.4. Antimicrobial Resistance Profiles of the Isolated Staphylococcus aureus (MRSA and MSSA)
Antimicrobial susceptibility testing was conducted on 30 S. aureus isolates from 29 patients, of which 2 (6.7%) were MRSA. Nearly all S. aureus strains (29/30, 96.7%) were phenotypically resistant to penicillin-G, 22/30 (73.3%) were resistant to trimethoprim, and 4/30 (13.3%) were resistant to tetracycline. There was phenotypic resistance to ciprofloxacin (2/30, 6.7%), clindamycin (6.7%), vancomycin (6.7%), and erythromycin (6.7%), cefoxitin (6.7%), oxacillin (6.7%), and gentamicin (1/30, 3.3%) (Figure 1). All isolates were susceptible to chloramphenicol, daptomycin, fusidic acid, linezolid, mupirocin, nitrofurantoin, rifampicin, teicoplanin and tigecycline.
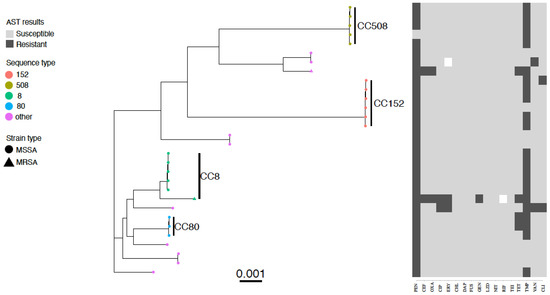
Figure 1.
Core genome phylogenetic tree and antibiogram of MSSA and MRSA isolates colonizing inpatients at Busia County Referral Hospital, Kenya May–July 2015. Predominant ST types are differentiated by colour, MRSA isolates are indicated by a triangle symbol and MSSA by circles. Phenotypic antimicrobial resistance is indicated by dark grey bars (resistant), white bars (intermediate), and light grey (susceptible). Antimicrobials: PEN—benzylpenicillin, CEF—cefoxitin, OXA—oxacillin, CIP—ciprofloxacin, ERY—erythromycin, CHL—chloramphenicol, DAP—daptomycin, FUS—fusidic acid, GEN—gentamicin, LZD- linezolid, NIT—nitrofurantoin, RIF—rifampicin, TEI—teicoplanin, TET—tetracycline, TMP—trimethoprim, VAN—vancomycin, CLI—clindamycin.
Twenty percent (6/30) of S. aureus isolates were phenotypically multidrug-resistant, the majority of which (4/6, 66.7%) were resistant to penicillin-G-trimethoprim-tetracycline (Figure 1). Highly resistant isolates (resistant to more than 5 classes of antibiotics) were also detected, including two MRSA isolates from two inpatients and one MSSA from one inpatient (Figure 1).
Genotypic resistance was detected using specific genes. The proportion of isolates with resistance to beta-lactamase (blaZ) was 30/30 (100%), tetracycline (tetK) 3/30 (10%), tetracycline (tetM) 2/30 (6.7%), trimethoprim (dfrG) 22/30 (73.3%), erythromycin (ermA) 1/30 (3.3%), erythromycin (ermC) 1/30 (3.3%), gentamicin (aacA-aphD) 1/30 (3.3%), and streptomycin (aad9) 1/30 (3.3%). The phenotypic and genotypic resistance patterns were almost 100% in agreement for all isolates.
There was no relationship between carriage of multi-drug resistant S. aureus and antimicrobial use. Of the 27 S. aureus isolates that were isolated from inpatients who had recently received antimicrobials 5 were MDR (18.5%) and of the three S. aureus isolates from inpatients who did not receive antimicrobials one was MDR (33.3%).
3.5. Clonal Complexes of the Staphylococcus aureus Isolates
Analysis of the 30 S. aureus isolates yielded 9 clonal complexes and 13 STs (Table 2). The predominant clonal complexes were CC152, CC8, CC80, and CC508. The predominant STs were ST 152 (n = 5), ST 8 (n = 5) and ST508 (n = 5) (Table 2). Other MSSA sequence types detected were ST5 (n = 1), ST22 (n = 2), ST25 (n = 1), ST80 (n = 3), ST188 (n = 2), ST573 (n = 1), ST580 (n = 2), ST1633 (n = 1). The MRSA sequence types were ST140 and a new sequence type ST241.

Table 2.
Sequence types, Clonal complexes, and presence of Panton Valentine leukocidin (pvl) and Toxic shock syndrome toxin {tsst-1} genes in Staphylococcus aureus strains recovered from nasal samples of patients admitted at Busia County Referral Hospital, Kenya, May–July 2015 (n = 30). * Virulence-associated sasX gene.
3.6. Virulence Factors in the Isolated Staphylococcus aureus
Six of 30 isolates were positive for the pvl gene (20%), associated with 3 sequence types (STs). The pvl-positive isolates belonged to ST152 (n = 4), ST5 (n = 1), and ST1633 (n = 1) (Table 2). Another 7 isolates tested positive for the tsst-1 gene (23.3%); these belonged to ST508 (n = 5), and ST22 (n = 2). SasX virulence gene was detected in a single MRSA ST241.
3.7. MRSA Isolates
One strain was MRSA ST 241, a single-locus variant of ST239 carrying associated SCCmec type III and previously associated with hospital acquired infection (33). This was isolated from a female patient who had stayed in the hospital for 7 days, the variant exhibited multiple phenotypic drug resistance to penicillin, cefoxitin, oxacillin, ciprofloxacin, erythromycin, gentamicin, tetracycline and trimethoprim, and genotypic resistance to beta-lactamase (blaZ), cefoxitin (mecA), tetracyclin (tetK/tetM), trimethoprim (dfrG), erythromycin (ermA), gentamicin (aacA-aphD) and streptomycin/spectinomycin (aad9). This strain also had a sasX gene.
The other strain was MRSA ST140 strain harboring SCCmec type IV cassette with phenotypic resistance to penicillin, cefoxitin, oxacillin, tetracycline and trimethoprim and hosting resistance genes to beta-lactamase (blaZ), cefoxitin (mecA), tetracycline (tetM), and trimethoprim (dfrG). This was isolated an adult male patient who had stayed in the surgical ward for 6 days, the strain has previously been associated with hospital transmission (34).
4. Discussion
In this study, 263 inpatients were screened for S. aureus nasal carriage; the overall carriage prevalence was 11.0%. The nasal carriage rate was slightly higher than that reported by a similar study conducted in a mid-sized government hospital in peri-urban Kenya, where the nasal carriage rate of S. aureus was 8.9% [36]. The prevalence reported in this study is lower than studies in other regions of Africa: 14% in Accra, Ghana, 37% in Bobo Dioulasso, Burkina Faso, and 29% in Uganda [37,38,39]. Other regions with higher nasal carriage rates include the Netherlands (26%), and United States (24%) [40,41]. The reduced prevalence of S. aureus carriage may be due to the sampling and isolation methods used. Previous research has indicated that the type of swab and the culture media (mannitol salt agar) may reduce the isolation of S. aureus [42,43].
Empirical therapy was found to be administered to the majority of inpatients; the most commonly used antimicrobials being ceftriaxone and metronidazole. The pattern of antimicrobial use is consistent with previous research in hospitals in Kenya [44,45], and the proportion of patients receiving antibiotics in this study (85.9%) was similar to a report of 80% in a study by Maina et al., 2020 [46]. Empirical antibiotic treatment has been linked to increased prevalence of infections with MDR bacterial pathogens. These pathogens pose a considerable threat to global public health, especially in hospitals like BCRH, where they could be the driving force behind increased antimicrobial resistance towards commonly prescribed empirical antimicrobial therapy. This limits treatment options, especially when considering the slow rate of new antibiotic discoveries [47].
This study identified S. aureus isolates carrying genes encoding for 3 virulence factors (pvl, tsst-1 and sasX). Busia County might be considered a pvl-endemic region since the prevalence estimate (20%) is consistent with pvl-positivity rates ranging from 17% to 74% reported in other parts of Africa [48,49,50]. Another virulence factor gene, tsst-1, was detected in almost one quarter of the S. aureus isolates, which is consistent with reports that tsst-1 is present in between 5 to 25% of S. aureus strains [51]. The report of an MRSA isolate carrying the sasX virulence gene, in this study, is the first in Africa; indicating the high chances that this strain is present in other nosocomial MRSA strains in Africa. The sasX virulence gene plays an important role in the organism’s colonization and pathogenesis, by significantly promoting nasal colonization, lung disease and abscess formation; it also supports immune system evasion [8].
This study has revealed that the majority of S. aureus strains were susceptible to linezolid, clindamycin, gentamicin, ciprofloxacin, and erythromycin; similar results have been reported in other studies in Kenya [52,53]. Nearly all S. aureus strains (96.7%) were resistant to penicillin G, 73.3% were resistant to trimethoprim and 13.3% were resistant to tetracycline. Previous studies in Kenya have reported high S. aureus antimicrobial resistance to penicillin, trimethoprim and tetracycline [36,54,55]. The proportion of S. aureus isolates that were identified as MRSA (6.7%) was consistent with other studies in Kenya 7.0% [36] and elsewhere: 9.5% in Ghana [38] and 9.7% in Uganda [37]. We did not detect an association between antimicrobial use and MDR S. aureus in this study which may be due to the small number of samples. Future research may focus on the source and evolution of AMR bacteria in this ecosystem.
Two MDR MRSA strains were identified and a MDR MSSA strain. The presence of antibiotic resistant pathogenic bacteria hosting resistant genes may promote transmission of genes to other pathogens, leading to more resistant pathogens and emergence of multi drug resistant strains [56,57]. The widespread use of empirical antimicrobial therapy in BCRH is likely to drive the selection of the small fraction of MDR resistant strains that exist in the hospital setting microbiota, by exerting selective pressure on susceptible microorganisms, thus giving a survival advantage to resistant strains. This compounds the problem of antimicrobial resistance in this region [58] and may render antibiotics ineffective, narrowing the therapeutic value of available antibiotics in the hospital [59].
The two MDR MRSA strains were ST241 and ST140. ST241 has previously been detected in a Nigerian hospital and ST140 in a health care institution in Angola [60], inferring a possibility of international transmission of these strains into Busia County or vice versa.
The population structure of MSSA has been reported to be more diverse than that of MRSA globally [19]. High clonal diversity among MSSA isolates was observed in this study. Methicillin-sensitive S. aureus clonal complex 152 (ST152 and ST1633) was the predominant lineage; with the majority of them harboring pvl gene (83%). This clonal complex has been reported to be endemic in Africa and the Caribbean, as opposed to Europe [61,62]. Other commonly identified clonal complexes in Busia were CC8, CC80, and CC508. A recent review indicated that reported clonal complexes in Kenya are CC5, CC7, CC8, CC22, CC88 and CC152 [63]. Previous research by these authors demonstrated S. aureus isolates from abattoir workers in western Kenya were predominantly CC152 and CC8 [64].
At the time of this research there was no information regarding the circulating S. aureus strains in animals in Kenya. The role of animals in the epidemiology of MSSA and MRSA has been highlighted in other regions with the documentation of MRSA and MDR MSSA in animals [65,66], and transmission to people both with and without animal contact [67,68]. The unregulated use of antimicrobials in animal and human medicine may result in increased drug resistance posing a threat to public health. Increased surveillance is required to understand the epidemiology of AMR bacteria in this region to develop appropriate integrated control strategies.
5. Conclusions
This study has identified inpatients as important reservoirs of S. aureus organisms which are resistant to a range of antimicrobials, including MDR S. aureus; MRSA included. Some of these strains also harbor disease causing virulence factors. Nasal carriage of MRSA and MSSA is a potential reservoir of nosocomial infections for susceptible patients in hospitals, with a potential to cause severe morbidity and mortality. Data from the genetic structure of S. aureus, from the inpatients of Busia County contributes to knowledge on molecular epidemiology of S. aureus in this region and may be useful when developing prevention and containment measures.
Author Contributions
Conceptualization, B.A.O.; Data curation, B.A.O., E.A.J.C., D.W., F.C. and E.M.H.; Formal analysis, B.A.O., E.A.J.C., R.N., D.W., J.M., B.B., F.C. and E.M.H.; Funding acquisition, E.M.F., S.-H.W., W.G. and S.J.P.; Investigation, B.A.O., R.N., D.W., J.M. and B.B.; Methodology, B.A.O.; Supervision, L.B., W.O., S.-H.W., W.G. and G.C.G.; Writing—original draft, B.A.O.; Writing—review and editing, E.A.J.C., E.M.H. and S.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
Benear Obanda’s research was supported in part by One Health Eastern Africa Research Training (OHEART) program, at the Ohio State University, Global One Health initiative (GOHi) through the financial support from National Institutes of Health (NIH) Fogarty International Center (grant number D43TW008650) awarded to GW and SHW. A Wellcome Trust grant (085308) was awarded to EMF and supported the People and their Zoonoses project (PAZ). Support was received from the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH), led by the International Food Policy Research Institute (IFPRI). We acknowledge the CGIAR Fund Donors (https://www.cgiar.org/funders/, accessed on 31 October 2022). This work was also supported by the Health Innovation Challenge Fund (WT098600, HICF-T5-342), a parallel funding partnership between the Department of Health and Wellcome Trust. The views expressed in this publication are those of the author(s) and not necessarily those of the Department of Health or Wellcome Trust and The APC was funded by the University of Liverpool.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Centre for Microbiology Research Centre Scientific Committee, Kenya Medical Research Institute scientific steering Committee and Ethical Review Committee (SSC No 2944 granted on 13 May 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Acknowledgments
Thank you to the Samuel Kariuki for his support and to the participants for their time.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Smith, T.C. Livestock-associated Staphylococcus aureus: The United States experience. PLoS Pathog. 2015, 11, e1004564. [Google Scholar] [CrossRef] [PubMed]
- El Aila, N.A.; Al Laham, N.A.; Ayesh, B.M. Nasal carriage of methicillin resistant Staphylococcus aureus among health care workers at Al Shifa hospital in Gaza Strip. BMC Infect. Dis. 2017, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Azeez, A. Global trend of Methicillin-resistant Staphlococcus aureus and emerging challenges for control. Afr. J. Clin. Exp. Microbiol. 2010, 11, 150–158. [Google Scholar] [CrossRef][Green Version]
- Reddy, P.N.; Srirama, K.; Dirisala, V.R. An Update on Clinical Burden, Diagnostic Tools, and Therapeutic Options of Staphylococcus aureus. Infect. Dis. 2017, 10, 1179916117703999. [Google Scholar] [CrossRef]
- McCormick, J.K.; Yarwood, J.M.; Schlievert, P.M. Toxic shock syndrome and bacterial superantigens: An update. Annu. Rev. Microbiol. 2001, 55, 77–104. [Google Scholar] [CrossRef]
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef]
- McGrath, B.; Rutledge, F.; Broadfield, E. Necrotising Pneumonia, Staphylococcus aureus and Panton-Valentine Leukocidin. J. Intensive Care Soc. 2008, 9, 170–172. [Google Scholar] [CrossRef]
- Li, M.; Du, X.; Villaruz, A.E.; Diep, B.A.; Wang, D.; Song, Y.; Tian, Y.; Hu, J.; Yu, F.; Lu, Y.; et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat. Med. 2012, 18, 816–819. [Google Scholar] [CrossRef]
- Kaur, D.C.; Chate, S.S. Study of Antibiotic Resistance Pattern in Methicillin Resistant Staphylococcus aureus with Special Reference to Newer Antibiotic. J. Glob. Infect. Dis. 2015, 7, 78–84. [Google Scholar] [CrossRef]
- Malhotra-Kumar, S.; Haccuria, K.; Michiels, M.; Ieven, M.; Poyart, C.; Hryniewicz, W.; Goossens, H. Current trends in rapid diagnostics for methicillin-resistant Staphylococcus aureus and glycopeptide-resistant enterococcus species. J. Clin. Microbiol. 2008, 46, 1577–1587. [Google Scholar] [CrossRef]
- Cheng, G.; Dai, M.; Ahmed, S.; Hao, H.; Wang, X.; Yuan, Z. Antimicrobial Drugs in Fighting against Antimicrobial Resistance. Front. Microbiol. 2016, 7, 470. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Rice, L.B. Empirical antibiotic choice for the seriously ill patient: Are minimization of selection of resistant organisms and maximization of individual outcome mutually exclusive? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2003, 36, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.; Aires-de-Sousa, M.; Boyce, J.; Tiemersma, E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006, 368, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, A.-C.; Otto, M.; Lowy, F.D.; DeLeo, F.R. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2014, 21, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Huang, Y.-C. New epidemiology of Staphylococcus aureus infection in Asia. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Schaumburg, F.; Alabi, A.S.; Peters, G.; Becker, K. New epidemiology of Staphylococcus aureus infection in Africa. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, 589–596. [Google Scholar] [CrossRef]
- Rasigade, J.-P.; Dumitrescu, O.; Lina, G. New epidemiology of Staphylococcus aureus infections. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, 587–588. [Google Scholar] [CrossRef]
- Deurenberg, R.H.; Vink, C.; Kalenic, S.; Friedrich, A.W.; Bruggeman, C.A.; Stobberingh, E.E. The molecular evolution of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2007, 13, 222–235. [Google Scholar] [CrossRef]
- Denis, O.; Deplano, A.; Nonhoff, C.; De Ryck, R.; de Mendonça, R.; Rottiers, S.; Vanhoof, R.; Struelens, M.J. National surveillance of methicillin-resistant Staphylococcus aureus in Belgian hospitals indicates rapid diversification of epidemic clones. Antimicrob. Agents Chemother. 2004, 48, 3625–3629. [Google Scholar] [CrossRef]
- Aires-de-Sousa, M.; Correia, B.; de Lencastre, H. Changing patterns in frequency of recovery of five methicillin-resistant Staphylococcus aureus clones in Portuguese hospitals: Surveillance over a 16-year period. J. Clin. Microbiol. 2008, 46, 2912–2917. [Google Scholar] [CrossRef]
- Anjum, M.F.; Marco-Jimenez, F.; Duncan, D.; Marín, C.; Smith, R.P.; Evans, S.J. Livestock-Associated Methicillin-Resistant Staphylococcus aureus From Animals and Animal Products in the UK. Front. Microbiol. 2019, 10, 2136. [Google Scholar] [CrossRef] [PubMed]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.O. Assessment of patient satisfaction levels in a County Referral Hospital: A case of County Referral Hospital. Baraton Interdiscip. Res. J. 2017, 7, 1–7. [Google Scholar]
- Wang, M.; Wei, H.; Zhao, Y.; Shang, L.; Di, L.; Lyu, C.; Liu, J. Analysis of multidrug-resistant bacteria in 3223 patients with hospital-acquired infections (HAI) from a tertiary general hospital in China. Bosn. J. Basic Med. Sci. 2019, 19, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Koletsi, P.K.; Bliziotis, I.A. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J. Med. Microbiol. 2006, 55, 1619–1629. [Google Scholar] [CrossRef]
- Kondo, Y.; Ito, T.; Ma, X.X.; Watanabe, S.; Kreiswirth, B.N.; Etienne, J.; Hiramatsu, K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: Rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 2007, 51, 264–274. [Google Scholar] [CrossRef]
- Lina, G.; Piémont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.-O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef]
- Jarraud, S.; Mougel, C.; Thioulouse, J.; Lina, G.; Meugnier, H.; Forey, F.; Nesme, X.; Etienne, J.; Vandenesch, F. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Coll, F.; Raven, K.E.; Knight, G.M.; Blane, B.; Harrison, E.M.; Leek, D.; Enoch, D.A.; Brown, N.M.; Parkhill, J.; Peacock, S.J. Definition of a genetic relatedness cutoff to exclude recent transmission of meticillin-resistant Staphylococcus aureus: A genomic epidemiology analysis. Lancet Microbe 2020, 1, e328–e335. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.J.; Bacigalupe, R.; Harrison, E.; Weinert, L.A.; Lycett, S.; Vrieling, M.; Robb, K.; Hoskisson, P.A.; Holden, M.T.G.; Feil, E.J.; et al. Gene exchange drives the ecological success of a multi-host bacterial pathogen. Nature Ecol. Evol. 2018, 2, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Yu, G. Using ggtree to Visualize Data on Tree-Like Structures. Curr. Protoc. Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Dai, Z.; Guo, P.; Fu, X.; Liu, S.; Zhou, L.; Tang, W.; Feng, T.; Chen, M.; Zhan, L.; et al. GgtreeExtra: Compact Visualization of Richly Annotated Phylogenetic Data. Mol. Biol. Evol. 2021, 38, 4039–4042. [Google Scholar] [CrossRef]
- Aiken, A.M.; Mutuku, I.M.; Sabat, A.J.; Akkerboom, V.; Mwangi, J.; Scott, J.A.G.; Morpeth, S.C.; Friedrich, A.W.; Grundmann, H. Carriage of Staphylococcus aureus in Thika Level 5 Hospital, Kenya: A cross-sectional study. Antimicrob. Resist. Infect. Control. 2014, 3, 22. [Google Scholar] [CrossRef]
- Bebell, L.M.; Ayebare, A.; Boum, Y.; Siedner, M.J.; Bazira, J.; Schiff, S.J.; Metlay, J.P.; Bangsberg, D.R.; Ttendo, S.; Firth, P.G. Prevalence and correlates of MRSA and MSSA nasal carriage at a Ugandan regional referral hospital. J. Antimicrob. Chemother. 2017, 72, 888–892. [Google Scholar] [CrossRef][Green Version]
- Egyir, B.; Guardabassi, L.; Sørum, M.; Nielsen, S.S.; Kolekang, A.; Frimpong, E.; Addo, K.K.; Newman, M.J.; Larsen, A.R. Molecular epidemiology and antimicrobial susceptibility of clinical Staphylococcus aureus from healthcare institutions in Ghana. PLoS ONE 2014, 9, e89716. [Google Scholar] [CrossRef]
- Ouedraogo, A.-S.; Dunyach-Remy, C.; Kissou, A.; Sanou, S.; Poda, A.; Kyelem, C.G.; Solassol, J.; Bañuls, A.-L.; Van De Perre, P.; Ouédraogo, R.; et al. High nasal carriage rate of Staphylococcus aureus containing panton-valentine leukocidin- and EDIN-encoding genes in community and hospital settings in Burkina Faso. Front. Microbiol. 2016, 7, 1406. [Google Scholar] [CrossRef]
- Weterings, V.; Veenemans, J.; van Rijen, M.; Kluytmans, J. Prevalence of nasal carriage of methicillin-resistant Staphylococcus aureus in patients at hospital admission in The Netherlands, 2010-2017, an observational study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2019, 25, e1–e1428. [Google Scholar] [CrossRef]
- Hidron, A.I.; Kourbatova, E.V.; Halvosa, J.S.; Terrell, B.J.; McDougal, L.K.; Tenover, F.C.; Blumberg, H.M.; King, M.D. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: Emergence of community-associated MRSA nasal carriage. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41, 159–166. [Google Scholar] [CrossRef]
- Han, Z.; Lautenbach, E.; Fishman, N.; Nachamkin, I. Evaluation of mannitol salt agar, CHROMagar Staph aureus and CHROMagar MRSA for detection of meticillin-resistant Staphylococcus aureus from nasal swab specimens. J. Med. Microbiol. 2007, 56, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, P.; Grattard, F.; Carricajo, A.; Pozzetto, B.; Berthelot, P. Better Detection of Staphylococcus aureus Nasal Carriage by Use of Nylon Flocked Swabs. J. Clin. Microbiol. 2010, 48, 4242–4244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mulwa, N.; Osanjo, G.; Ndwigah, S.; Kaburi, A.; Muriuki, G. Patterns of Prescribing Practices in Makueni County Referral Hospital, Kenya. Afr. J. Pharmacol. Ther. 2015, 4, 161–168. [Google Scholar]
- Okoth, C.; Opanga, S.; Okalebo, F.; Oluka, M.; Baker Kurdi, A.; Godman, B. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: Findings and implications. Pract. 2018, 46, 128–136. [Google Scholar] [CrossRef]
- Maina, M.; Mwaniki, P.; Odira, E.; Kiko, N.; McKnight, J.; Schultsz, C.; English, M.; Tosas-Auguet, O. Antibiotic use in Kenyan public hospitals: Prevalence, appropriateness and link to guideline availability. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 99, 10–18. [Google Scholar] [CrossRef]
- Paramythiotou, E.; Routsi, C. Association between infections caused by multidrug-resistant gram-negative bacteria and mortality in critically ill patients. World J. Crit. Care Med. 2016, 5, 111–120. [Google Scholar] [CrossRef]
- Oosthuysen, W.F.; Orth, H.; Lombard, C.J.; Sinha, B.; Wasserman, E. Population structure analyses of Staphylococcus aureus at Tygerberg Hospital, South Africa, reveals a diverse population, a high prevalence of Panton-Valentine leukocidin genes, and unique local methicillin-resistant S. aureus clones. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, 652–659. [Google Scholar] [CrossRef][Green Version]
- Breurec, S.; Zriouil, S.; Fall, C.; Boisier, P.; Brisse, S.; Djibo, S.; Etienne, J.; Fonkoua, M.; Perrier-Gros-Claude, J.; Pouillot, R.; et al. Epidemiology of methicillin-resistant Staphylococcus aureus lineages in five major African towns: Emergence and spread of atypical clones. Clin. Microbiol. Infect. 2011, 17, 160–165. [Google Scholar] [CrossRef]
- Schaumburg, F.; Ngoa, U.A.; Sters, K.K.; Köck, R.; Adegnika, A.; Kremsner, P.; Lell, B.; Peters, G.; Mellmann, A.; Becker, K. Virulence factors and genotypes of Staphylococcus aureus from infection and carriage in Gabon. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2011, 17, 1507–1513. [Google Scholar] [CrossRef]
- Zumla, A.; Superantigens, T. Cells, and Microbes. Clin. Infect. Dis. 1992, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kyany’A, C.; Nyasinga, J.; Matano, D.; Oundo, V.; Wacira, S.; Sang, W.; Musila, L. Phenotypic and genotypic characterization of clinical Staphylococcus aureus isolates from Kenya. BMC Microbiol. 2019, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Gitau, W.; Masika, M.; Musyoki, M.; Museve, B.; Mutwiri, T. Antimicrobial susceptibility pattern of Staphylococcus aureus isolates from clinical specimens at Kenyatta National Hospital. BMC Res. Notes 2018, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Omuse, G.; Kariuki, S.; Revathi, G. Unexpected absence of meticillin-resistant Staphylococcus aureus nasal carriage by healthcare workers in a tertiary hospital in Kenya. J. Hosp. Infect. 2012, 80, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Maina, E.K.; Kiiyukia, C.; Wamae, C.N.; Waiyaki, P.G.; Kariuki, S. Characterization of methicillin-resistant Staphylococcus aureus from skin and soft tissue infections in patients in Nairobi, Kenya. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2013, 17, e115–e119. [Google Scholar] [CrossRef][Green Version]
- van Hoek, A.H.A.M.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J.M. Acquired antibiotic resistance genes: An overview. Front. Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef]
- Avershina, E.; Shapovalova, V.; Shipulin, G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front. Microbiol. 2021, 12, 707330. [Google Scholar] [CrossRef]
- Karam, G.; Chastre, J.; Wilcox, M.H.; Vincent, J.-L. Antibiotic strategises in the era of multidrug resistance. Crit. Care 2016, 20, 136. [Google Scholar] [CrossRef]
- Mehl, A.; Åsvold, B.O.; Kümmel, A.; Lydersen, S.; Paulsen, J.; Haugan, I.; Solligård, E.; Damås, J.K.; Harthug, S.; Edna, T.-H. Trends in antimicrobial resistance and empiric antibiotic therapy of bloodstream infections at a general hospital in Mid-Norway: A prospective observational study. BMC Infect. Dis. 2017, 17, 116. [Google Scholar] [CrossRef]
- Aires-de-Sousa, M.; Rodrigues, S.; Conceição, T.; de Lencastre, H. Evaluation of Different Screening Methodologies for the Detection of Methicillin-Resistant Staphylococcus aureus from Environmental Surfaces: Swabs, Gauzes, and Polywipes. Microb. Drug Resist. 2018, 24, 585–589. [Google Scholar] [CrossRef]
- Sowash, M.G.; Uhlemann, A.-C. Community-associated methicillin-resistant Staphylococcus aureus case studies. Methods Mol. Biol. 1085, 25–69. [Google Scholar] [CrossRef]
- Ruimy, R.; Maiga, A.; Armand-Lefevre, L.; Maiga, I.; Diallo, A.; Koumaré, A.K.; Ouattara, K.; Soumaré, S.; Gaillard, K.; Lucet, J.-C.; et al. The carriage population of Staphylococcus aureus from Mali is composed of a combination of pandemic clones and the divergent Panton-Valentine leukocidin-positive genotype ST152. J. Bacteriol. 2008, 190, 3962–3968. [Google Scholar] [CrossRef]
- Lawal, O.U.; Ayobami, O.; Abouelfetouh, A.; Mourabit, N.; Kaba, M.; Egyir, B.; Abdulgader, S.M.; Shittu, A.O. A 6-Year Update on the Diversity of Methicillin-Resistant Staphylococcus aureus Clones in Africa: A Systematic Review. Front. Microbiol. 2022, 13, 860436. [Google Scholar] [CrossRef] [PubMed]
- OObanda, B.A.; Gibbons, C.L.; Fèvre, E.M.; Bebora, L.; Gitao, G.; Ogara, W.; Wang, S.-H.; Gebreyes, W.; Ngetich, R.; Blane, B.; et al. Multi-Drug Resistant Staphylococcus aureus Carriage in Abattoir Workers in Busia, Kenya. Antibiotics 2022, 11, 1726. [Google Scholar] [CrossRef]
- Kalee, N.E.; Gahamanyi, N.; Hoza, A.S. Prevalence and antimicrobial susceptibility profiles of Staphylococcus aureus from raw bovine milk in dairy and pastoral farms in Morogoro region, Tanzania. Ger. J. Vet. Res. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Benrabia, I.; Hamdi, T.M.; Shehata, A.A.; Neubauer, H.; Wareth, G. Methicillin-resistant Staphylococcus aureus (MRSA) in poultry species in algeria: Long-term study on prevalence and antimicrobial resistance. Vet. Sci. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Petersen, A.; Sørum, M.; Stegger, M.; van Alphen, L.; Valentiner-Branth, P.; Knudsen, L.K.; Larsen, L.S.; Feingold, B.; Price, L.B.; et al. Meticillin-resistant Staphylococcus aureus CC398 is an increasing cause of disease in people with no livestock contact in Denmark, 1999 to 2011. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2015, 20, 30021. [Google Scholar] [CrossRef]
- Pantosti, A. Methicillin-resistant Staphylococcus aureus associated with animals and its relevance to human health. Front. Microbiol. 2012, 3, 127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).