Preparation and Characterization of Plasters with Photodegradative Action
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
- Identification of the S/A ratio optimal (S = base mortar, A = water) according to the different quantities of additives, through compression resistance tests.
- Characterization of systems with only one additive.
- Characterization of systems with multiple additives.
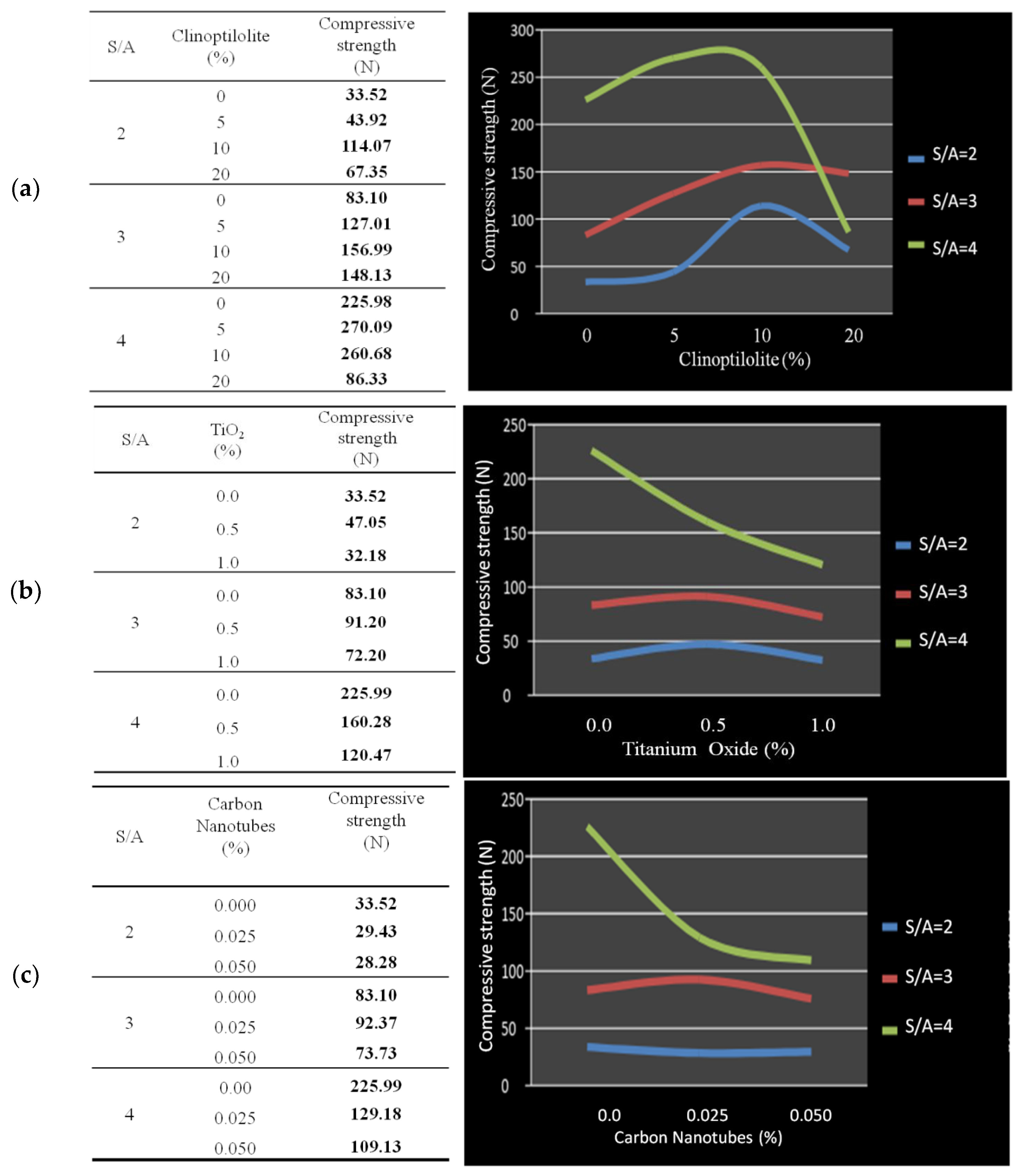
3.1. Identification of the S/A Ratio and the Quantity of Optimal Additives
3.2 Characterization of Systems with a Single Additive
3.2.1. Colorimetric Tests of Photoactivity
3.2.2. Water Absorption Coefficient
3.2.3 Determination of the Tear Adhesion Strength
3.3. Characterization of Systems With Multiple Additives
3.3.1. Compressive Strength
3.3.2. Colorimetric Tests of Photoactivity
3.3.3. Water Absorption Coefficient
3.3.4. Determination of Tear Adhesion Force
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franzoni, E. Materials selection for green buildings: Which tools for engineers and architects? Procedia Eng. 2001, 21, 883–890. [Google Scholar] [CrossRef]
- Scofield, J.H. Letters: Green building technology. Environ. Sci. Technol. 2000, 34, 448A. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Carbone, I.; Nagy, B.J. Green building materials: A review of state of the art studies of innovative materials. J. Green Build. 2017, 12, 141–161. [Google Scholar] [CrossRef]
- Warren, L.P.; Peter, A.T. A comparison of occupant comfort and satisfaction between a green building and a conventional building. Build. Environ. 2008, 43, 1858–1870. [Google Scholar]
- Ljungberg, L.Y. Materials selection and design for development of sustainable products. Mater. Des. 2007, 28, 466–479. [Google Scholar] [CrossRef]
- Govindan, K.; Madan Shankar, K.; Kannan, D. Sustainable material selection for construction industry—A hybrid multi criteria decision making approach. Renew. Sustain. Energy Rev. 2016, 55, 1274–1288. [Google Scholar] [CrossRef]
- Magnone, G.; De Luca, P.; Salituro, A.; Cosenza, M.G. Assessment of reduction strategies for the mitigation of the environmental impact of the primary packaging within the large scale retail trade. Procedia Environ. Sci. Eng. Manag. 2014, 1, 93–97. [Google Scholar]
- Jahan, A.; Ismail, M.Y.; Mustapha, F.; Sapuan, S.M. Material selection based on ordinal data. Mater. Des. 2010, 31, 3180–3187. [Google Scholar] [CrossRef]
- Jahan, A.; Ismail, M.Y.; Sapuan, S.M.; Mustapha, F. Material screening and choosing methods—a review. Mater. Des. 2010, 31, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Norhidayah, A.; Lee, C.K.; Azhar, M.K.; Nurulwahida, S. Indoor air quality and sick building syndrome in three selected buildings. Procedia Eng. 2013, 53, 93–98. [Google Scholar] [CrossRef]
- Stolwijsk, J.A.J. Sick building syndrome. Environ. Health Perspect. 1991, 95, 99–100. [Google Scholar]
- Redlich, C.A.; Spare, J.; Cullen, M.R. Sick-building syndrome. Lancet 1997, 349, 1013–1016. [Google Scholar] [CrossRef]
- Cooley, J.D.; Woong, W.C.; Jumper, C.A.; Straus, D.C. Correlation between the prevalence of certain fungi and sick building syndrome. Occup. Environ. Med. 1998, 55, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apter, A.; Bracker, A.; Hodgson, M.; Sidman, J.; Wing-Yan Leung, B.S. Epidemiology of the sick building syndrome. J. Allergy Clin. Immun. 1998, 94, 277–288. [Google Scholar] [CrossRef]
- Hodgson, M.; Levin, H.; Wolkoff, P. Volatile organic compounds and indoor air. J. Allergy Clin. Immun. 1994, 94, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.D.; Grédiac, M.; Michaud, P. Bio-based adhesives. In Biopolymers and Biotech Admixtures for Eco-efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Elsevier Science and Technology: Cambridge, UK, 19 February 2016; ISBN 978-0-08-100214-8. [Google Scholar]
- De Luca, P.; Roberto, B.; Vuono, D.; Siciliano, C.; Nagy, J.B. Preparation and optimization of natural glues based on Laricio pine resin. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012071. [Google Scholar] [CrossRef]
- De Luca, P.; Pane, L.; Vuono, D.; Siciliano, C.; Candamano, S.; Nagy, J.B. Preparation and characterization of natural glues with carbon nanotubes. Environ. Eng. Manag. J. 2017, 16, 1659–1672. [Google Scholar] [CrossRef]
- Packham, D.E. Adhesive technology and sustainability. Int. J. Adhes. Adhes. 2009, 29, 248–252. [Google Scholar] [CrossRef]
- Ding, G.K.C. Sustainable construction—The role of environmental assessment tools. J. Environ. Manag. 2008, 86, 451–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatarama Reddy, B.V. Sustainable materials for low carbon buildings. Int. J. Low-Carbon Technol. 2009, 4, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable bio-composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Sudin, R.; Swamy, N. Bamboo and wood fibre cement composites for sustainable infrastructure regeneration. J. Mater. Sci. 2006, 41, 6917–6924. [Google Scholar] [CrossRef]
- Nastro, V.; Vuono, D.; Guzzo, M.; Niceforo, G.; Bruno, I.; De Luca, P. Characterisation of raw materials for production of ceramics. J. Therm. Anal. Calorim. 2006, 84, 181–184. [Google Scholar] [CrossRef]
- Chi-Liang, Y.; Dyi-Hwa, T.; Tung-Tsan, L. Characterization of eco-cement paste produced from waste sludges. Chemosphere 2011, 84, 220–226. [Google Scholar]
- Yiming, L.; Shaoqi, Z.; Fuzhen, L.; Yixiao, L. Utilization of municipal sewage sludge as additives for the production of eco-cement. J. Hazard. Mater. 2012, 30, 457–465. [Google Scholar]
- Kizinievič, O.; Žurauskienė, R.; Kizinievič, V.; Žurauskas, R. Utilisation of sludge waste from water treatment for ceramic products. Constr. Build. Mater. 2013, 41, 464–473. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Ou, J. Light-Emitting Concrete. In Smart and Multifunctional Concrete toward Sustainable Infrastructures; Springer: Gateway East, Singapore, 12 June 2017. [Google Scholar]
- De Luca, P.; Chiodo, A.; Nagy, J.B. Activated ceramic materials with deposition of photocatalytic titano-silicate micro-crystals. Sustain. Chem. 2011, 154, 155–165. [Google Scholar]
- Husken, G.; Hunger, M.; Brouwers, H.J.H. Experimental study of photocatalytic concrete products for air purification. Build. Environ. 2009, 44, 2463–2474. [Google Scholar] [CrossRef]
- Ballari, M.M.; Hunger, M.; Husken, G.; Brouwers, H.J.H. NOx photocatalytic degradation employing concrete pavement containing titanium dioxide. Appl. Catal. B-Environ. 2010, 95, 245–254. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A.; Dirkx, I.; Bams, V. Durability of cementitious photocatalytic building materials. Catal. Today 2017, 287, 196–202. [Google Scholar] [CrossRef]
- Chuck, W.F.Y.; Jeong, T.K. Building Pathology, Investigation of Sick Buildings—VOC Emissions. Indoor Built Environ. 2010, 12, 30–39. [Google Scholar]
- Englert, N. Fine particles and human health—A review of epidemiological studies. Toxicol. Lett. 2004, 149, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Marcazzan, G.M.; Valli, G.; Vecchi, R. Factors influencing mass concentration and chemical composition of fine aerosols during a PM high pollution episode. Sci. Total Environ. 2002, 298, 65–79. [Google Scholar] [CrossRef]
- Filice, M.; De Luca, P.; Guido, G.P. Particular matter pollution in university area: Traffic flow analysis. Environ. Eng. Manag. J. 2009, 8, 1407–1412. [Google Scholar] [CrossRef]
- Pekkanen, J.; Timonen, K.L.; Ruuskanen, J.; Reponen, A.; Mirme, A. Effects of ultrafine and fine particles in urban air on peak expiratory flow among children with asthmatic symptoms. Environ. Res. 1997, 74, 24–33. [Google Scholar]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Di Paola, A.; Garcia-Lopez, E.; Marci, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, Z.; Zhang, J.; Zhang, B. Application of TiO2 photocatalyst to the stone conservation. Mater. Res. Innov. 2015, 19, 51–54. [Google Scholar] [CrossRef]
- Reddy, V.R.; Hwang, D.W.; Lee, J.S. Photocatalytic water splitting over ZrO2 prepared by precipitation method. Korean J. Chem. 2003, 20, 1026–1029. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, S.; Maity, J.; Mukherjee, R. Synthesis and characterization of ZnO nanoparticles using moringa oleifera leaf extract: Investigation of photocatalytic and antibacterial activity. Int. J. Nanosci. Nanotechnol. 2018, 14, 111–119. [Google Scholar]
- Ghosh, M.; Biswas, K.; Sundaresan, A.; Rao, C.N.R. MnO and NiO nanoparticles: Synthesis and magnetic properties. J. Mater Chem. 2006, 16, 106–111. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, L.M.; Cozzoli, D.P.; Mascolo, G.; Agostiano, A. UV induced photocatalytic degradation of azo dyes by organic-capped ZnO nanocrystals immobilized onto substrates. Appl. Catal. B Environ. 2005, 60, 1–11. [Google Scholar] [CrossRef]
- Zhai, J.; Tao, X.; Pu, Y.; Zeng, F.X.; Chen, F.X. Core/shell structured ZnO/SiO2 nanoparticles: Preparation, characterization and photocatalytic property. Appl. Surface Sci. 2010, 257, 393–397. [Google Scholar] [CrossRef]
- Ramirez, A.M.; Demeestere, K.; De Belie, N.; Mantyla, T.; Levanen, E. Titanium dioxide coated cementitious materials for air purifying purposes: Preparation, characterization and toluene removal potential. Build. Environ. 2010, 45, 832–838. [Google Scholar] [CrossRef]
- Marwa, M.H.; Heather, D.; Louay, N.M.; Tyson, R. Evaluation of the durability of titanium dioxide photocatalyst coating for concrete pavement. Constr. Build. Mater. 2010, 24, 1456–1461. [Google Scholar]
- Shough, A.M.; Douglas, J.; Doren, M.N.; Raul, F.L. Effects of vanadium substitution on the structure and photocatalytic behavior of ETS-10. J. Phys. Chem. C 2007, 111, 1776–1782. [Google Scholar] [CrossRef]
- Ren, Y.; Gu, M.; Hu, Y.; Yue, B.; Jiang, L.; Kong, Z.; He, H. Preparation and photocatalytic activity of lanthanide loaded microporous titanosilicate ETS-10 catalysts. Chin. J. Catal. 2012, 33, 33,123–128. [Google Scholar] [CrossRef]
- Labrés i Xamena, F.X.; Calza, P.; Lamberti, C.; Prestipino, C.; Damin, A.; Bordiga, S.; Pelizzetti, E.; Zecchina, A. Enhancement of the ETS-10 titanosilicate activity in the shape-selective photocatalytic degradation of large aromatic molecules by controlled defect production. J. Am. Chem. Soc. 2003, 125, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Krisnandi, Y.K.; Southon, P.D.; Adesina, A.A.; Howe, R.F. ETS-10 as a photocatalyst. Int. J. Photoenergy 2003, 5, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Turta, N.A.; De Luca, P.; Bilba, N.; Nagy, J.B. Synthesis of titanosilicate ETS-10 in presence of cetyltrimethylammonium bromide. Microporous Mesoporous Mater. 2008, 112, 425–431. [Google Scholar] [CrossRef]
- De Luca, P.; Vuono, D.; Filice, M. Self bonded ETS-10 containing iron. Environ. Eng. Manag. J. 2009, 8, 1009–1015. [Google Scholar] [CrossRef]
- Turta, N.A.; Veltri, M.; Vuono, D.; De Luca, P.; Bilba, N.; Nastro, A. Effect of crystallization temperature on the synthesis of ETS-4 and ETS-10 titanosilicates. J. Porous Mater. 2009, 16, 527–536. [Google Scholar] [CrossRef]
- Vuono, D.; Guzzo, M.; De Luca, P.; Nagy, J.B. Physico-chemical characterization of zirconium-based self-bonded ETS-4 pellets. J. Therm. Anal. Calorim. 2014, 116, 169–182. [Google Scholar] [CrossRef]
- De Raffele, G.; Aloise, A.; De Luca, P.; Vuono, D.; Tagarelli, A.; Nagy, J.B. Kinetic and thermodynamic effects during the adsorption of heavy metals on ETS-4 and ETS-10 microporous materials. J. Porous Mater. 2016, 23, 389–400. [Google Scholar] [CrossRef]
- Anson, A.; Wang, Y.; Lin, C.C.H.; Kuznicki, T.M.; Kuznicki, S.M. Adsorption of ethane and ethylene on modified ETS-10. Chem. Eng. Sci. 2008, 63, 4171–4175. [Google Scholar] [CrossRef]
- De Luca, P.; Poulsen, T.G.; Salituri, A.; Tedeschi, A.; Vuono, D.; Kònya, Z.; Madaràsz, D.; Nagy, J.B. Evaluation and comparison of the ammonia adsorption capacity of titanosilicates ETS-4 and ETS-10 and aluminotitanosilicates ETAS-4 and ETAS-10. J. Therm. Anal. Calorim. 2015, 122, 1257–1267. [Google Scholar] [CrossRef]
- Canpolat, F.; Yılmaz, K.; Kose, M.M.; Sumer, M.; Yurdusev, M.A. Use of zeolite, coal bottom ash and fly ash as replacement materials in cement production. Cem. Concr. Res. 2004, 34, 731–735. [Google Scholar] [CrossRef]
- De Luca, P.; Mastroianni, C.; Nagy, J.B. Synthesis of self-bonded pellets of ETS-4 phase by new methodology of preparation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012003. [Google Scholar] [CrossRef]
- Nai-Qian, F.; Gai-Fei, P. Applications of natural zeolite to construction and building materials in China. Constr. Build. Mater. 2005, 19, 579–584. [Google Scholar]
- Sabet, F.A.; Libre, N.A.; Shekarchi, M. Mechanical and durability properties of self consolidating high performance concrete incorporating natural zeolite, silica fume and fly ash. Constr. Build. Mater. 2013, 44, 175–184. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Sharma, M.; Sharma, P.; Tomar, R. Synthesis and surfactant modification of clinoptilolite and montmorillonite for the removal of nitrate and preparation of slow release nitrogen fertilizer. J. Hazard. Mater. 2012, 15, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Doula, M.K. Synthesis of a clinoptilolite–Fe system with high Cu sorption capacity Chemosphere. Chemosphere 2007, 67, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Takeuchi, Y. Clinoptilolite: The distribution of potassium atoms and its role in thermal stability. Z. Krist.-Cryst. Mater. 1977, 145, 216–239. [Google Scholar]
- Li, Y.H.; Wang, S.; Luan, Z.; Ding, J.; Xu, C.; Wu, D. Adsorption of cadmium(II) from aqueous solution by surface oxidized carbon nanotubes. Carbon 2003, 41, 1057–1062. [Google Scholar] [CrossRef] [Green Version]
- Policicchio, A.; Vuono, D.; Rugiero, T.; De Luca, P.; Nagy, J.B. Study of MWCNTs adsorption perfomances in gas processes. J. CO2 Util. 2015, 10, 30–39. [Google Scholar] [CrossRef]
- Walraven, J.C. High performance fiber reinforced concrete: Progress in knowledge and design codes. Mater. Struct. 2009, 42, 1247. [Google Scholar] [CrossRef]
- Wille, K.; Naaman, A.E.; El-Tawil, S.; Parra-Montesinos, G.J. Ultra-high performance concrete and fiber reinforced concrete: Achieving strength and ductility without heat curing. Mater. Struct. 2012, 45, 309–324. [Google Scholar] [CrossRef]
- De Luca, P.; Nappo, G.; Siciliano, C.; Nagy, J.B. The role of carbon nanotubes and cobalt in the synthesis of pellets of titanium silicates. J. Porous Mater. 2018, 25, 283–296. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci Technol. 2001, 16, 1899–1912. [Google Scholar] [CrossRef]
- UNI ENI 1348:2000; Italian National Unification: Milano, Italy, 2008.
- EN 1015-12; British Standards Institution (BSI): London, UK, 2016.

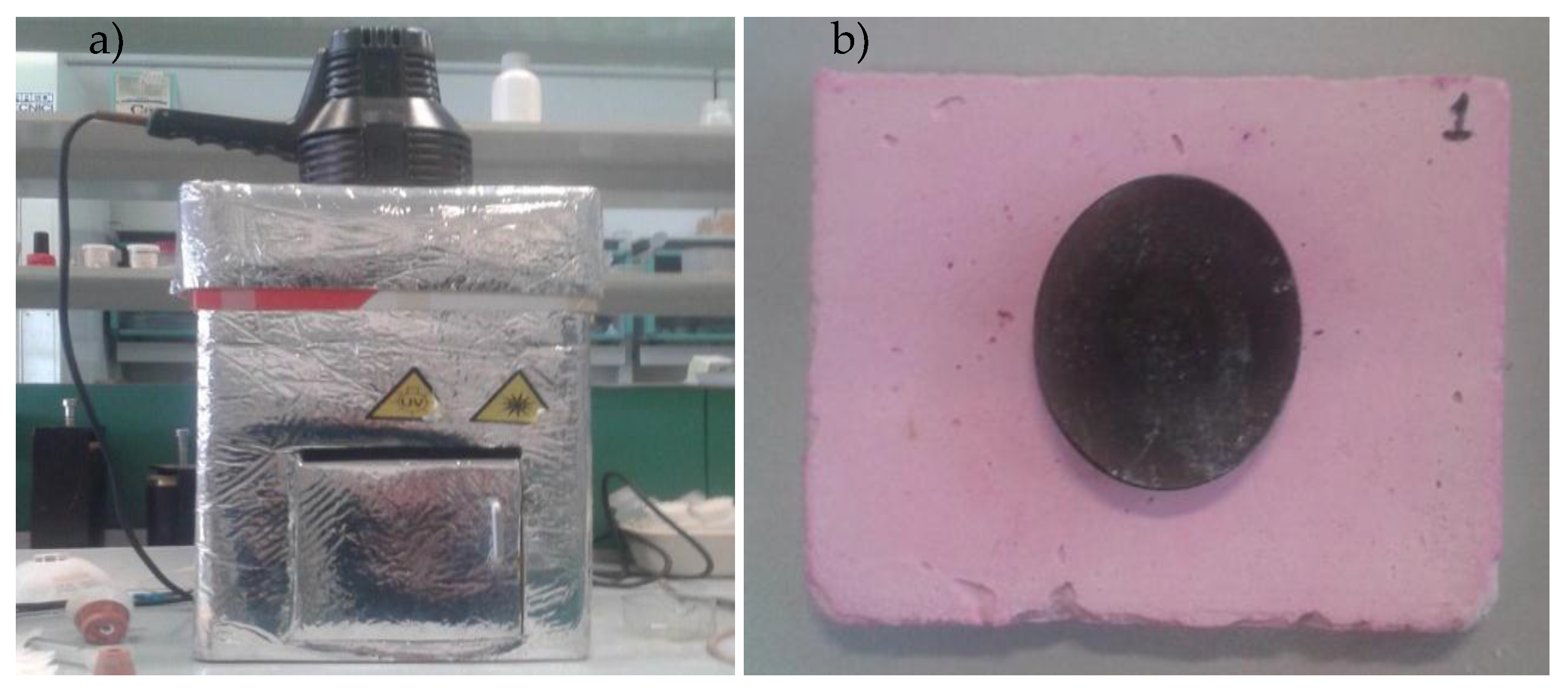

| Systems | S/A | Clinoptilolite (%) | TiO2 (%) | Carbon Nanotubes (%) |
|---|---|---|---|---|
| 1 | 3 | 0 | 0.0 | 0.000 |
| 2 | 3 | 5 | 0.0 | 0.000 |
| 3 | 3 | 0 | 0.5 | 0.000 |
| 4 | 3 | 0 | 0.0 | 0.025 |
| Systems | Before | After |
|---|---|---|
| 1 (without additive) |  |  |
| 2 (with clinoptilolite) |  |  |
| 3 (with TiO2) |  |  |
| 4 (with Carbon Nanotubes) |  |  |
| Systems | (kg/m2 min0.5) |
|---|---|
| 1 (without additive) | 0.4 |
| 2 (with clinoptilolite) | 0.3 |
| 3 (with TiO2) | 0.4 |
| 4 (with Carbon Nanotubes) | 0.5 |
| Systems | Adhesion Strength (N/mm2) |
|---|---|
| 1 (without additive) | 0.498 |
| 2 (with clinoptilolite) | 0.585 |
| 3 (with TiO2) | 0.241 |
| 4 (with Carbon nanotubes) | - |
| Systems | S/A | Clinoptilolite (%) | TiO2 (%) | Carbon Nanotubes (%) |
|---|---|---|---|---|
| 5 | 3 | 5 | 0.5 | 0.025 |
| 6 | 3 | 5 | 0.5 | - |
| 7 | 3 | 5 | - | 0.025 |
| 8 | 3 | - | 0.5 | 0.025 |
| Systems | S/A | Clinoptilolite (%) | TiO2 (%) | Carbon Nanotubes (%) | Compressive Strength (N) |
|---|---|---|---|---|---|
| 1 | 3 | 0 | 0.0 | 0.000 | 83.10 |
| 5 | 3 | 5 | 0.5 | 0.025 | 66.68 |
| 6 | 3 | 5 | 0.5 | 0.000 | 72.39 |
| 7 | 3 | 5 | 0.0 | 0.025 | 87.31 |
| 8 | 3 | 0 | 0.5 | 0.025 | 78.02 |
| Systems | Before | After |
|---|---|---|
| 5 (Clinoptilolite + TiO2 + Carbon Nanotubes) |  |  |
| 6 (Clinoptilolite + TiO2) |  |  |
| 7 (Clinoptilolite + Carbon Nanotubes) |  |  |
| 8 (TiO2 + Carbon Nanotubes) |  |  |
| Systems | Clinoptilolite (%) | TiO2 (%) | Carbon Nanotubes (%) | Water Absorption Coefficient (kg/m2 min0.5) |
|---|---|---|---|---|
| 1 | 0 | 0.0 | 0.000 | 0.4 |
| 5 | 5 | 0.5 | 0.025 | 0.7 |
| 6 | 5 | 0.5 | 0.000 | 0.2 |
| 7 | 5 | 0.0 | 0.025 | 0.3 |
| 8 | 0 | 0.5 | 0.025 | 0.5 |
| Systems | Clinoptilolite (%) | TiO2 (%) | Carbon Nanotubes (%) | Adhesion Strength (N/mm2) |
|---|---|---|---|---|
| 1 | 0 | 0.0 | 0.000 | 0.498 |
| 5 | 5 | 0.5 | 0.025 | 0.532 |
| 6 | 5 | 0.5 | 0.000 | 0.413 |
| 7 | 5 | 0.0 | 0.025 | - |
| 8 | 0 | 0.5 | 0.025 | - |
| Systems | Clinoptil (%) | TiO2 (%) | Carbon Nanotubes (%) | Compressive Strength (N) | Absorption Coefficient (kg/m2 min 0.5) | Adhesion Strength (N/mm2) | Photoactivity |
|---|---|---|---|---|---|---|---|
| 1 | - | - | - | 83.1 | 0.4 | 0.498 | |
| 2 | 5 | - | - | 127.01 | 0.3 | 0.585 | |
| 3 | - | 0.5 | - | 91.20 | 0.4 | 0.241 | |
| 4 | - | - | 0.025 | 92.37 | 0.5 | - | |
| 5 | 5 | 0.5 | 0.025 | 66.68 | 0.7 | 0.532 | |
| 6 | 5 | 0.5 | - | 72.39 | 0.2 | 0.413 | |
| 7 | 5 | - | 0.025 | 87.31 | 0.3 | - | |
| 8 | - | 0.5 | 0.025 | 78.02 | 0.5 | - |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, P.; De Luca, P.; Candamano, S.; Macario, A.; Crea, F.; Nagy, J.B. Preparation and Characterization of Plasters with Photodegradative Action. Buildings 2018, 8, 122. https://doi.org/10.3390/buildings8090122
De Luca P, De Luca P, Candamano S, Macario A, Crea F, Nagy JB. Preparation and Characterization of Plasters with Photodegradative Action. Buildings. 2018; 8(9):122. https://doi.org/10.3390/buildings8090122
Chicago/Turabian StyleDe Luca, Pierantonio, Pasquale De Luca, Sebastiano Candamano, Anastasia Macario, Fortunato Crea, and Jànos B. Nagy. 2018. "Preparation and Characterization of Plasters with Photodegradative Action" Buildings 8, no. 9: 122. https://doi.org/10.3390/buildings8090122






