Temperature-Time Superposition Effect on Compressive Properties of AZ31B Magnesium Composite Foams
Abstract
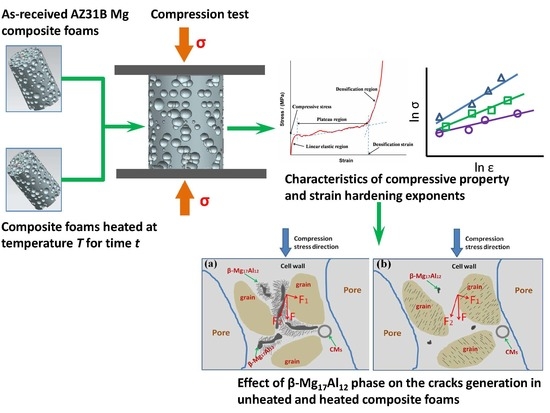
:1. Introduction
2. Experimental Procedures
2.1. Specimens Fabrication
2.2. Thermal Treatment
2.3. Microstructure Observation
2.4. Mechanical Property Test
3. Results
3.1. Pore Structure and Morphology
3.2. Microstructure and Phase Composition
3.3. Micro-Hardness
3.4. Compression Characteristics
3.5. Strain Hardening Exponent
4. Discussion
5. Conclusions
- (1)
- With the addition of CMs and Ca, obvious β-Mg17Al12 phases are detected in AZ31B magnesium composite foams, which is due to the changes of solidification behaviors of AZ31B melt that is caused by CMs and Ca.
- (2)
- With heating temperature and enduring time increasing, β-Mg17Al12 phases gradually dissolve into the matrix, resulting in solution strengthening effect. While the release of internal stress leads to the decrease of the compressive strength and the micro-hardness of the foams. Their combined effect is responsible for the evolution of mechanical properties under the experimental conditions.
- (3)
- When comparing with the unheated foams, almost all of the heated composite foams possess higher strain hardening exponents, while exhibiting lower micro-hardness, yield strength, and energy absorption capacity.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mondal, D.P.; Majumder, J.D.; Jha, N.; Badkul, A.; Das, S.; Patel, A. Titanium-cenosphere syntactic foam made through powder metallurgy route. Mater. Des. 2012, 34, 82–89. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Jiang, Q.; Xia, X.C.; Qiu, C.R.; Ding, J.; Zhao, W.M. A novel bubble nucleation particle for magnesium composite foam. Mater. Lett. 2017, 193, 187–190. [Google Scholar] [CrossRef]
- Tao, X.F.; Zhang, L.P.; Zhao, Y.Y. Al matrix syntactic foam fabricated with bimodal ceramic microspheres. Mater. Des. 2009, 30, 2732–2736. [Google Scholar] [CrossRef]
- Orbulov, I.N. Compressive properties of aluminium matrix syntactic foams. Mater. Sci. Eng. A 2012, 555, 52–56. [Google Scholar] [CrossRef]
- Xia, X.C.; Chen, X.W.; Zhang, Z.; Chen, X.; Zhao, W.M.; Liao, B.; Hur, B.Y. Compressive properties of closed-cell aluminum foams with different contents of ceramic microspheres. Mater. Des. 2014, 56, 353–358. [Google Scholar] [CrossRef]
- Gupta, N.; Luong, D.D.; Cho, K. Magnesium matrix composite foams—Density, mechanical properties, and applications. Metals 2012, 2, 238–252. [Google Scholar] [CrossRef]
- Hartmann, M.; Reindel, K.; Singer, R.F. Fabrication and properties of syntactic magnesium foams. MRS Proc. 1998, 521. [Google Scholar] [CrossRef]
- Daoud, A.; El-Khair, M.T.A.; Abdel-Aziz, M.; Rohatgi, P. Fabrication, microstructure and compressive behavior of ZC63 Mg–microballoon foam composites. Compos. Sci. Technol. 2007, 67, 1842–1853. [Google Scholar] [CrossRef]
- Steinacher, M.; Mrvar, P.; Zupanič, F. Interaction between AE44 magnesium alloy and SiC-Al2O3-SiO2 ceramic foam. Trans. Nonferr. Met. Soc. China 2015, 25, 1011–1019. [Google Scholar] [CrossRef]
- Xia, X.C.; Feng, J.L.; Ding, J.; Song, K.H.; Chen, X.W.; Zhao, W.M.; Liao, B.; Hur, B.Y. Fabrication and characterization of closed-cell magnesium-based composite foams. Mater. Des. 2015, 74, 36–43. [Google Scholar] [CrossRef]
- Huang, L.; Wang, H.; Yang, D.H.; Ye, F.; Lu, Z.P. Effects of scandium additions on mechanical properties of cellular al-based foams. Intermetallics 2012, 28, 71–76. [Google Scholar] [CrossRef]
- Yang, D.H.; Hur, B.Y.; Yang, S.R. Study on fabrication and foaming mechanism of Mg foam using CaCO3 as blowing agent. J. Alloys Compd. 2008, 461, 221–227. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Yu, S.R. Microstructure characterization on the formation of in situ Mg2Si and MgO reinforcements in AZ91D/Flyash composites. J. Alloys Compd. 2011, 509, 311–315. [Google Scholar] [CrossRef]
- Xu, S.W.; Matsumoto, N.; Kamado, S.; Honma, T.; Kojima, Y. Effect of Mg17Al12 precipitates on the microstructural changes and mechanical properties of hot compressed AZ91 magnesium alloy. Mater. Sci. Eng. A 2009, 523, 47–52. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, B.K.; Jun, J.H.; Shin, K.; Kim, K.T.; Jung, W.J. Microstructure and heat resistance of Mg-Al-Zn alloys containing metastable phase. Mater. Sci. Eng. A 2007, 449–451, 326–329. [Google Scholar] [CrossRef]
- Xu, S.W.; Matsumoto, N.; Kamado, S.; Honma, T.; Kojima, Y. Dynamic microstructural changes in Mg-9Al-1Zn alloy during hot compression. Scr. Mater. 2009, 61, 249–252. [Google Scholar] [CrossRef]
- Xia, X.; Zhao, W.; Feng, X.; Feng, H.; Zhang, X. Effect of homogenizing heat treatment on the compressive properties of closed-cell Mg alloy foams. Mater. Des. 2013, 49, 19–24. [Google Scholar] [CrossRef]
- Xia, X.C.; Chen, X.W.; Zhao, W.M.; Xue, H.T.; Liao, B.; Hur, B.Y.; Wang, Z.F. Corrosion behavior of closed-cell AZ31 Mg alloy foam in NaCl aqueous solutions. Corros. Sci. 2014, 80, 247–256. [Google Scholar] [CrossRef]
- Li, X.; Jiao, F.; Al-Samman, T.; Chowdhury, S.G. Influence of second-phase precipitates on the texture evolution of Mg-Al-Zn alloys during hot deformation. Scr. Mater. 2012, 66, 159–162. [Google Scholar] [CrossRef]
- Yang, D.H.; Yang, S.R.; Wang, H.; Ma, A.B.; Jiang, J.H.; Chen, J.Q.; Wang, D.L. Compressive properties of cellular Mg foams fabricated by melt-foaming method. Mater. Sci. Eng. A 2010, 527, 5405–5409. [Google Scholar] [CrossRef]
- Xu, Z.G.; Fu, J.W.; Luo, T.J.; Yang, Y.S. Effects of cell size on quasi-static compressive properties of Mg alloy foams. Mater. Des. 2012, 34, 40–44. [Google Scholar] [CrossRef]
- Tahreen, N.; Chen, D.L.; Nouri, M.; Li, D.Y. Effects of aluminum content and strain rate on strain hardening behavior of cast magnesium alloys during compression. Mater. Sci. Eng. A 2014, 594, 235–245. [Google Scholar] [CrossRef]
- Wilson, D.V. Relationships between microstructure and behavior in the uniaxial tensile test. J. Phys. D Appl. Phys. 1974, 7, 954–968. [Google Scholar] [CrossRef]
- Dong, J.G.; Zhang, D.F.; Sun, J.; Dai, Q.W.; Pan, F.S. Effects of different stretching routes on microstructure and mechanical properties of AZ31B magnesium alloy sheets. J. Mater. Sci. Technol. 2015, 31, 935–940. [Google Scholar] [CrossRef]
- Pan, H.C.; Wang, F.H.; Li, J.; Feng, M.L.; Dong, J. Mechanical behavior and microstructure evolution of a rolled magnesium alloy AZ31B under low stress triaxiality. J. Mater. Sci. Technol. 2016, 32, 1282–1288. [Google Scholar] [CrossRef]
- Lou, S.; Northwood, D.O. Effect of strain aging on the strength coefficient and strain-hardening exponent of construction-grade steels. J. Mater. Eng. Perform. 1994, 3, 344–349. [Google Scholar] [CrossRef]
- Hollomon, J.H. Tensile deformation. Trans. AIME 1945, 162, 268–290. [Google Scholar]
- Antunes, R.A.; Oliveira, M.C.L.D. Materials selection for hot stamped automotive body parts: An application of the Ashby approach based on the strain hardening exponent and stacking fault energy of materials. Mater. Des. 2014, 63, 247–256. [Google Scholar] [CrossRef]
- Wang, C.X.; Jiang, C.H.; Ji, V. Thermal stability of residual stresses and work hardening of shot peened tungsten cemented carbide. J. Mater. Process. Technol. 2017, 240, 98–103. [Google Scholar] [CrossRef]
- Juijerm, P.; Altenberger, I. Residual stress relaxation of deep-rolled Al-Mg-Si-Cu alloy during cyclic loading at elevated temperatures. Scr. Mater. 2006, 55, 1111–1114. [Google Scholar] [CrossRef]
- Nikitin, I.; Besel, M. Residual stress relaxation of deep-rolled austenitic steel. Scr. Mater. 2008, 58, 239–242. [Google Scholar] [CrossRef]
- Liu, J.A.; Yu, S.R.; Huang, Z.Q.; Ma, G.; Liu, Y. Microstructure and compressive property of in situ Mg2Si reinforced Mg-microballoon composites. J. Alloys Compd. 2012, 537, 12–18. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Daoud, A.; Schultz, B.F.; Puri, T. Microstructure and mechanical behavior of die casting AZ91D-Fly ash cenosphere composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 883–896. [Google Scholar] [CrossRef]
- Xu, C.; Sheng, G.G.; Wang, H.; Feng, K.; Yuan, X.J. Tungsten inert gas welding-brazing of AZ31B magnesium alloy to TC4 titanium alloy. J. Mater. Sci. Technol. 2016, 32, 167–171. [Google Scholar] [CrossRef]
- Gupta, N.; Luong, D.D.; Rohatgi, P.K. A method for intermediate strain rate compression testing and study of compressive failure mechanism of Mg-Al-Zn alloy. J. Appl. Phys. 2011, 109, 103512. [Google Scholar] [CrossRef]
- Lü, Y.Z.; Wang, Q.D.; Ding, W.J.; Zeng, X.Q.; Zhu, Y.P. Fracture behavior of AZ91 magnesium alloy. Mater. Lett. 2000, 44, 265–268. [Google Scholar] [CrossRef]
- Li, B.; Pan, Q.L.; Shi, Y.J.; Chen, L.I.; Yin, Z.M. Microstructural evolution of Al-Zn-Mg-Zr alloy with trace amount of Sc during homogenization treatment. Trans. Nonferr. Met. Soc. China 2013, 23, 3568–3574. [Google Scholar] [CrossRef]












| Mg | Al | Zn | Mn | Fe | Si | Cu | Ca | Total Imp. |
|---|---|---|---|---|---|---|---|---|
| Balance | 3.1148 | 0.8109 | 0.3197 | 0.0002 | 0.0081 | 0.0003 | 0.0002 | 0.1 |
| Al2O3 (wt %) | SiO2 (wt %) | Stacking Desity (g/cm3) | Size Range (μm) | Wall Thickness (μm) |
|---|---|---|---|---|
| ~10 | ~90 | 0.42 | 40–150 | 7.5 ± 0.8 |
| Temperature (°C) | Time (h) | Porosity (%) | Pore Size (mm) | Specimens Size (mm) |
|---|---|---|---|---|
| 150 | 1 | 67.40 | 1~2 | Φ 10 × 15 |
| 2 | 67.30 | |||
| 4 | 67.20 | |||
| 6 | 66.70 | |||
| 24 | 67.22 | |||
| 250 | 1 | 66.56 | 1~2 | Φ 10 × 15 |
| 2 | 67.39 | |||
| 4 | 66.90 | |||
| 6 | 67.40 | |||
| 24 | 67.53 | |||
| 320 | 1 | 67.41 | 1~2 | Φ 10 × 15 |
| 2 | 67.50 | |||
| 4 | 67.25 | |||
| 6 | 67.31 | |||
| 24 | 67.19 | |||
| 400 | 1 | 67.30 | 1~2 | Φ 10 × 15 |
| 2 | 67.27 | |||
| 4 | 66.90 | |||
| 6 | 67.07 | |||
| 24 | 67.10 | |||
| 500 | 1 | 67.20 | 1~2 | Φ 10 × 15 |
| 2 | 67.40 | |||
| 4 | 67.28 | |||
| 6 | 66.90 | |||
| 24 | 67.20 | |||
| As-received | - | 67.36 | 1~2 | Φ 10 × 15 |
| Holding Time | As-Received | 1 h | 2 h | 4 h | 6 h | 24 h |
|---|---|---|---|---|---|---|
| K | 29.781 | 31.572 | 20.825 | 32.761 | 42.136 | 41.622 |
| n | 0.377 | 0.657 | 0.444 | 0.534 | 0.509 | 0.531 |
| Holding Temperature | As-Received | 150 °C | 250 °C | 320 °C | 400 °C | 500 °C |
|---|---|---|---|---|---|---|
| K | 29.781 | 28.048 | 29.780 | 20.825 | 31.049 | 40.548 |
| n | 0.377 | 0.543 | 0.544 | 0.444 | 0.588 | 0.558 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Wang, J.; Peng, Y.; Wang, N.; He, X.; Qiu, C.; Ding, J.; Chen, X. Temperature-Time Superposition Effect on Compressive Properties of AZ31B Magnesium Composite Foams. Metals 2018, 8, 434. https://doi.org/10.3390/met8060434
Xia X, Wang J, Peng Y, Wang N, He X, Qiu C, Ding J, Chen X. Temperature-Time Superposition Effect on Compressive Properties of AZ31B Magnesium Composite Foams. Metals. 2018; 8(6):434. https://doi.org/10.3390/met8060434
Chicago/Turabian StyleXia, Xingchuan, Jing Wang, Yuanyi Peng, Nannan Wang, Xin He, Chuanrong Qiu, Jian Ding, and Xueguang Chen. 2018. "Temperature-Time Superposition Effect on Compressive Properties of AZ31B Magnesium Composite Foams" Metals 8, no. 6: 434. https://doi.org/10.3390/met8060434
APA StyleXia, X., Wang, J., Peng, Y., Wang, N., He, X., Qiu, C., Ding, J., & Chen, X. (2018). Temperature-Time Superposition Effect on Compressive Properties of AZ31B Magnesium Composite Foams. Metals, 8(6), 434. https://doi.org/10.3390/met8060434