ZnO Nanoparticles Anchored on a N-Doped Graphene-Coated Separator for High Performance Lithium/Sulfur Batteries
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Reagents
2.2. Synthesis of ZnO/NDG Composite
2.3. Characterization
2.4. Electrochemical Measurements
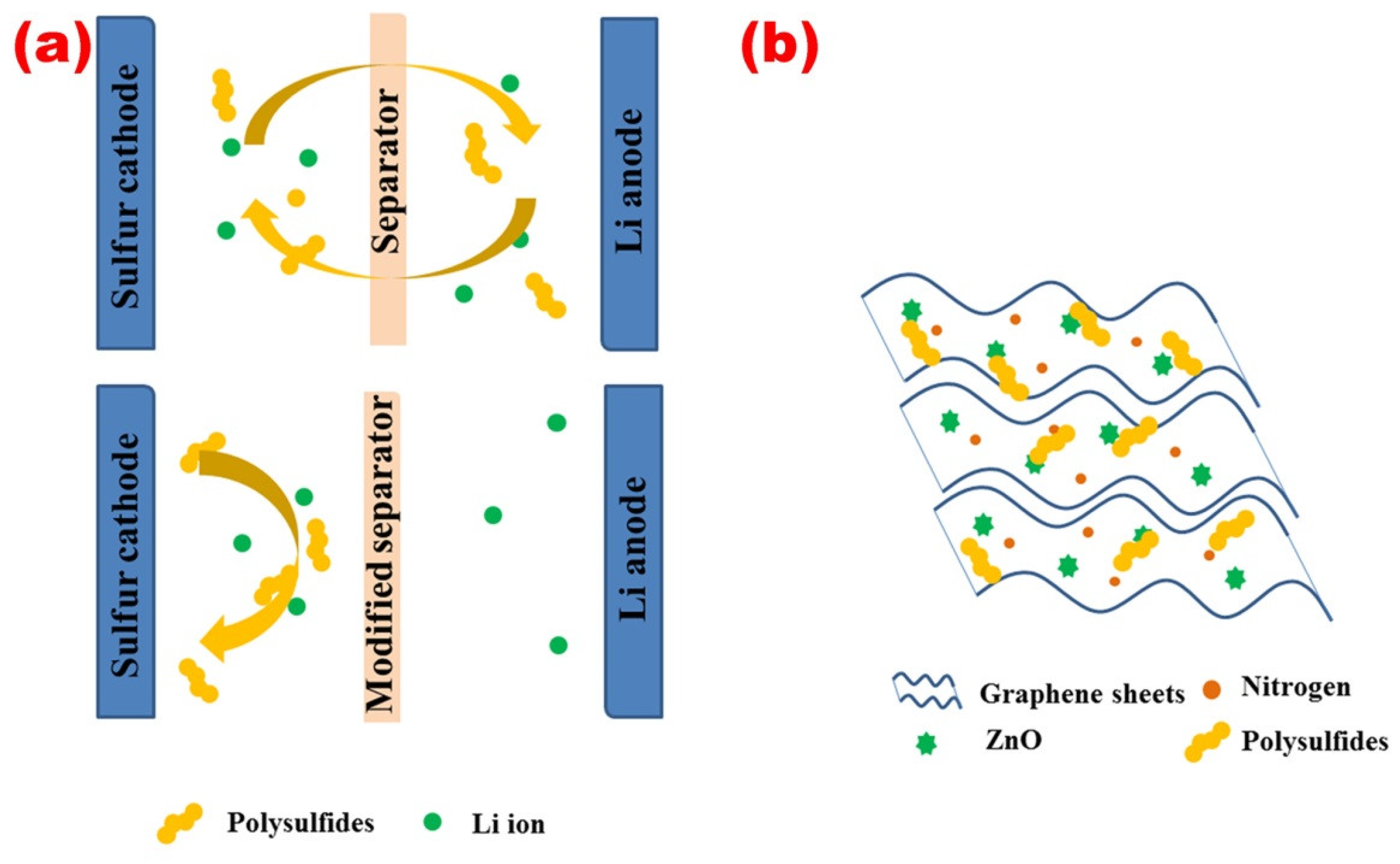
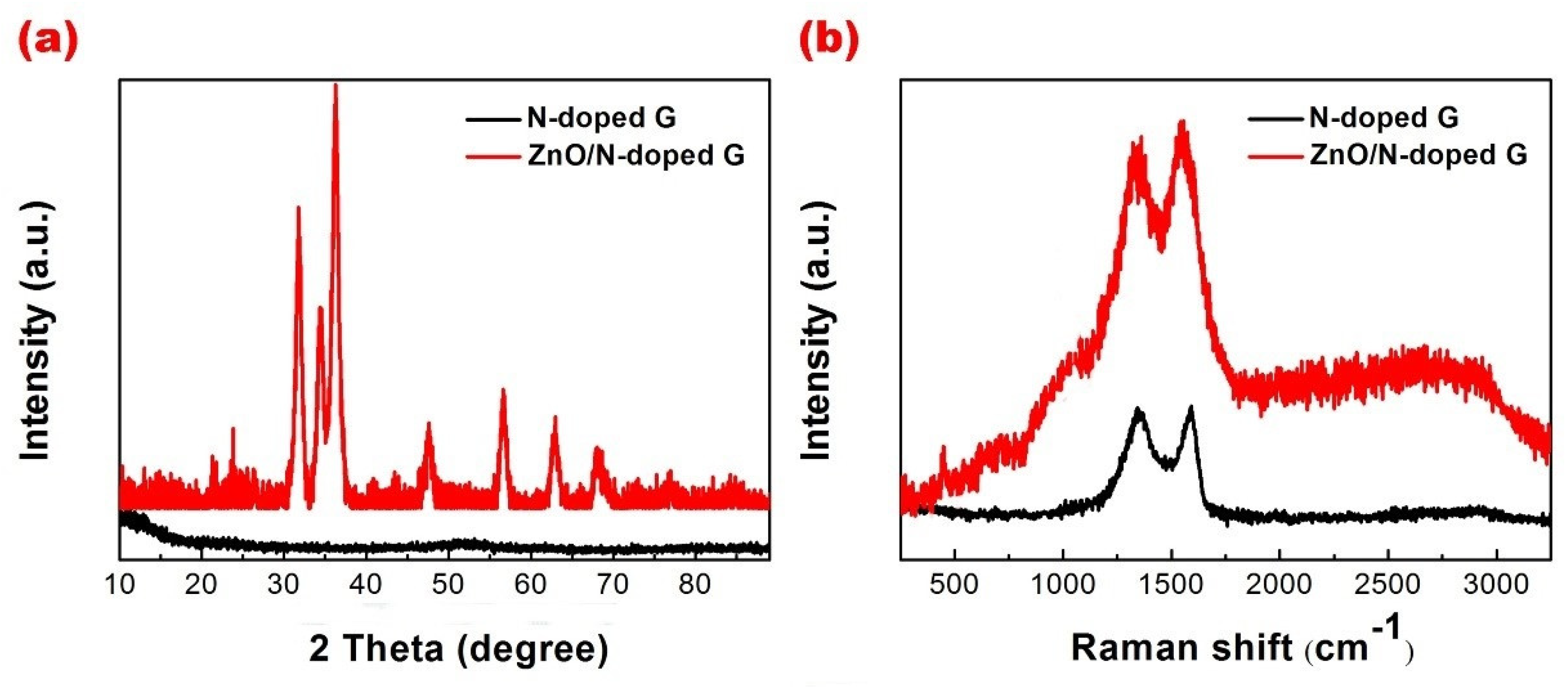
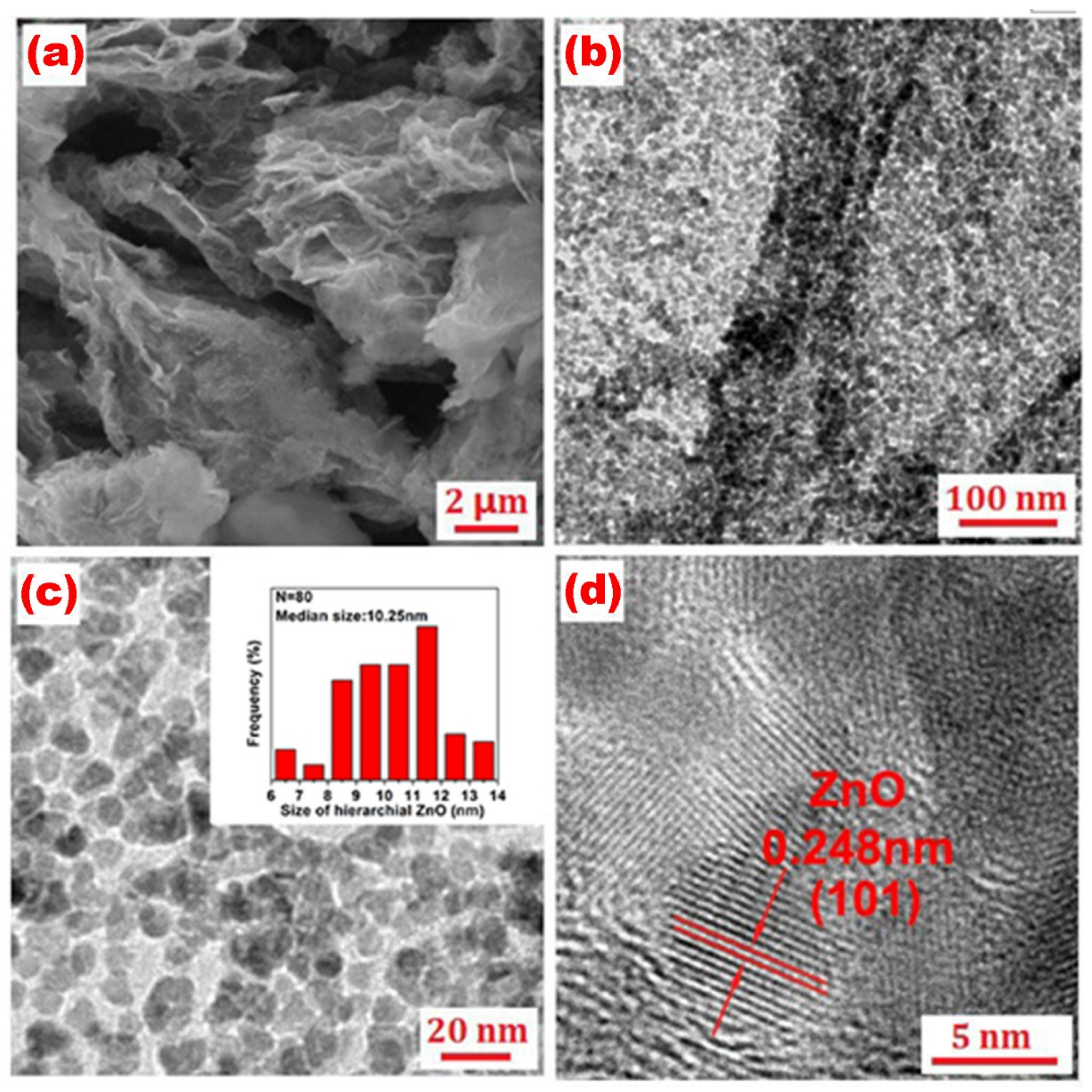
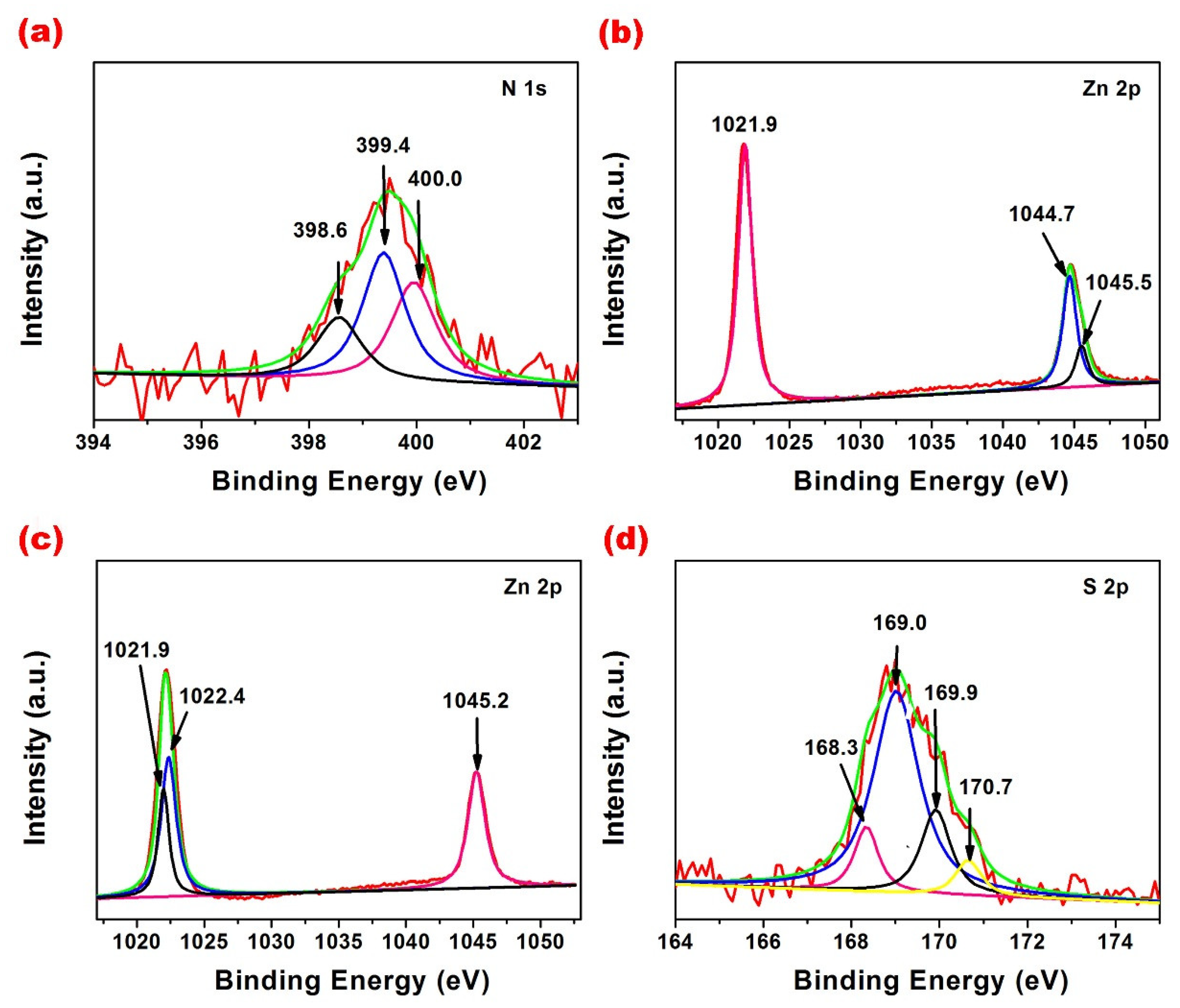
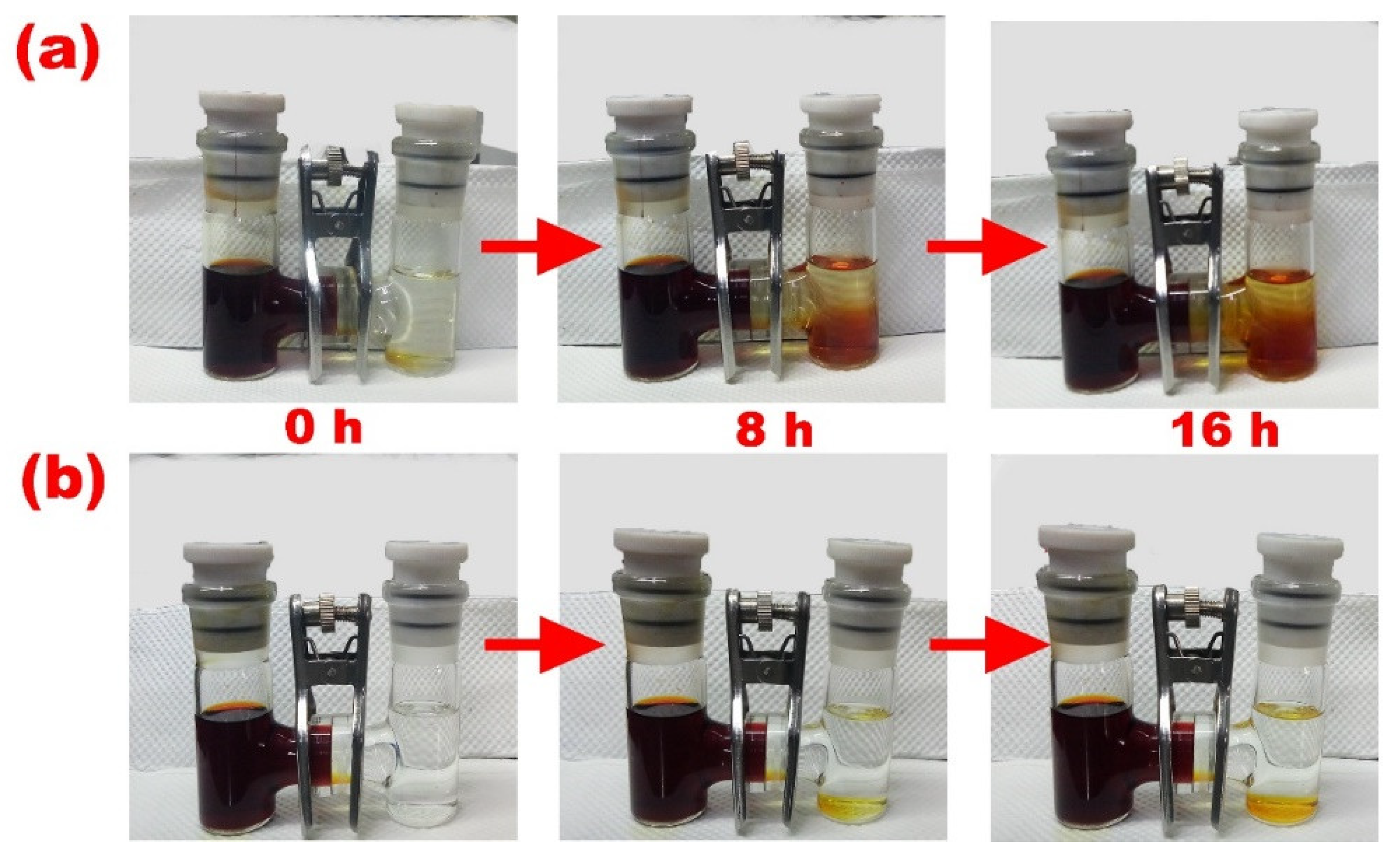
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Hardwick, L.J.; Abraham, K.M. Lithium-air and lithium-sulfur batteries. Mrs. Bull. 2011, 36, 506–512. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, Y.; Zhang, Z.; Li, J. A functional carbon layer-coated separator for high performance lithium sulfur batteries. Solid State Ionics 2015, 278, 166–171. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F.; Gao, Y.; Wang, Y.; Wang, S.; Gao, Q.; Jiao, Z.; Zhao, B.; Chen, Z. Inhibiting the shuttle effect of Li-S battery with a graphene oxide coating separator: Performance improvement and mechanism study. J. Power Sources 2017, 342, 929–938. [Google Scholar] [CrossRef]
- Evers, S.; Yim, T.; Nazar, L.F. Understanding the nature of absorption/adsorption in nanoporous polysulfide sorbents for the Li-S battery. J. Phys. Chem. C 2012, 116, 19653–19658. [Google Scholar] [CrossRef]
- Ji, X.; Evers, S.; Black, R.; Nazar, L.F. Stabilizing lithium-sulphur cathodes using polysulphide reservoirs. Nat. Commun. 2011, 2, 325. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Huang, Y.; Zhang, W.; Sun, X.; Yang, Y.; Wang, L.; Zong, M. Lock of sulfur with carbon black and a three-dimensional graphene@carbon nanotubes coated separator for lithium-sulfur batteries. J. Alloy. Compd. 2017, 708, 743–750. [Google Scholar] [CrossRef]
- Chai, L.; Wang, J.; Wang, H.; Zhang, L.; Yu, W.; Mai, L. Porous carbonized graphene-embedded fungus film as an interlayer for superior Li-S batteries. Nano Energy 2015, 17, 224–232. [Google Scholar] [CrossRef]
- Han, P.; Manthiram, A. Boron- and nitrogen- doped reduced graphene oxide coated separators for high-performance Li-S batteries. J. Power Sources 2017, 369, 87–94. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Zhang, Y.; Tan, T.; Wang, G.; Bakenov, Z. Enhanced cycle performance of Li/S battery with the reduced graphene oxide/activated carbon functional interlayer. J. Energy Chem. 2017, 26, 1276–1281. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. High-Performance Li-S batteries with an Ultra-lightweight MWCNT-Coated Separator. J. Phys. Chem. Lett. 2015, 5, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Song, X.; Wang, S.; Wang, Y.; Gao, T.; Ding, L.X.; Wang, H. A multifunctional separator modified with cobalt and nitrogen co-doped porous carbon nanofibers for Li-S batteries. J. Membr. Sci. 2018, 548, 247–253. [Google Scholar] [CrossRef]
- Zhu, J.; Ge, Y.; Kim, D.; Lu, Y.; Chen, C.; Jiang, M.; Zhang, X. A novel separator coated by carbon for achieving exceptional high performance lithium-sulfur batteries. Nano Energy 2016, 20, 176–184. [Google Scholar] [CrossRef]
- Balach, J.; Jaumann, T.; Mühlenhoff, S.; Eckert, J.; Giebeler, L. Enhanced polysulphide redox reaction using a RuO2 nanoparticle-decorated mesoporous carbon as functional separator coating for advanced lithium-sulphur batteries. Chem. Commun. 2016, 52, 8134–8137. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zuo, C.; Xu, X.; Wan, Y.; Wang, L.; Zhou, D.; Chen, Z. A thin TiO2 NTs/GO hybrid membrane applied as an interlayer for lithium-sulfur batteries. RSC Adv. 2018, 8, 429–434. [Google Scholar] [CrossRef]
- Shao, H.; Wang, W.; Zhang, H.; Wang, A.; Chen, X.; Huang, Y. Nano-TiO2 decorated carbon coating on the separator to physically and chemically suppress the shuttle effect for lithium-sulfur battery. J. Power Sources 2018, 378, 537–545. [Google Scholar] [CrossRef]
- Wei, S.Z.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; Mcdowell, M.T.; Hsu, P.C.; Cui, Y. Sulphur-TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium-sulphur batteries. Nat. Commun. 2013, 4, 1331. [Google Scholar]
- Tang, Q.; Li, H.; Zhang, J.; Lin, Z.; Pan, Y.; Hu, Q.; You, Y.; Ye, Y. A dual-faced interlayer prepared by electron beam evaporation for enhanced-performance lithium-sulfur batteries. Rsc Adv. 2017, 7, 44035–44042. [Google Scholar] [CrossRef]
- Xu, Q.; Hu, G.C.; Bi, H.L.; Xiang, H.F. A trilayer carbon nanotube/Al2O3/polypropylene separator for lithium-sulfur batteries. Ionics 2014, 21, 981–986. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, L.; Yuan, K.; Li, Z.; Hao, Z.; Xiang, J.; Huang, Y. SnO2 as high-efficiency polysulfide trap in lithium-sulfur battery. Nanoscale 2016, 8, 13638–13645. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Q.; Qin, X.; Liang, G.; Han, W.; Zhou, D.; He, Y.B.; Li, B.; Kang, F. Suppressing self-discharge and shuttle effect of lithium-sulfur batteries with V2O5-decorated carbon nanofiber interlayer. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yan, L.; Luo, Y.; Wang, D.; Jiang, K.; Li, Q.; Fan, S.; Wang, J. Li-S batteries: Ultrathin MnO2/graphene oxide/carbon nanotube interlayer as efficient polysulfide-trapping shield for high-performance Li-S batteries. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Ma, G.Q.; Wen, Z.Y.; Wang, Q.S.; Jin, J.; Wu, X.W.; Zhang, J.C. Effects of CeO2 Nano-crystal on electrochemical properties of Lithium/Sulfur batteries. J. Inorg. Mater. 2015, 30, 913–918. [Google Scholar]
- Kong, Y.; Luo, J.; Jin, C.; Yuan, H.; Sheng, O.; Zhang, L.; Fang, C.; Zhang, W.; Huang, H.; Xia, Y. Enhanced sulfide chemisorption by conductive Al-doped ZnO decorated carbon nanoflakes for advanced Li-S batteries. Nano Res. 2018, 11, 477–489. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Zhang, Y.; Zhang, C.; Wang, G.; Zhao, Y.; Yin, F.; Bakenov, Z. In situ sol-gel synthesis of ultrafine ZnO nanocrystals anchored on graphene as anode material for Lithium-ion batteries. Ceram. Int. 2016, 42, 12371–12377. [Google Scholar] [CrossRef]
- Yin, F.; Liu, X.; Zhang, Y.; Zhao, Y.; Menbayeva, A.; Bakenov, Z.; Wang, X. Well-dispersed sulfur anchored on interconnected polypyrrole nanofiber network as high performance cathode for lithium-sulfur batteries. Solid State Sci. 2017, 66, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Holloway, B.C.; Kraft, O.; Shuh, D.K.; Kelly, M.A.; Nix, W.D. Interpretation of X-ray photoelectron spectra of elastic amorphous carbon nitride films. Appl. Phys. Lett. 1999, 74. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Gao, F.; Ma, R.; Du, A.; Tan, T.; Du, M.; Zhao, X.; Fan, Y.; Wen, M. ZnO Nanoparticles Anchored on a N-Doped Graphene-Coated Separator for High Performance Lithium/Sulfur Batteries. Metals 2018, 8, 755. https://doi.org/10.3390/met8100755
Wang S, Gao F, Ma R, Du A, Tan T, Du M, Zhao X, Fan Y, Wen M. ZnO Nanoparticles Anchored on a N-Doped Graphene-Coated Separator for High Performance Lithium/Sulfur Batteries. Metals. 2018; 8(10):755. https://doi.org/10.3390/met8100755
Chicago/Turabian StyleWang, Suyu, Fan Gao, Ruina Ma, An Du, Taizhe Tan, Miao Du, Xue Zhao, Yongzhe Fan, and Ming Wen. 2018. "ZnO Nanoparticles Anchored on a N-Doped Graphene-Coated Separator for High Performance Lithium/Sulfur Batteries" Metals 8, no. 10: 755. https://doi.org/10.3390/met8100755



