Study of a Bimetallic Interfacial Bonding Process Based on Ultrasonic Quantitative Evaluation
Abstract
:1. Introduction
2. Materials and Methods
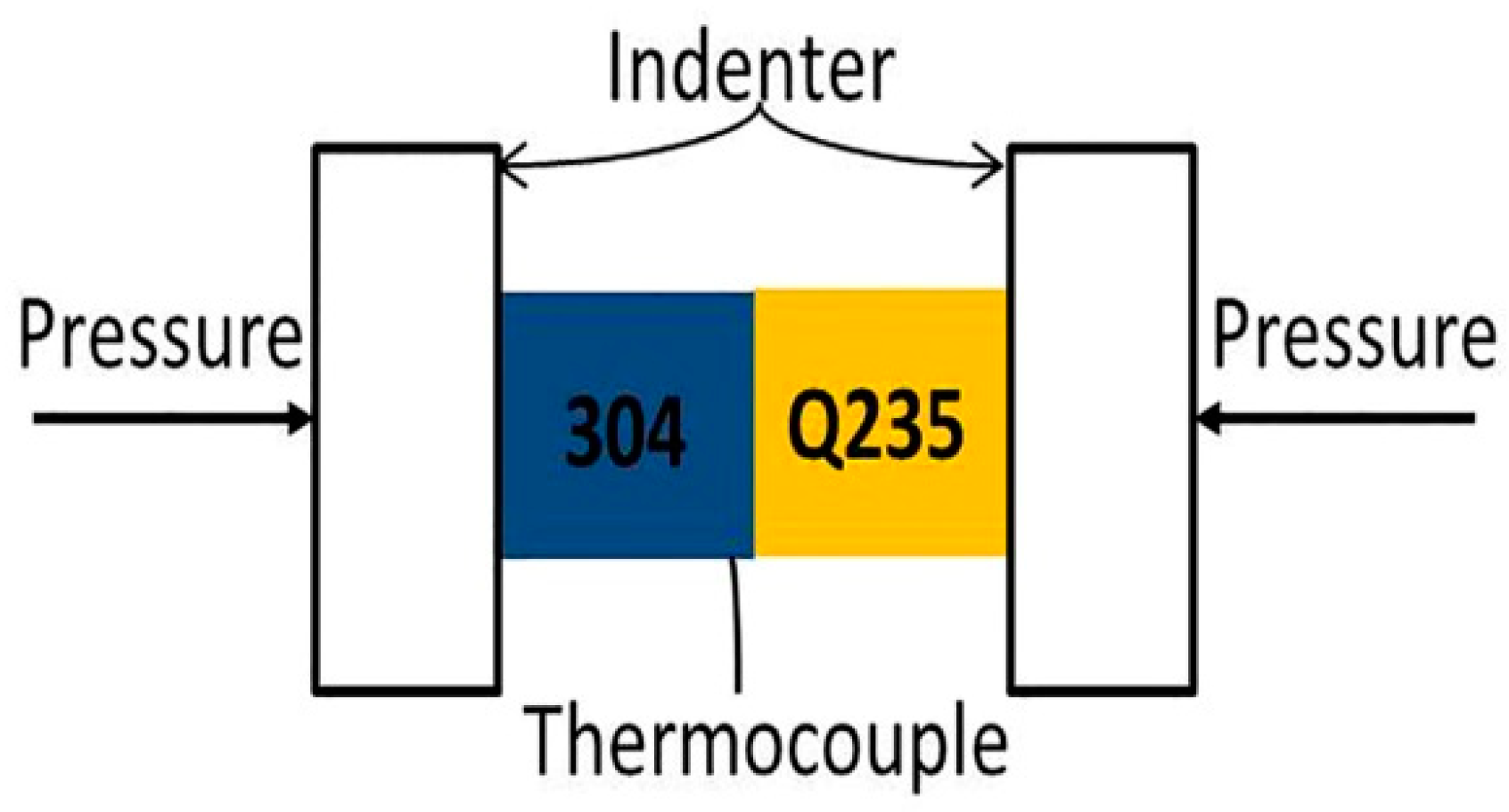
2.1. Bonding Process
2.2. Heat Preservation
2.3. Tensile Test
3. Results and Discussion
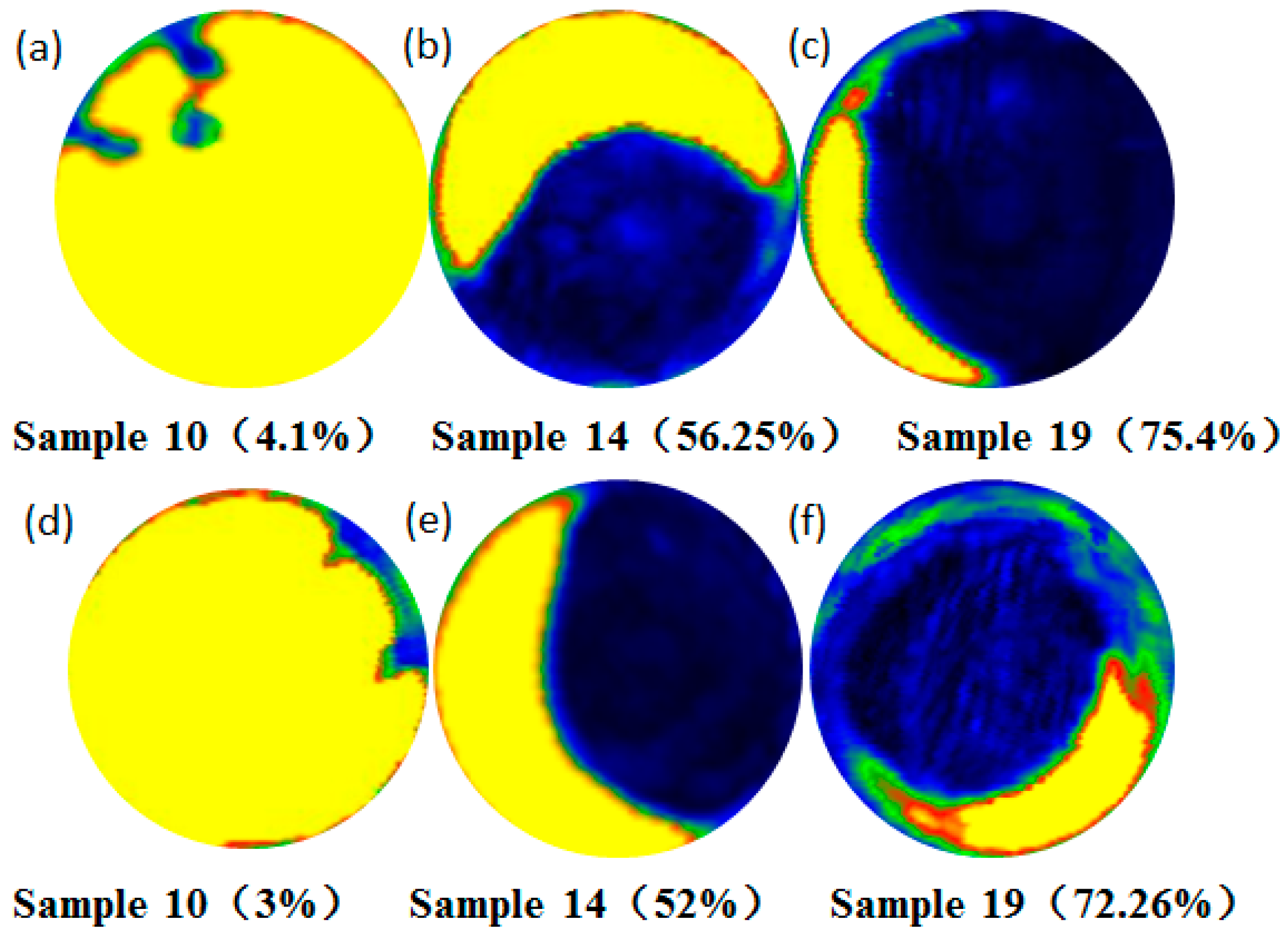
3.1. Effect of Deformation Bonding Parameters on Interfacial Bonding Rate
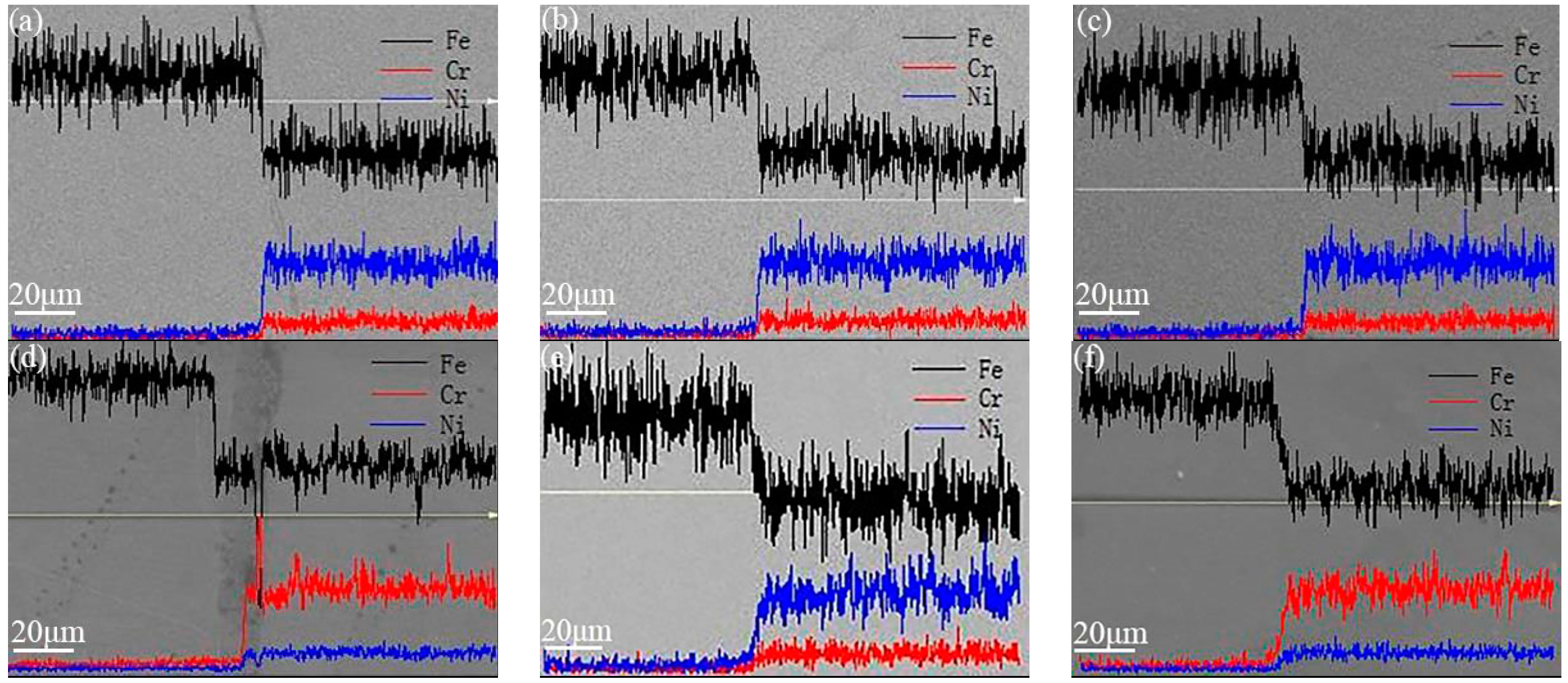
3.2. Effect of Heat Preservation on the Interfacial Bonding Rate
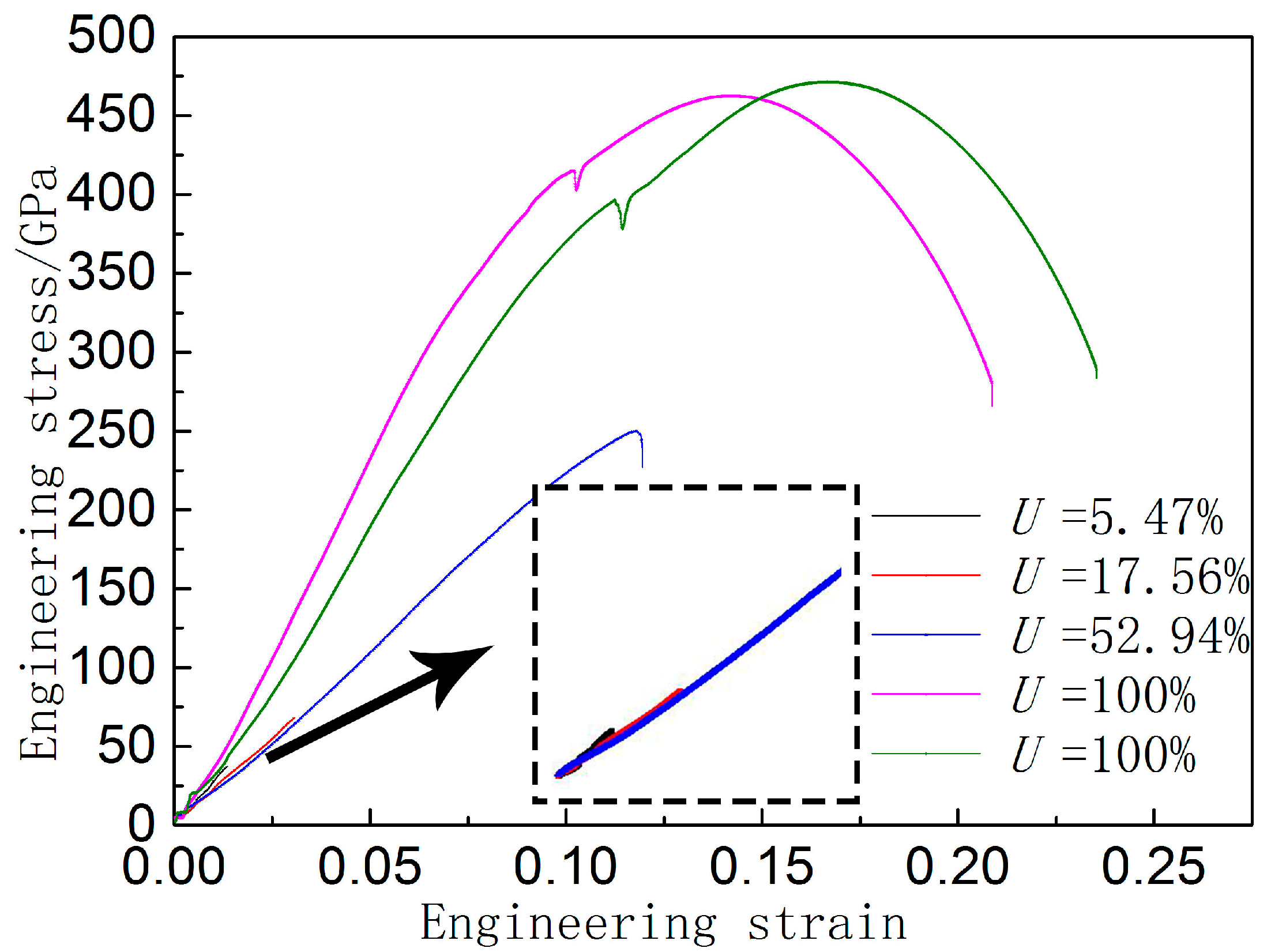
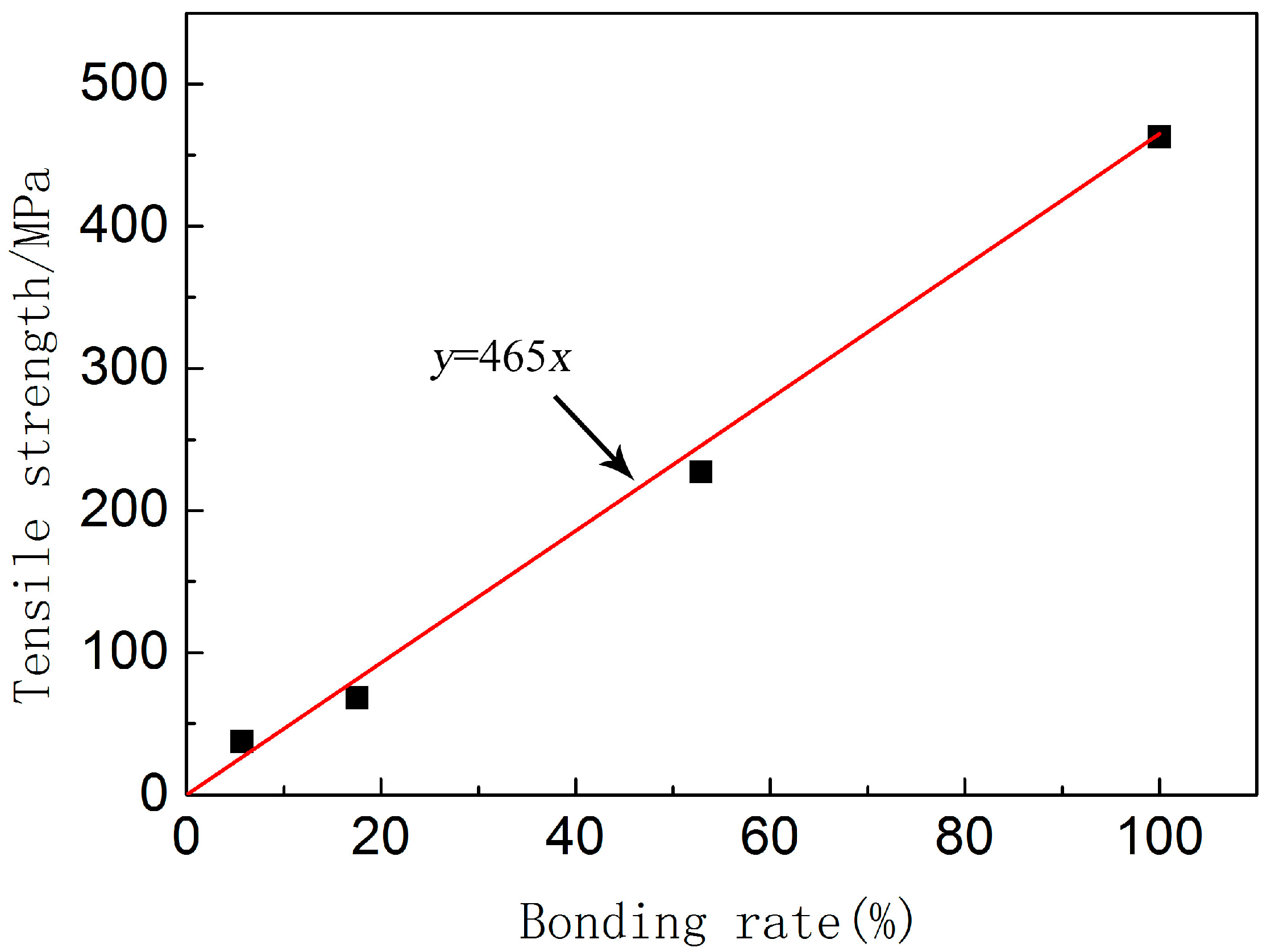
3.3. Relationship between Bonding Rate and Mechanical Properties
4. Conclusions
- During the deformation bonding production of stainless-steel and carbon-steel joints, the deformation temperature has the most significant linear correlation with bonding rate, but has little influence on the thickness of the diffusion layer. Only shallow diffusion can be realized, owing to the short contact time.
- The thickness of the diffusion layer can be improved by the heat preservation treatment, whereas the bonding rate of the joint decreases slightly due to the difference in thermal expansion coefficients between two materials.
- The interfacial bonding rate of the joint has a great influence on the mechanical properties of stainless steel/carbon steel composite. It is found that the bonding strength between the diffusion layer and both sides of the metal is higher than that of the carbon steel matrix, and the strength of the composite is only related to the bonding rate and the strength of the relatively soft material.
- The interfacial bonding rate also has a great influence on the failure mode of stainless steel/carbon steel. When the bonding rate is low, the crack initiates at the junction of the unbonded and bonded areas of the interface and extends to the binding region. When the bonding rate is high, no obvious gap occurs at the interface, and the crack source is located in the interior of the carbon steel. Moreover, with the increase in bonding rate, the fracture mode changes from cleavage brittle fracture, to ductile-brittle mixed-mode fracture, and then to ductile fracture.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Yang, Z.; Jha, B.; Xia, G.; Stevenson, J.W. Clad metals, roll bonding and their applications for SOFC interconnects. J. Power Sources 2005, 152, 40–45. [Google Scholar] [CrossRef]
- Ju, Y.J.; Sun, I.H. Effect of heat treatment on tensile deformation characteristics and properties of Al3003/STS439 clad composite. Mater. Sci. Eng. A 2014, 596, 1–8. [Google Scholar]
- Knezevic, M.; Jahedi, M.; Korkolis, Y.P.; Beyerlein, I.J. Material-based design of the extrusion of bimetallic tubes. Comput. Mater. Sci. 2014, 95, 63–73. [Google Scholar] [CrossRef]
- Shen, W.N.; Feng, L.J.; Feng, H.; Cao, Y.; Liu, L.; Cao, M.; Ge, Y.F. Preparation and characterization of 304 stainless steel/q235 carbon steel composite material. Results Phys. 2017, 7, 529–534. [Google Scholar] [CrossRef]
- Gomez, X.; Echeberria, J. Microstructure and mechanical properties of carbon steel A210/superalloy Sanicro 28 bimetallic tubes. Mater. Sci. Eng. A 2003, 348, 180–191. [Google Scholar] [CrossRef]
- Masahashi, N.; Watanabe, S.; Nomura, N.; Semboshi, S.; Hanada, S. Laminates based on an iron aluminide intermetallic alloy and a CrMo steel. Intermetallics 2005, 13, 717–726. [Google Scholar] [CrossRef]
- Kurt, B. The interface morphology of diffusion bonded dissimilar stainless steel and medium carbon steel couples. J. Mater. Process. Technol. 2007, 190, 138–141. [Google Scholar] [CrossRef]
- Velmurugan, C.; Senthilkumar, V.; Sarala, S.; Arivarasan, J. Low temperature diffusion bonding of Ti-6Al-4 V and duplex stainless steel. J. Mater. Process. Technol. 2016, 234, 272–279. [Google Scholar] [CrossRef]
- Sheng, G.M.; Huang, J.W.; Qin, B.; Zhou, B.; Qiu, S.Y.; Li, C. An experimental investigation of phase transformation superplastic diffusion bonding of titanium alloy to stainless steel. J. Mater. Sci. 2005, 40, 6385–6390. [Google Scholar] [CrossRef]
- Zotti, L.A.; Sanvito, S.; O’Regan, D.D. A simple descriptor for energetics at fcc-bcc metal interfaces. Mater. Des. 2018, 142, 158–165. [Google Scholar] [CrossRef]
- Weng, S.Y.; Ning, H.M.; Hu, N.; Yan, C.; Fu, T.; Peng, X.H.; Fu, S.Y.; Zhang, J.Y.; Xu, C.H.; Sun, D.Y.; et al. Strengthening effects of twin interface in Cu/Ni multilayer thin films—A molecular dynamics study. Mater. Des. 2016, 111, 1–8. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, Y.J.; Park, K.T.; Nersisyan, H.H.; Jeong, H.G.; Lee, J.H. Controlling Al/Cu composite diffusion layer during hydrostatic extrusion by using colloidal Ag. J. Mater. Process. Technol. 2013, 213, 487–494. [Google Scholar] [CrossRef]
- Shao, X.; Guo, X.; Han, Y.; Lu, W.; Qin, J.; Zhang, D. Characterization of the diffusion bonding behavior of pure Ti and Ni with different surface roughness during hot pressing. Mater. Des. 2015, 65, 1001–1010. [Google Scholar] [CrossRef]
- Chen, S.; Ke, F.; Min, Z.; Bai, Y. Atomistic investigation of the effects of temperature and surface roughness on diffusion bonding between Cu and Al. Acta Mater. 2007, 55, 3169–3175. [Google Scholar] [CrossRef]
- Ahmed, T.; Belova, I.V.; Murch, G.E. Finite Difference Solution of the Diffusion Equation and Calculation of the Interdiffusion Coefficient using the Sauer-Freise and Hall Methods in Binary Systems. Procedia Eng. 2015, 105, 570–575. [Google Scholar] [CrossRef]
- Wang, T.M.; Cao, F.; Zhou, P.; Kang, H.J.; Chen, Z.N.; Fu, Y.N.; Xiao, T.Q.; Huang, W.X.; Yuan, Q.X. Study on diffusion behavior and microstructural evolution of Al/Cu bimetal interface by synchrotron X-ray radiography. J. Alloys Compd. 2014, 616, 550–555. [Google Scholar] [CrossRef]
- Sun, C.Y.; Li, L.; Fu, M.W.; Zhou, Q.J. Element diffusion model of bimetallic hot deformation in metallurgical bonding process. Mater. Des. 2016, 94, 433–443. [Google Scholar] [CrossRef]
- Sun, C.Y.; Cong, Y.P.; Zhang, Q.D.; Fu, M.W.; Li, L. Element diffusion model with variable coefficient in bimetallic bonding process. J. Mater. Process. Technol. 2017, 253, 99–108. [Google Scholar] [CrossRef]
- Ben, B.S.; Ben, B.A.; Ratnam, C.; Yang, S.H. Ultrasonic based method for damage identification in composite materials. Int. J. Mech. Mater. Des. 2012, 8, 297–309. [Google Scholar] [CrossRef]
- Juan, C.; Nicolas, B.; Manuel, C.; Sergio, C.; Guillermo, R. A multilevel Bayesian method for ultrasound-based damage identification in composite laminates. Mech. Syst. Signal Process. 2017, 88, 462–477. [Google Scholar]
- Zhou, H.M.; Liu, G.W. A model-based ultrasonic quantitative evaluation method for bonding quality of multi-layered material. Measurement 2012, 45, 1414–1423. [Google Scholar] [CrossRef]
- Luan, Y.L.; Sun, T.; Feng, J.C.; Gang, T. Ultrasonic evaluation of TiAl and 40Cr diffusion bonding quality based on time-scale characteristics extraction. NDT & E Int. 2011, 44, 786–796. [Google Scholar]
- Suresh Kumar, S.; Krishnamoorthi, J.; Ravisankar, B.; Balusamy, V. Assessing quality of diffusion bonded joints with interlayer using ultrasonic/ultrasound. J. Mater. Process. Technol. 2017, 242, 139–146. [Google Scholar] [CrossRef]
- Suresh Kumar, S.; Ravisankar, B. An evaluation of quality of joints in two dissimilar metals diffusion bonded using ultrasonic. Mater. Manuf. Process. 2016, 31, 2084–2090. [Google Scholar] [CrossRef]
- Lesuer, D.R.; Syn, C.K.; Sherby, O.D.; Wadsworth, J.; Lewandowski, J.J.; Hun, W.H. Mechanical behaviour of laminated metal composites. Mater. Rev. 1996, 41, 169–197. [Google Scholar] [CrossRef]
- Wang, D.W.; Xiu, S.C. Effect of bonding temperature on the interfacial microstructure and performance of mild steel/austenite stainless steel diffusion-bonded joint. Acta Metall. Sin. 2017, 53, 567–574. [Google Scholar] [CrossRef]
- Liu, H.T.; Sun, L.Z. Effects of thermal residual stresses on effective elastoplastic behavior of metal matrix composites. Int. J. Solids Struct. 2004, 41, 2189–2203. [Google Scholar] [CrossRef]
- Dave, V.R.; Beyerlein, I.J.; Hartman, D.A.; Barbieri, J.M. A probabilistic diffusion weld modeling framework. Weld. J. 2003, 82, 170–178. [Google Scholar]






| Material | Cr | Ni | C | Si | Mn | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| AISI304 | 18.97 | 8.86 | 0.04 | 1.00 | 2.00 | 0.035 | 0.03 | Bal |
| Q235A | - | - | 0.22 | 0.30 | 0.43 | 0.04 | 0.05 | Bal |
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Temperature/°C | 600 | 600 | 600 | 600 | 600 | 700 | 700 | 700 | 700 | 700 | 800 | 800 | 800 |
| Engineering strain rate/s−1 | 0.1 | 0.5 | 1 | 5 | 10 | 0.1 | 0.5 | 1 | 5 | 10 | 0.1 | 0.5 | 1 |
| Engineering strain | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.2 | 0.3 | 0.4 | 0.5 | 0.1 | 0.3 | 0.4 | 0.5 |
| Number | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | - |
| Temperature/°C | 600 | 600 | 600 | 600 | 600 | 700 | 700 | 700 | 700 | 700 | 800 | 800 | - |
| Engineering strain rate/s−1 | 5 | 10 | 0.1 | 0.5 | 1 | 5 | 10 | 0.1 | 0.5 | 1 | 5 | 10 | - |
| Engineering strain | 0.1 | 0.2 | 0.4 | 0.5 | 0.1 | 0.2 | 0.3 | 0.5 | 0.1 | 0.2 | 0.3 | 0.4 | - |
| Bonding Parameters | Temperature | Engineering Strain Rate | Engineering Strain |
|---|---|---|---|
| Range value | 100 | 19.95 | 27.45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, S.; Liu, J.; Wang, Y.; Zhang, B.; Zhang, L.-Y. Study of a Bimetallic Interfacial Bonding Process Based on Ultrasonic Quantitative Evaluation. Metals 2018, 8, 329. https://doi.org/10.3390/met8050329
Zhang Q, Li S, Liu J, Wang Y, Zhang B, Zhang L-Y. Study of a Bimetallic Interfacial Bonding Process Based on Ultrasonic Quantitative Evaluation. Metals. 2018; 8(5):329. https://doi.org/10.3390/met8050329
Chicago/Turabian StyleZhang, Qingdong, Shuo Li, Jiyang Liu, Yanan Wang, Boyang Zhang, and Li-Yuan Zhang. 2018. "Study of a Bimetallic Interfacial Bonding Process Based on Ultrasonic Quantitative Evaluation" Metals 8, no. 5: 329. https://doi.org/10.3390/met8050329
APA StyleZhang, Q., Li, S., Liu, J., Wang, Y., Zhang, B., & Zhang, L.-Y. (2018). Study of a Bimetallic Interfacial Bonding Process Based on Ultrasonic Quantitative Evaluation. Metals, 8(5), 329. https://doi.org/10.3390/met8050329







