DFT Modelling of Cu Segregation in Al-Cu Alloys Covered by an Ultrathin Oxide Film and Possible Links with Passivity
Abstract
:1. Introduction
2. Methods
2.1. Calculations
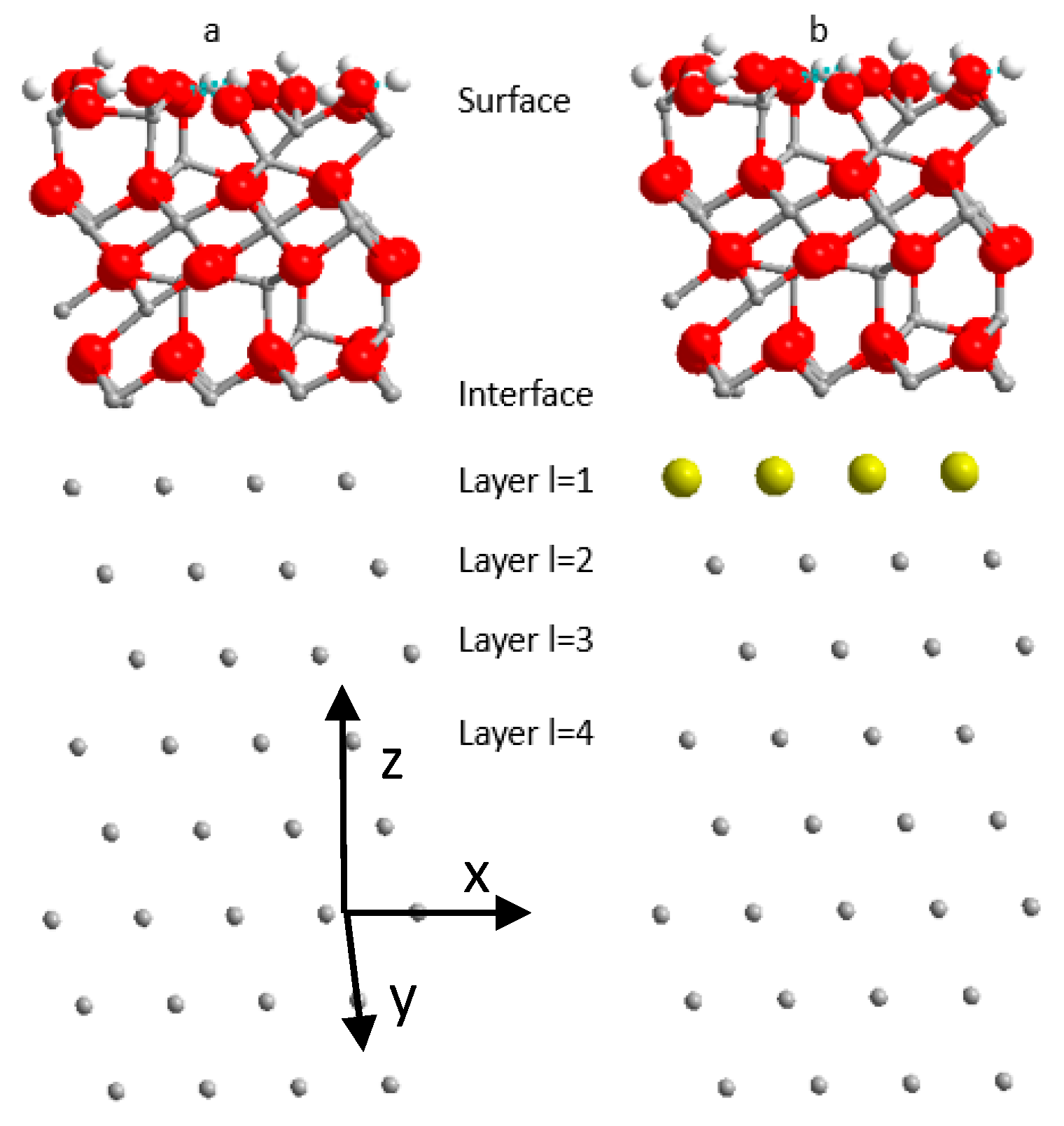
2.2. Models
2.3. Electronic and Charge Analysis
2.4. Energetics
3. Results
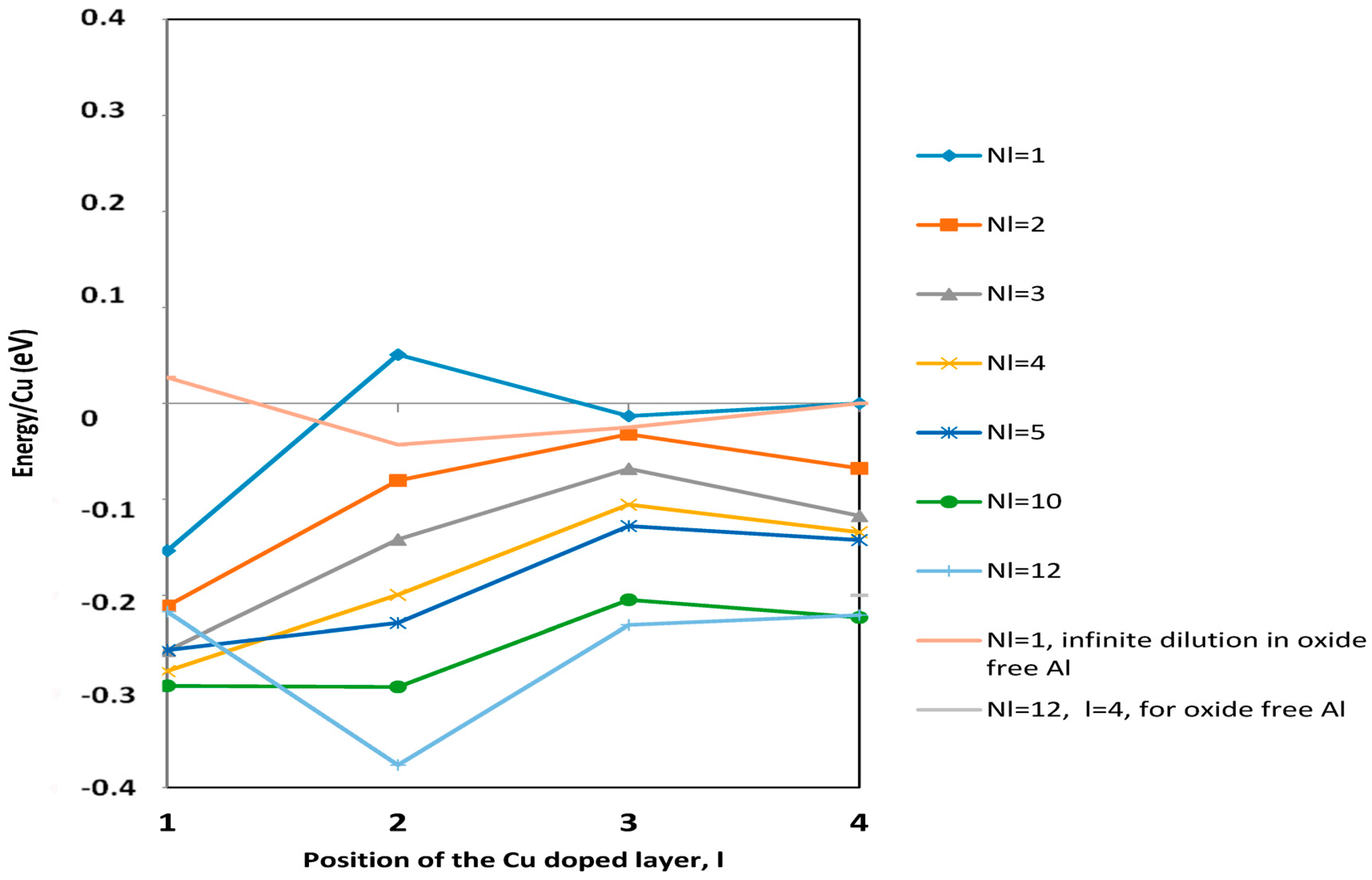
3.1. Energetics of Cu in Al(111) Covered by an Oxide Layer as a Function of Cu Concentration and Location
3.2. Copper Segregation at the Interface with the Passive Film
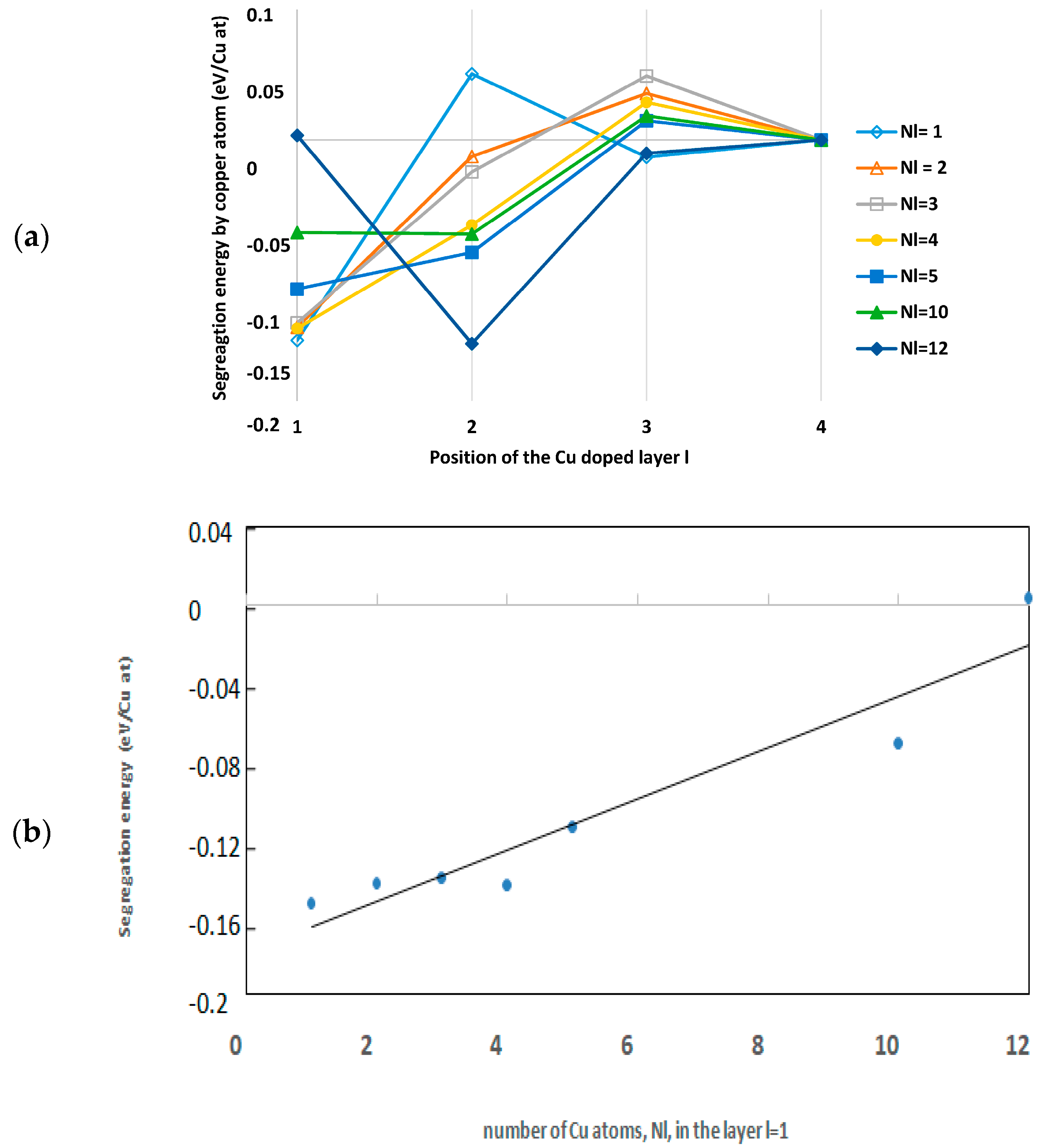
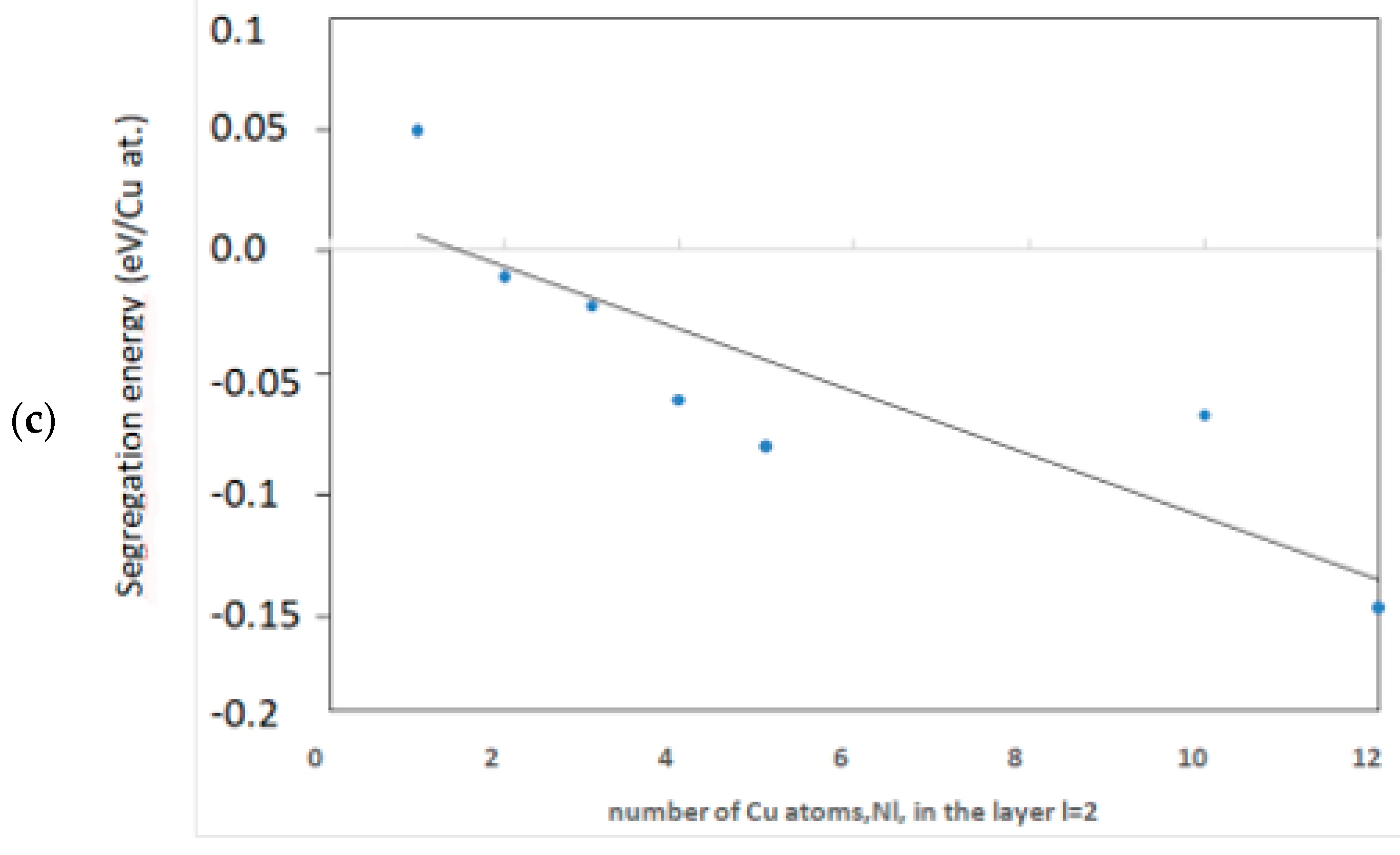
3.2.1. Energy of Segregation
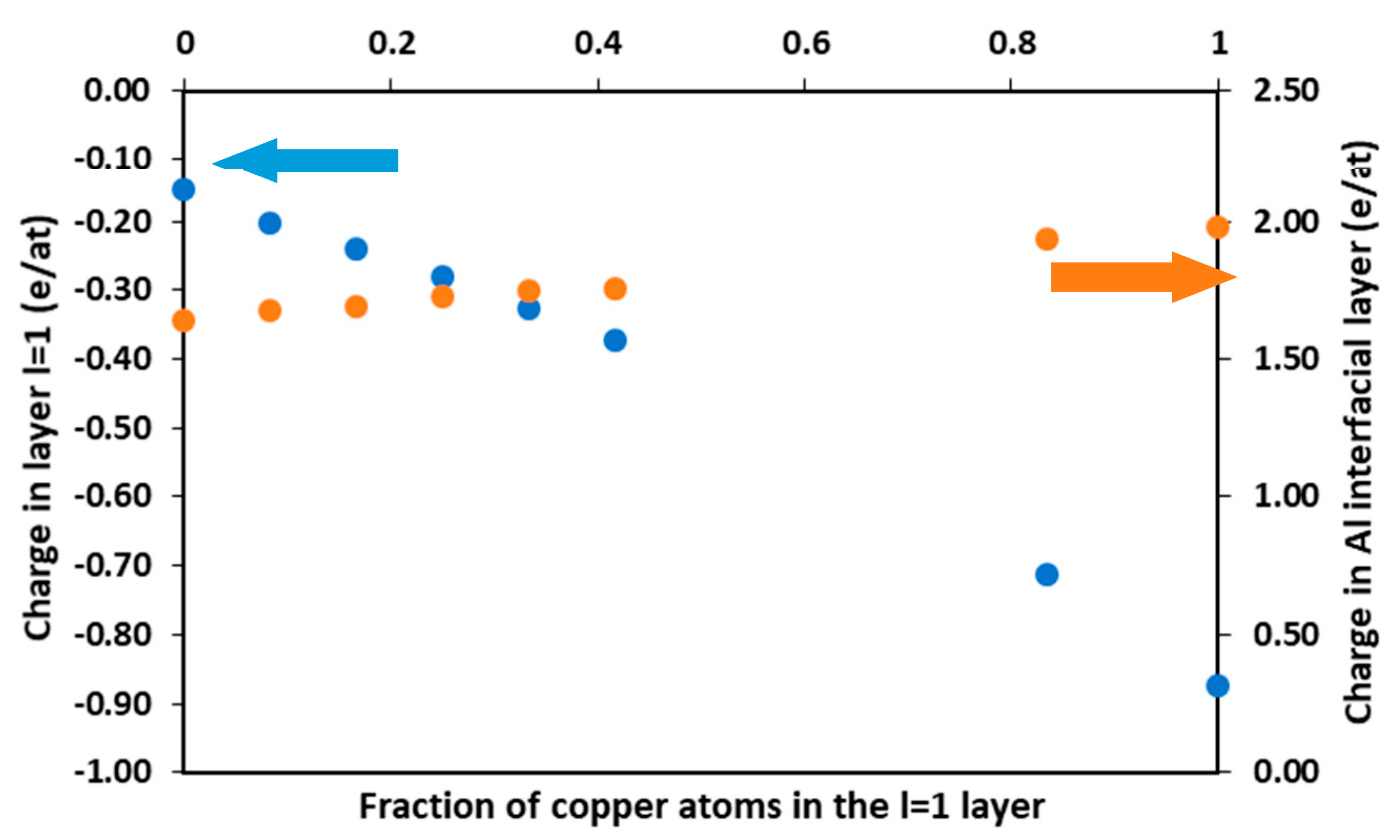
3.2.2. Charge Analysis and Electronic Workfunction
Cu Located in the l = 1 Layer
Full Cu Layer at Depths l = 2, 3, 4
Cu at Low Concentration
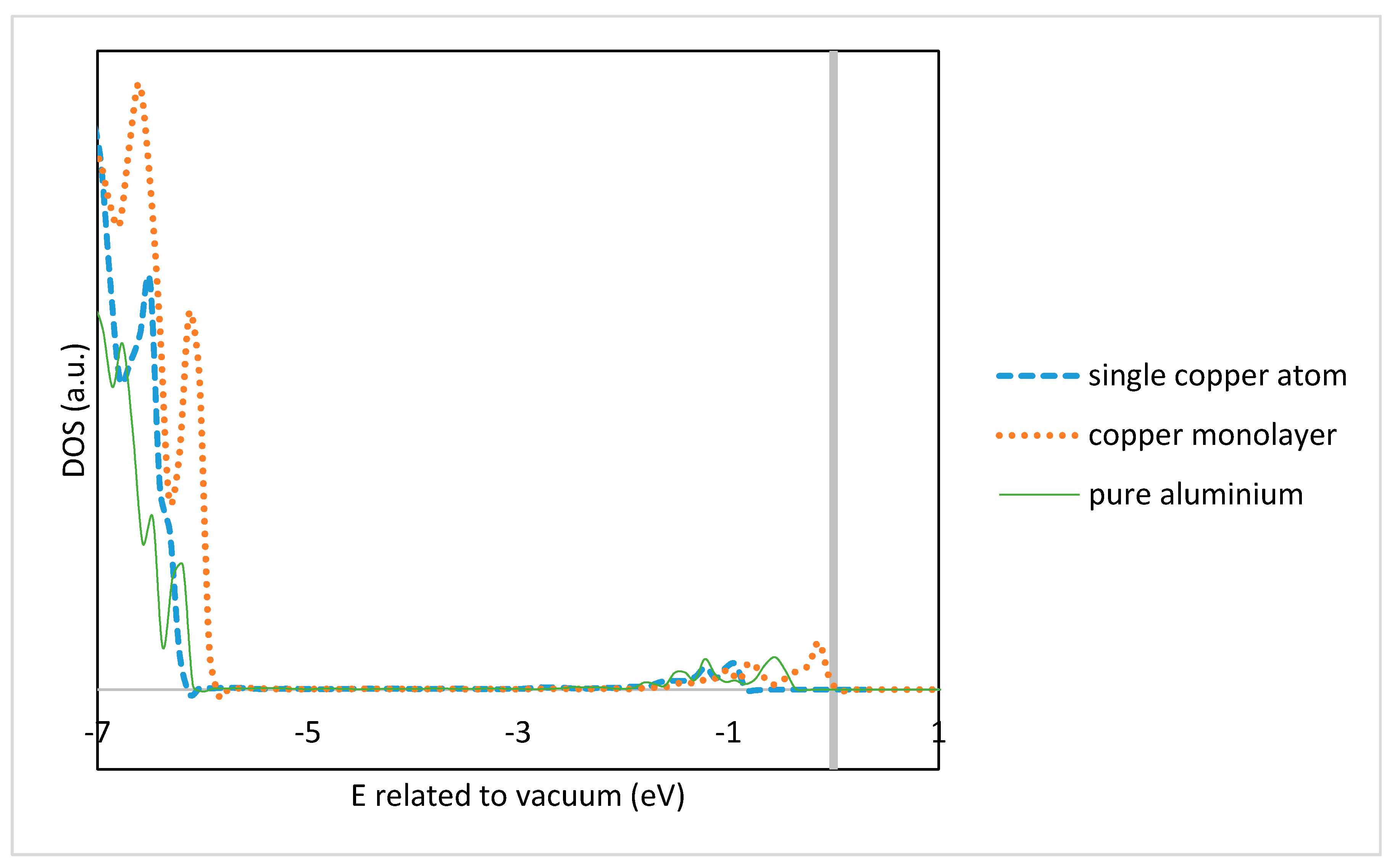
3.3. Electronic Density of States Analysis
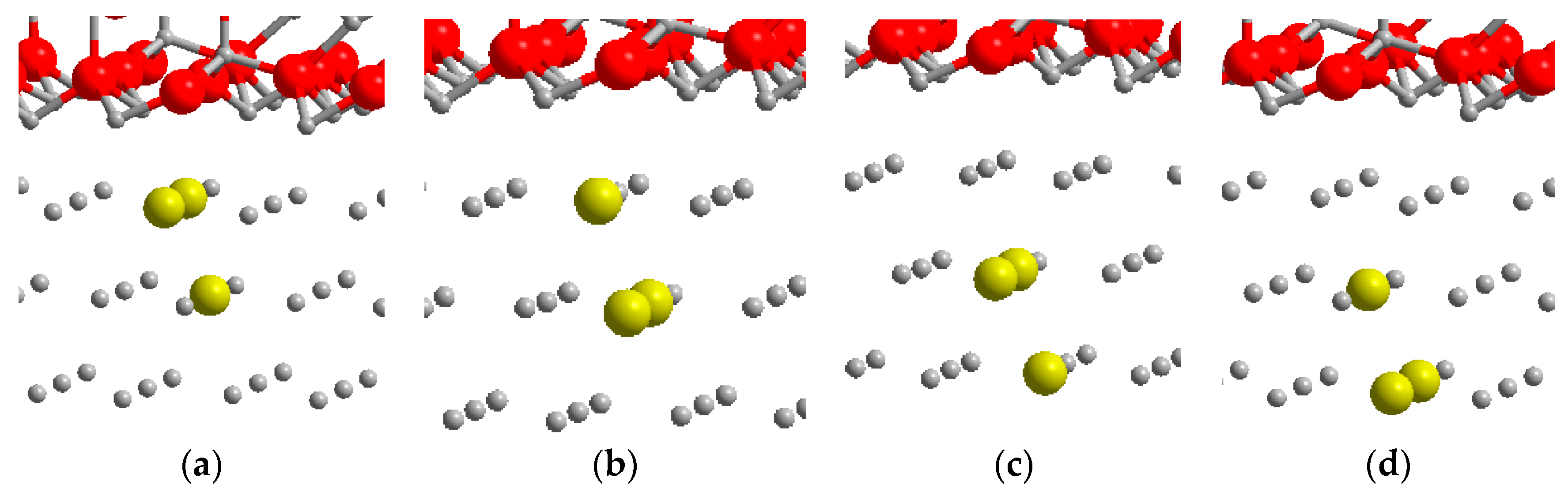
3.4. Copper Segregation in GP Zones
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vargel, C. Corrosion of Aluminium, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Strohmeier, B.R. An ESCA method for determining the oxide thickness on aluminum alloys. Surf. Interface Anal. 1990, 15, 51–56. [Google Scholar] [CrossRef]
- Van den Brand, J.; Sloof, W.G.; Terryn, H.; De Wit, J.H.W. Correlation between hydroxyl fraction and O/Al atomic ratio as determined from XPS spectra of aluminium oxide layers. Surf. Interface Anal. 2004, 36, 81–88. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion De L’aluminium; Dunod: Paris, France, 1999. [Google Scholar]
- Costa, D.; Ribeiro, T.; Mercuri, F.; Pacchioni, G.; Marcus, P. Atomistic Modeling of Corrosion Resistance: A First Principles Study of O2 Reduction on the Al(111) Surface Covered with a Thin Hydroxylated Alumina Film. Adv. Mater. Interfaces 2014, 1, 1300072. [Google Scholar] [CrossRef]
- Marcus, P. (Ed.) Corrosion Mechanisms in Theory and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Taylor, C.D.; Marcus, P. Theoretical Methods in Modeling Corrosion; Wiley and Sons: New York, NY, USA, 2015. [Google Scholar]
- Kokalj, A.; Peljhan, S.; Finsgar, M.; Milosev, I. What Determines the Inhibition Effectiveness of ATA, BTAH, and BTAOH Corrosion Inhibitors on Copper? J. Am. Chem. Soc. 2010, 132, 16657–16668. [Google Scholar] [CrossRef] [PubMed]
- Milosev, I.; Kovacevic, N.; Kovac, J.; Kokalj, A. The roles of mercapto, benzene and methyl groups in the corrosion inhibition of imidazoles on copper: I. Experimental characterization. Corros. Sci. 2015, 98, 107–118. [Google Scholar] [CrossRef]
- Taylor, C.D. Atomistic Modeling of Corrosion Events at the Interface between a Metal and Its Environment. Int. J. Corros. 2012, 2012, 204640. [Google Scholar] [CrossRef]
- Chiter, F.; Lacaze-Dufaure, C.; Tang, H.; Pebere, N. DFT studies of the bonding mechanism of 8-hydroxyquinoline and derivatives on the (111) aluminum surface. Phys. Chem. Chem. Phys. 2015, 17, 22243–22258. [Google Scholar] [CrossRef] [PubMed]
- Poberžnik, M.; Kokalj, A. Origin of Surprising Attractive Interactions between Electronegative Oxygen Adatoms on Aluminum Surfaces. J. Phys. Chem. C 2016, 120, 25915–25922. [Google Scholar] [CrossRef]
- Hoshino, T.; Fujima, N.; Asato, M.; Tamura, R. Medium-ranged interactions of transition-metal (3d and 4d) impurity pairs in Al and atomic structures of Al-rich Al-transition-metal alloys. J. Alloys Compd. 2007, 434–435, 572–576. [Google Scholar] [CrossRef]
- Benali, A.; Lacaze-Dufaure, C.; Morillo, J. Density functional study of copper segregation in aluminum. Surf. Sci. 2011, 605, 341–350. [Google Scholar] [CrossRef]
- Braunovic, M.; Alexandrov, N. Intermetallic compounds at aluminum-to-copper electrical interfaces: Effect of temperature and electric current. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1994, 17, 78–85. [Google Scholar] [CrossRef]
- Lee, M.J.G.; Gensch, M.; Shkrebtii, A.I.; Herrmann, T.; Richter, W.; Esser, N.; Hofmann, P. Surface states and resonances on Al(110): Ultraviolet photoemission spectroscopy and ab initio calculations. Phys. Rev. B 2005, 72, 85408. [Google Scholar] [CrossRef]
- Vaithyanathan, V.; Wolverton, C.; Chen, L.Q. Multiscale Modeling of Precipitate Microstructure Evolution. Phys. Rev. Lett. 2002, 88, 125503. [Google Scholar] [CrossRef] [PubMed]
- Wolverton, C.; Ozoliņš, V. Entropically Favored Ordering: The Metallurgy of Al2Cu Revisited. Phys. Rev. Lett. 2001, 86, 5518–5521. [Google Scholar] [CrossRef] [PubMed]
- Wolverton, C.; Ozoliņš, V. First-principles aluminum database: Energetics of binary Al alloys and compounds. Phys. Rev. B 2006, 73, 144104. [Google Scholar] [CrossRef]
- Wolverton, C.; Yan, X.-Y.; Vijayaraghavan, R.; Ozoliš, V. Incorporating first-principles energetics in computational thermodynamics approaches. Acta Mater. 2002, 50, 2187–2197. [Google Scholar] [CrossRef]
- Vaithyanathan, V.; Wolverton, C.; Chen, L.Q. Multiscale modeling of θ′ precipitation in Al-Cu binary alloys. Acta Mater. 2004, 52, 2973–2987. [Google Scholar] [CrossRef]
- Wolverton, C.; Ozolins, V.; Zunger, A. Short-range-order types in binary alloys: A reflection of coherent phase stability. J. Phys. Condens. Matter. 2000, 12, 2749. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, L.; Li, B.; Song, Q.; Wu, P. Structural, Elastic, and Electronic Properties of Al-Cu Intermetallics from First-Principles Calculations. J. Electron. Mater. 2009, 38, 356–364. [Google Scholar] [CrossRef]
- Wang, S.Q.; Schneider, M.; Ye, H.Q.; Gottstein, G. First-principles study of the formation of Guinier-Preston zones in Al-Cu alloys. Scr. Mater. 2004, 51, 665–669. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, Y.; Li, L.; Liu, R.; Qiu, M.; Xu, C.; Chen, X. A periodic density functional theory calculation: The structure of isolated copper (I) oxide species on γ-Al2O3 (110) surface and its adsorption ability toward thiophene and benzene. Appl. Surf. Sci. 2015, 346, 165–171. [Google Scholar] [CrossRef]
- Wang, J.; Wolverton, C.; Müller, S.; Liu, Z.-K.; Chen, L.-Q. First-principles growth kinetics and morphological evolution of Cu nanoscale particles in Al. Acta Mater. 2005, 53, 2759–2764. [Google Scholar] [CrossRef]
- Mohamed, I.F.; Yonenaga, Y.; Lee, S.; Edalati, K.; Horita, Z. Age hardening and thermal stability of Al-Cu alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2015, 627, 111–118. [Google Scholar] [CrossRef]
- Lanthony, C.; Ducéré, J.M.; Rouhani, M.D.; Hemeryck, A.; Estève, A.; Rossi, C. On the early stage of aluminum oxidation: An extraction mechanism via oxygen cooperation. J. Chem. Phys. 2012, 137, 94707. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.D.; Grönbeck, H.; Hellman, A. Mechanism for Limiting Thickness of Thin Oxide Films on Aluminum. Phys. Rev. Lett. 2014, 112, 146103. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.C.; Lu, H.; Li, D.Y. Understanding the corrosion behavior of isomorphous Cu–Ni alloy from its electron work function. Mater. Chem. Phys. 2016, 173, 238–245. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoftpseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Ribeiro, T.; Cornette, P.; Marcus, P. DFT Modeling of Corrosion Inhibition by Organic Molecules: Carboxylates as Inhibitors of Aluminum Corrosion. J. Phys. Chem. C 2016, 120, 28607–28616. [Google Scholar] [CrossRef]
- Eastment, R.M.; Mee, C.H.B. Work function measurements on (100), (110) and (111) surfaces of aluminium. J. Phys. F Met. Phys. 1973, 3, 1738. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Hoshino, T.; Asato, M.; Tanaka, S.; Nakamura, F.; Fujima, N. First-principles calculations for stability of atomic structures of Al-rich AlX (X = Sc-Zn) alloys, including AlMn quasicrystal: II. Medium-ranged interactions of X pairs in Al. Intermetallics 2006, 14, 913–916. [Google Scholar] [CrossRef]
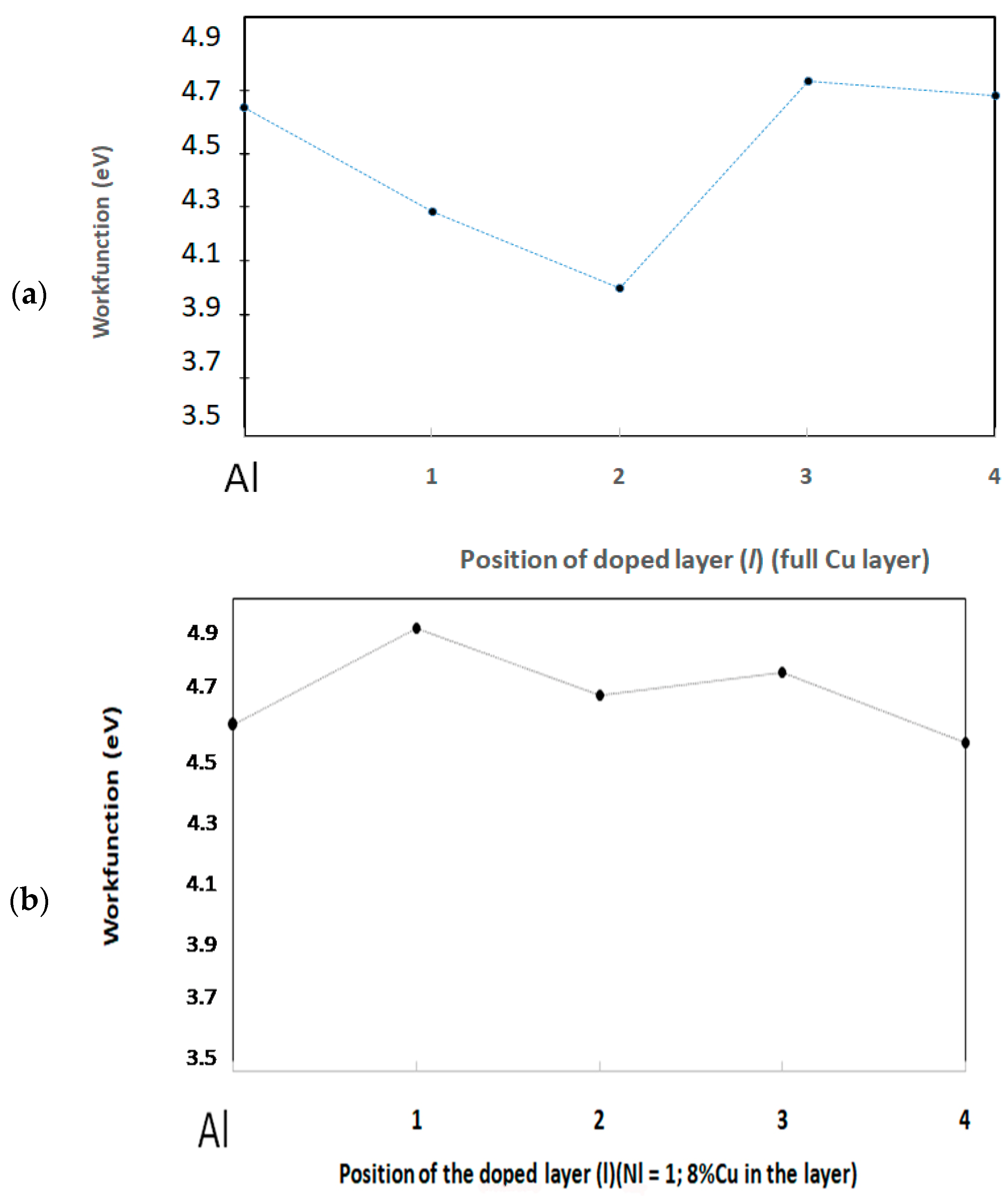
| Composition of the Metal Layers Under the Oxide Film | OxideBand Gap (eV) | Valence Band Level/Vacuum (eV) | Workfunction φe (eV) |
|---|---|---|---|
| Pure Al | 4.48 | −6.00 | 4.60 |
| Nl = 1 Cu | 4.53 | −6.09 | 4.91 |
| Nl= 12 Cu, l = 1 | 4.37 | −5.84 | 4.25 |
| Nl = 12 Cu, l = 2 | 4.78 * | −6.44 * | 4.00 * |
| Configuration | Position 1 | Position 1 in Absence of Oxide | Position 2 | Position 2 in Absence of Oxide | Bulk Position |
|---|---|---|---|---|---|
| Configuration 1 | −0.16 | −0.08 | −0.12 | −0.13 | −0.06 |
| Configuration 2 | −0.11 | −0.08 | −0.09 | −0.12 | −0.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornette, P.; Costa, D.; Marcus, P. DFT Modelling of Cu Segregation in Al-Cu Alloys Covered by an Ultrathin Oxide Film and Possible Links with Passivity. Metals 2017, 7, 366. https://doi.org/10.3390/met7090366
Cornette P, Costa D, Marcus P. DFT Modelling of Cu Segregation in Al-Cu Alloys Covered by an Ultrathin Oxide Film and Possible Links with Passivity. Metals. 2017; 7(9):366. https://doi.org/10.3390/met7090366
Chicago/Turabian StyleCornette, Pauline, Dominique Costa, and Philippe Marcus. 2017. "DFT Modelling of Cu Segregation in Al-Cu Alloys Covered by an Ultrathin Oxide Film and Possible Links with Passivity" Metals 7, no. 9: 366. https://doi.org/10.3390/met7090366




