Effect of Wood Hemicellulose Composition on Binding Interactions with Caffeine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experiment Procedure
2.3. Chemical Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panshin, A.J.; de Zeeuw, D. Textbook of Wood Technology, 4th ed.; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Svatoň, J. Ochrana Dřeva, 1st ed.; Mendel University in Brno: Brno, Czech Republic, 2000. [Google Scholar]
- Carpita, N.C.; Giheaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the wall during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Gibsen, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef] [PubMed]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Kobetičová, K.; Černý, R. Terrestrial eutrophication of building materials and buildings: An emerging topic in environmental studies. Sci. Total Environ. 2019, 686, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.S.; Ohlan, D. In vitro studies on antifungal activity of tea (Camellia sinensis) and coffee (Coffea arabica) against wood-rotting fungi. J. Basic Microbiol. 1997, 37, 159–165. [Google Scholar] [CrossRef]
- Ratajczak, I.; Wozniak, M.; Kwasniewska-Sip, P.; Szentner, K.; Cofta, G.; Mazela, B. Chemical characterization of wood treated with a formulation based on propolis, caffeine and organosilanes. Eur. J. Wood Wood Prod. 2018, 76, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Kwasniewska-Sip, P.; Cofta, G.; Nowak, P.B. Resistance of fungal growth on Scots pine treated with caffeine. Int. Biodeterior. Biodegrad. 2018, 132, 178–184. [Google Scholar] [CrossRef]
- Kwasniewska-Sip, P.; Bartkowiak, M.; Cofta, G.; Nowak, P.B. Resistance of Scots Pine (Pinus sylvestris L.) after Treatment with Caffeine and Thermal Modification against Aspergillus niger. Bioresources 2019, 14, 1890–1898. [Google Scholar]
- Kobetičová, K.; Böhm, M.; Černý, R. Mutual interactions of fungi and molds on woods treated with a caffeine solution: A preliminary study. AIP Conf. Proc. 2020, 2275, 020010. [Google Scholar]
- Kobetičová, K.; Nábělková, J.; Ďurišová, K.; Šimůnková, K.; Černý, R. Antifungal Activity of Methylxanthines in Relation to their Properties. Bioresources 2020, 15, 8110–8120. [Google Scholar] [CrossRef]
- Pánek, M.; Šimůnková, K.; Novák, D.; Dvořák, O.; Schönfelder, O.; Šedivka, P.; Kobetičová, K. Caffeine and TiO2 Nanoparticles Treatment of Spruce and Beech Wood for Increasing Transparent Coating Resistance against UV-Radiation and Mould Attacks. Coatings 2020, 10, 1141. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, L.; Zhang, Z. Antifungal activity of caffeine against fungal pathogens of tea plant. J. Nanjing. Agric. Univ. 2020, 2, 63–67. [Google Scholar]
- Šimůnková, K.; Reinprecht, L.; Nábělková, J.; Hýsek, Š.; Kindl, J.; Borůvka, V.; Lišková, T.; Šobotník, J.; Pánek, M. Caffeine—Perspective natural biocide for wood protection against decaying fungi and termites. J. Clean. Prod. 2021, 304, 127110. [Google Scholar] [CrossRef]
- Kobetičová, K.; Ďurišová, K.; Nábělková, J. Caffeine Interactions with Wood Polymers. Forests 2021, 12, 533. [Google Scholar] [CrossRef]
- Tavagnacco, L.; Engström, O.; Schnupf, U.; Saboungi, M.-L.; Himmel, M.; Widmalm, G.; Cesàro, A.; Brady, J.W. Caffeine and Sugars Interact in Aqueous Solutions: A Simulation and NMR Study. J. Phys. Chem. B 2020, 116, 11701–11711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertini, B.; Melegari, C.; Bertoni, S.; Dolci, L.S.; Passerini, N. A Novel Approach for Dry Powder Coating of Pellets with Ethylcellulose. Part II: Evaluation of Caffeine Release. AAPS Pharm. Sci. Tech. 2018, 19, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Aslam, N.; Khan, A.Y. Food-Drug Interactions. Oman Med. J. 2011, 26, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Tavagnacco, L.; Brady, J.W.; Cesáro, A. The Interaction of Sorbitol with Caffeine in Aqueous Solution. Food Biophys. 2013, 8, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blažej, A.; team of authors. Tenzidy, 1st ed.; Alfa, SNTL: Prague, Czech Republic, 1977. [Google Scholar]
- Kwasniewska-Sip, P.; Wozniak, M.; Jankowski, W.; Ratajczak, I.; Cofta, G. Chemical Changes of Wood Treated with Caffeine. Materials 2021, 14, 497. [Google Scholar] [CrossRef] [PubMed]
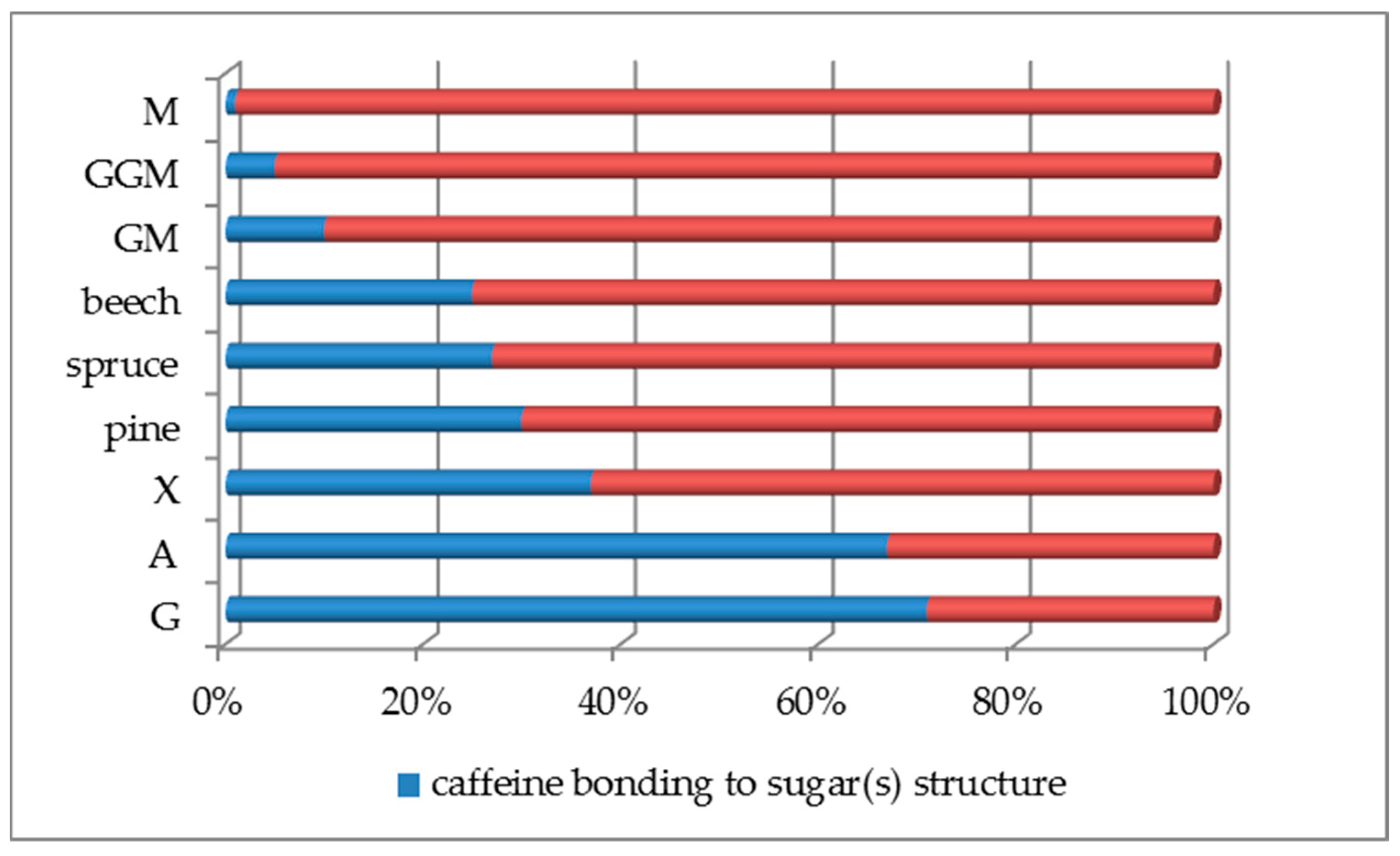
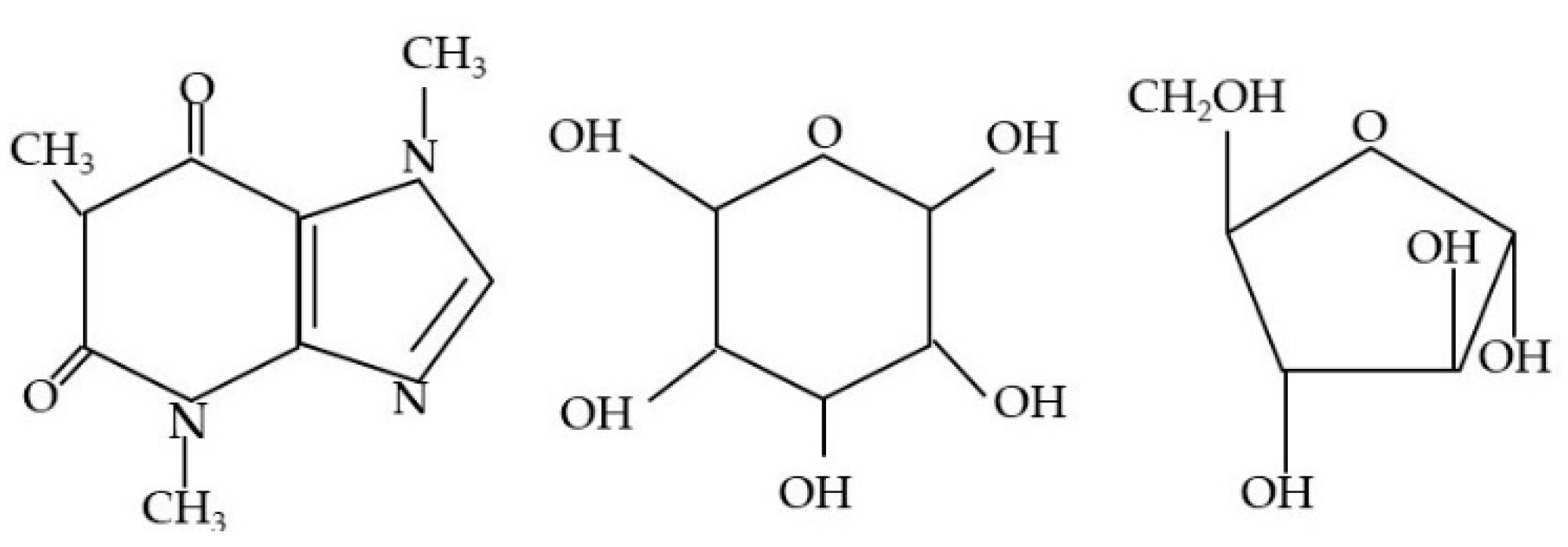
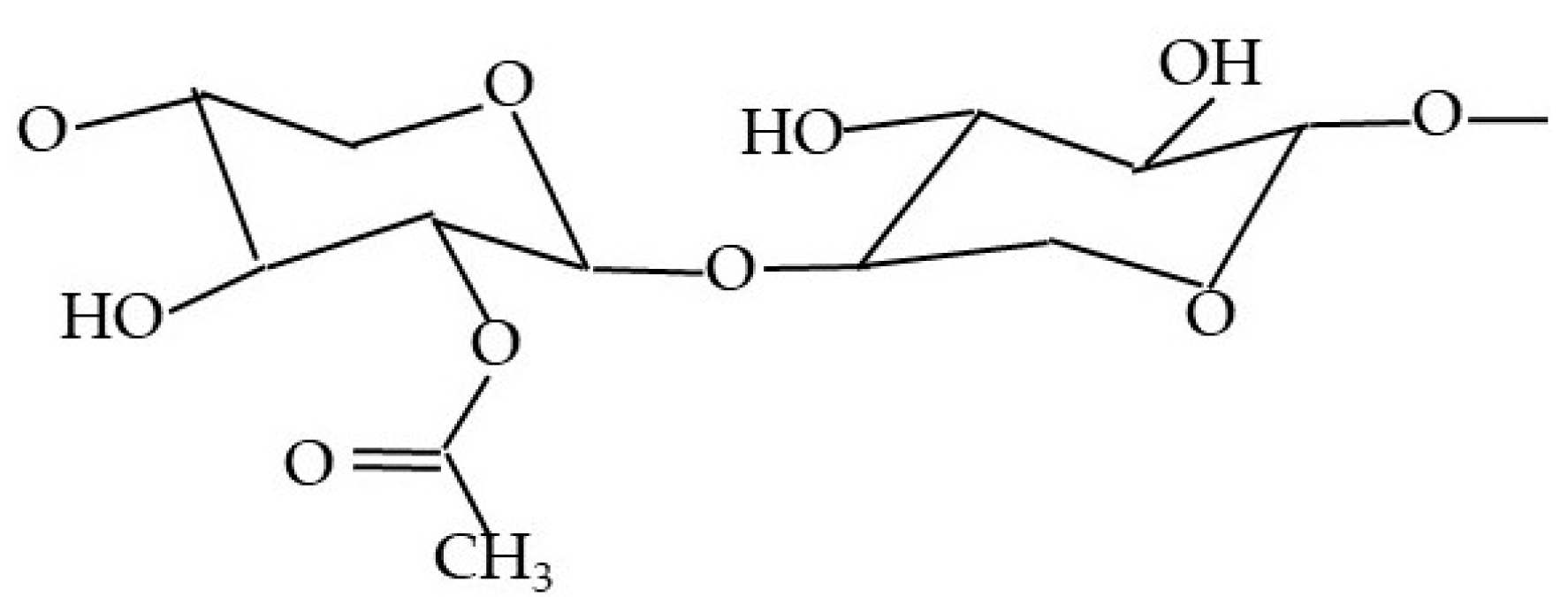
| Substance | 1. [A] | 2. [A] | 3. [A] | Average [A] | SD [A] | A-CF: (IIb—IIa to VIIb—VIIa) [A] | Average CF—(A-CF) [A] | |
|---|---|---|---|---|---|---|---|---|
| I | CF | 0.737 | 0.748 | 0.749 | 0.745 | 0.007 | 0.745 | - |
| IIa | HC | 0.028 | 0.025 | 0.022 | 0.025 | 0.003 | ||
| IIb | CF + HC | 0.725 | 0.732 | 0.738 | 0.732 | 0.007 | 0.707 | 0.038 |
| IIIa | GM | 0.059 | 0.059 | 0.050 | 0.055 | 0.005 | ||
| IIIb | CF + GM | 0.729 | 0.741 | 0.738 | 0.736 | 0.006 | 0.681 | 0.065 |
| IVa | M | 0 | 0 | 0 | 0 | 0 | ||
| IVb | CF + M | 0.736 | 0.743 | 0.735 | 0.738 | 0.004 | 0.738 | 0.007 |
| Va | G | 0.020 | 0.019 | 0.020 | 0.020 | 0.001 | ||
| Vb | CF + G | 0.236 | 0.236 | 0.235 | 0.235 | 0.001 | 0.215 | 0.529 |
| VIa | A | 0.051 | 0.054 | 0.054 | 0.053 | 0.002 | ||
| VIb | CF + A | 0.300 | 0.303 | 0.304 | 0.302 | 0.002 | 0.249 | 0.495 |
| VIIa | X | 0.455 | 0.460 | 0.464 | 0.460 | 0.005 | ||
| VIIb | CF + X | 0.928 | 0.923 | 0.937 | 0.929 | 0.007 | 0.469 | 0.275 |
| VIIIa | spruce | 0.265 | 0.268 | 0.261 | 0.265 | 0.003 | ||
| VIIIb | CF + spruce | 0.805 | 0.815 | 0.805 | 0.810 | 0.006 | 0.545 | 0.199 |
| IXa | pine | 0.363 | 0.360 | 0.364 | 0.362 | 0.002 | ||
| IXb | CF + pine | 0.869 | 0.889 | 0.894 | 0.884 | 0.013 | 0.522 | 0.223 |
| Xa | beech | 0.276 | 0.277 | 0.281 | 0.278 | 0.003 | ||
| Xb | CF + beech | 0.842 | 0.833 | 0.837 | 0.837 | 0.005 | 0.559 | 0.185 |
| Comparison | Difference | Significance | p Value |
|---|---|---|---|
| CF vs. GGM | 3.000 | n.s. | >0.05 |
| CF vs. GM | 6.000 | n.s. | >0.05 |
| CF vs. G | 24.000 | ** | <0.01 |
| CF vs. A | 21.000 | * | <0.05 |
| CF vs. X | 18.000 | n.s. | >0.05 |
| CF vs. spruce | 12.000 | n.s. | <0.05 |
| CF vs. pine | 15.000 | n.s. | >0.05 |
| CF vs. beech | 9.000 | n.s. | >0.05 |
| GGM vs. GM | 3.000 | n.s. | >0.05 |
| GGM vs. G | 21.000 | * | >0.05 |
| GGM vs. A | 18.000 | n.s. | >0.05 |
| GGM vs. X | 15.000 | n.s. | >0.05 |
| GGM vs. spruce | 9.000 | n.s. | <0.05 |
| GGM vs. pine | 12.000 | n.s. | >0.05 |
| GGM vs. beech | 6.000 | n.s. | >0.05 |
| GM vs. G | 18.000 | n.s. | >0.05 |
| GM vs. A | 15.000 | n.s. | >0.05 |
| GM vs. X | 12.000 | n.s. | >0.05 |
| GM vs. spruce | 6.000 | n.s. | >0.05 |
| GM vs. pine | 9.000 | n.s. | >0.05 |
| GM vs. beech | 3.000 | n.s. | >0.05 |
| G vs. A | −3.000 | n.s. | >0.05 |
| G vs. X | −6.000 | n.s. | >0.05 |
| G vs. spruce | −12.000 | n.s. | >0.05 |
| G vs. pine | −9.000 | n.s. | >0.05 |
| G vs. beech | −15.000 | n.s. | >0.05 |
| A vs. X | −3.000 | n.s. | >0.05 |
| A vs. spruce | −9.000 | n.s. | >0.05 |
| A vs. pine | −6.000 | n.s. | >0.05 |
| A vs. beech | −12.000 | n.s. | >0.05 |
| X vs. spruce | −6.000 | n.s. | >0.05 |
| X vs. pine | −3.000 | n.s. | >0.05 |
| X vs. beech | −9.000 | n.s. | >0.05 |
| spruce vs. pine | 3.000 | n.s. | >0.05 |
| spruce vs. beech | −3.000 | n.s. | >0.05 |
| pine vs. beech | −6.000 | n.s. | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobetičová, K.; Nábělková, J. Effect of Wood Hemicellulose Composition on Binding Interactions with Caffeine. Buildings 2021, 11, 515. https://doi.org/10.3390/buildings11110515
Kobetičová K, Nábělková J. Effect of Wood Hemicellulose Composition on Binding Interactions with Caffeine. Buildings. 2021; 11(11):515. https://doi.org/10.3390/buildings11110515
Chicago/Turabian StyleKobetičová, Klára, and Jana Nábělková. 2021. "Effect of Wood Hemicellulose Composition on Binding Interactions with Caffeine" Buildings 11, no. 11: 515. https://doi.org/10.3390/buildings11110515





