Effects of the Preparation Solvent on the Catalytic Properties of Cobalt–Boron Alloy for the Hydrolysis of Alkaline Sodium Borohydride
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of the Co–B Samples
2.2. Hydrogen Generation Measurement
3. Results and Discussion
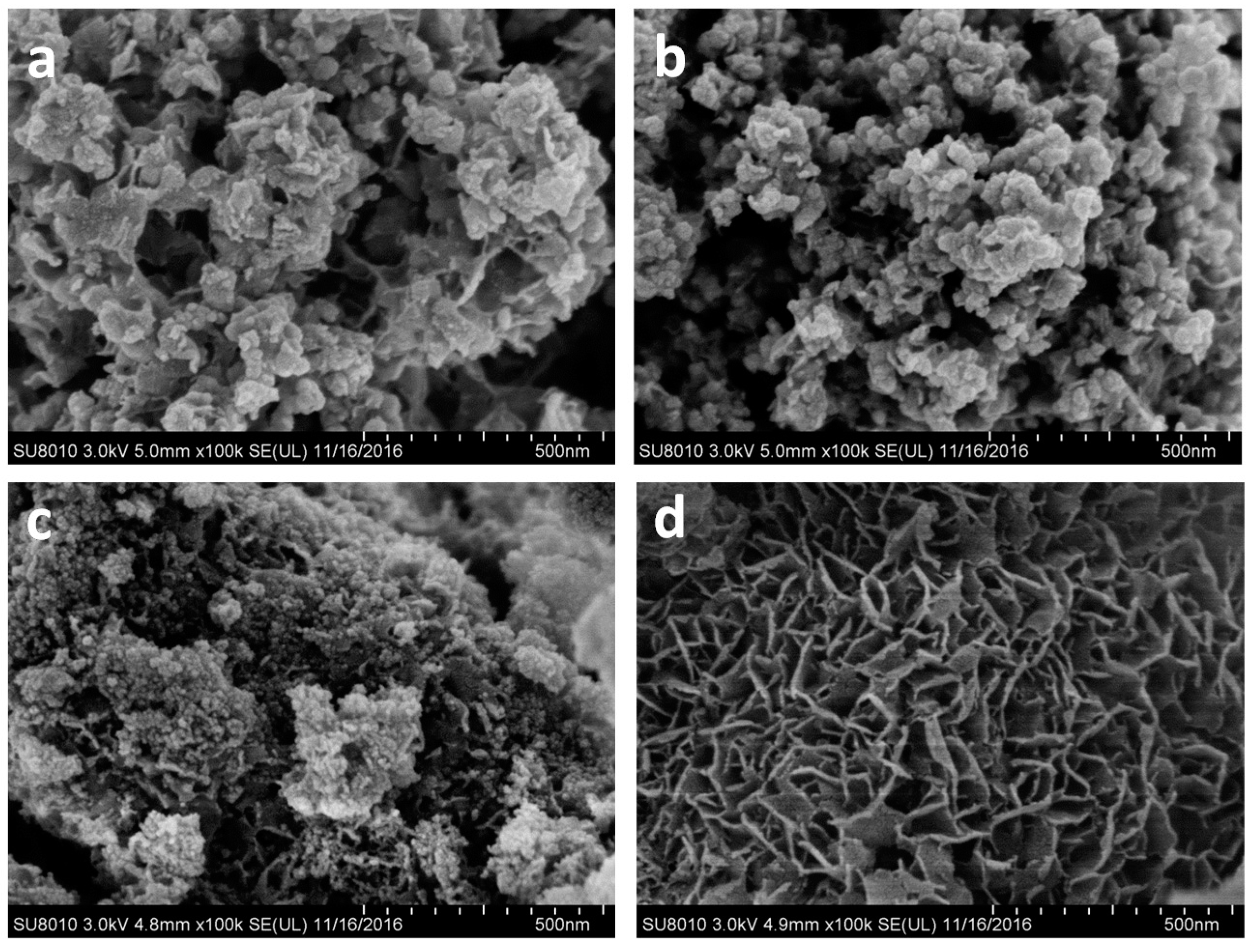
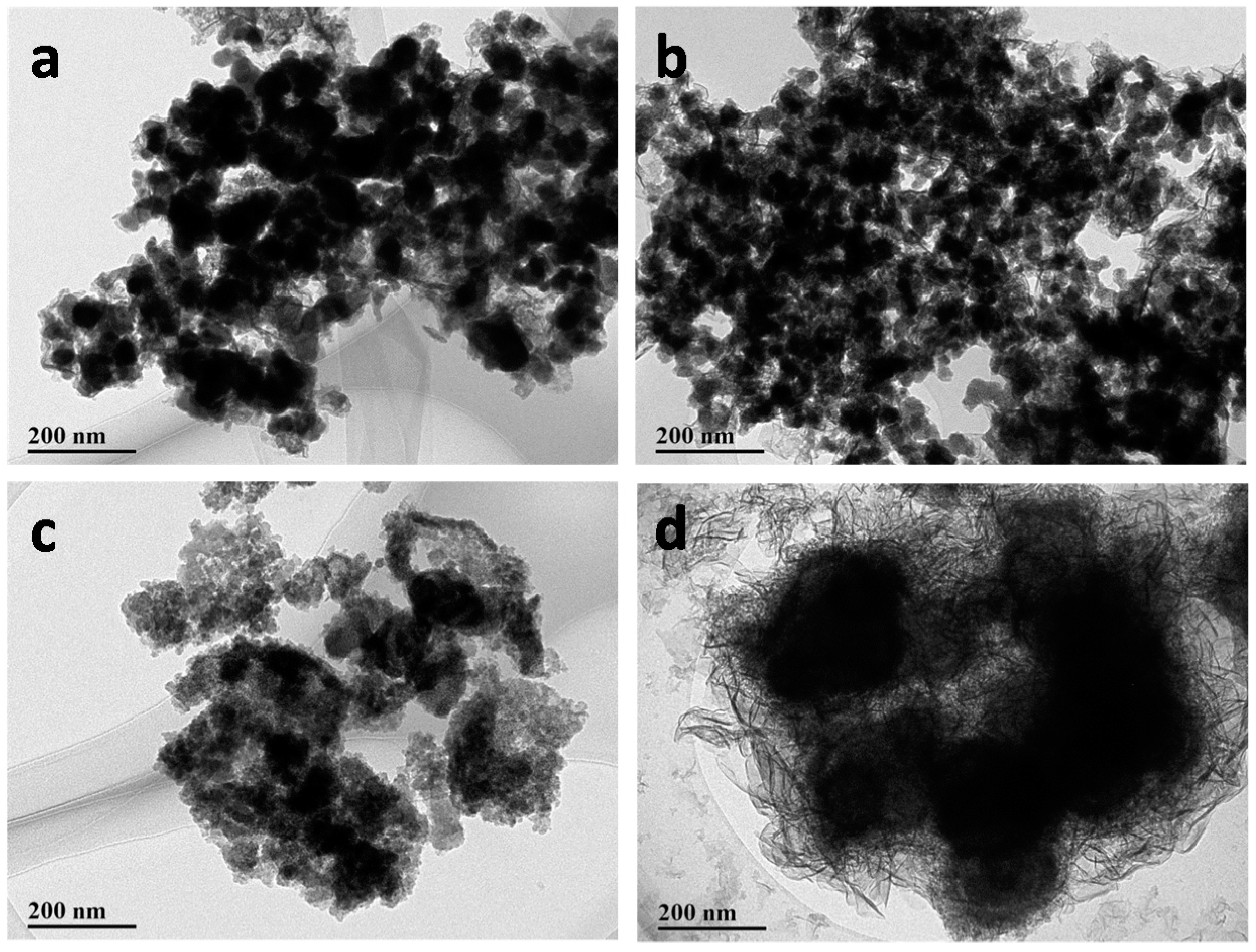
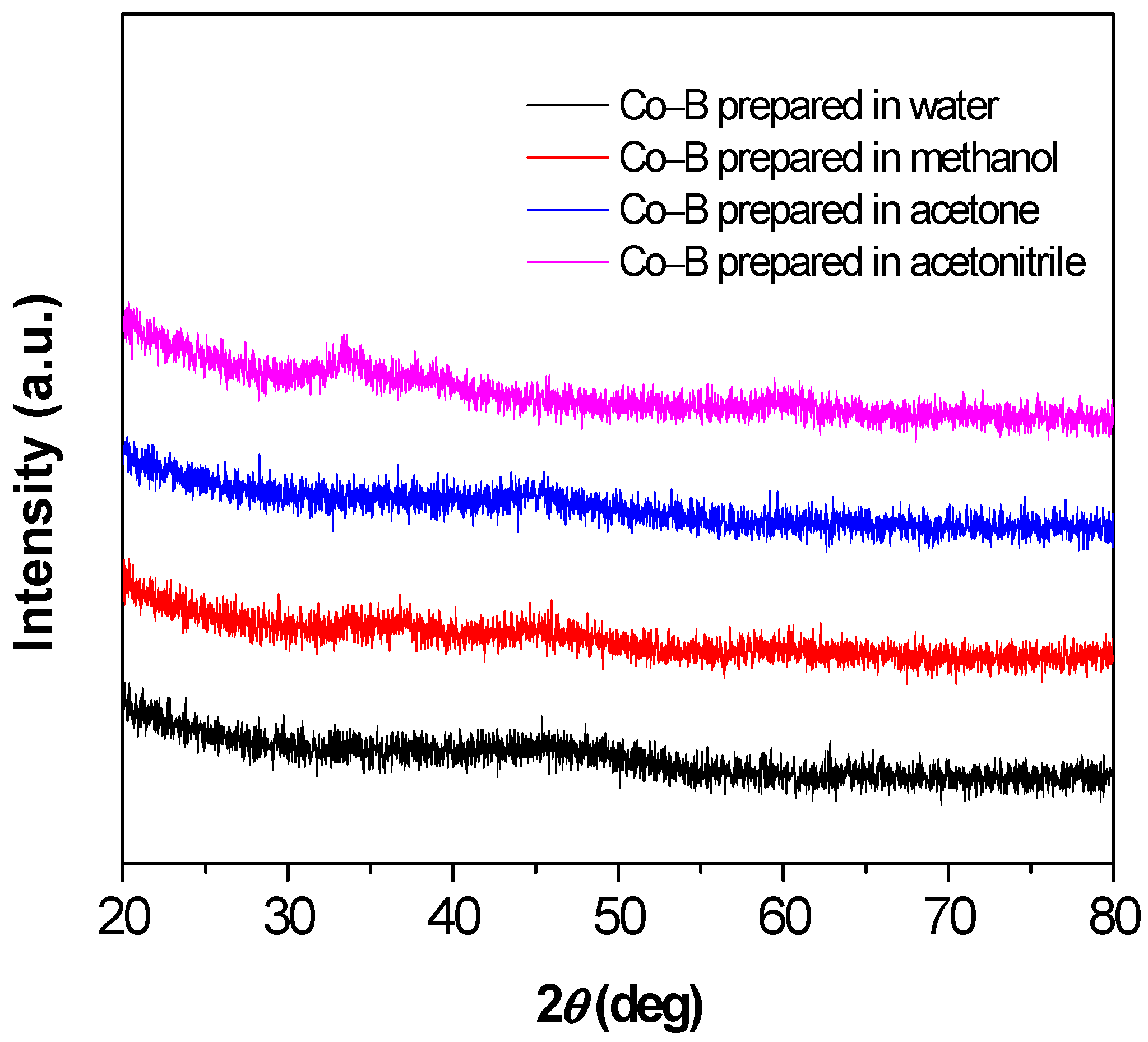
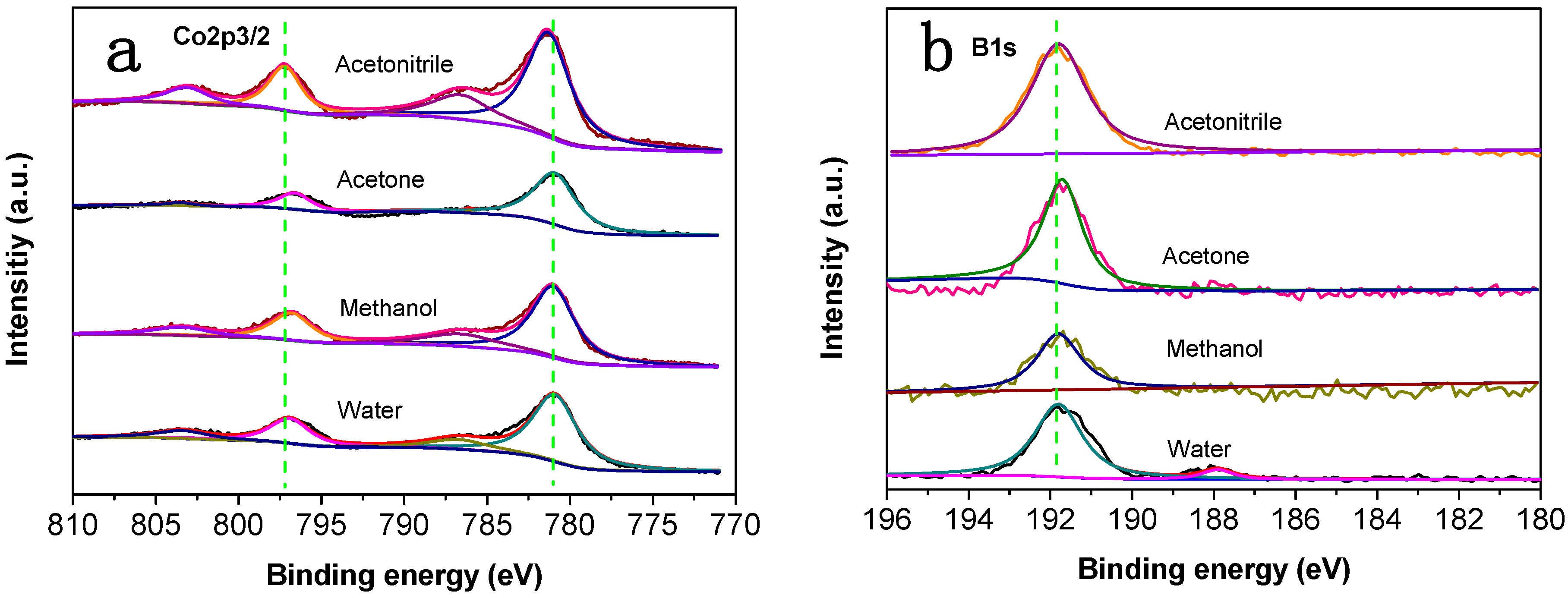
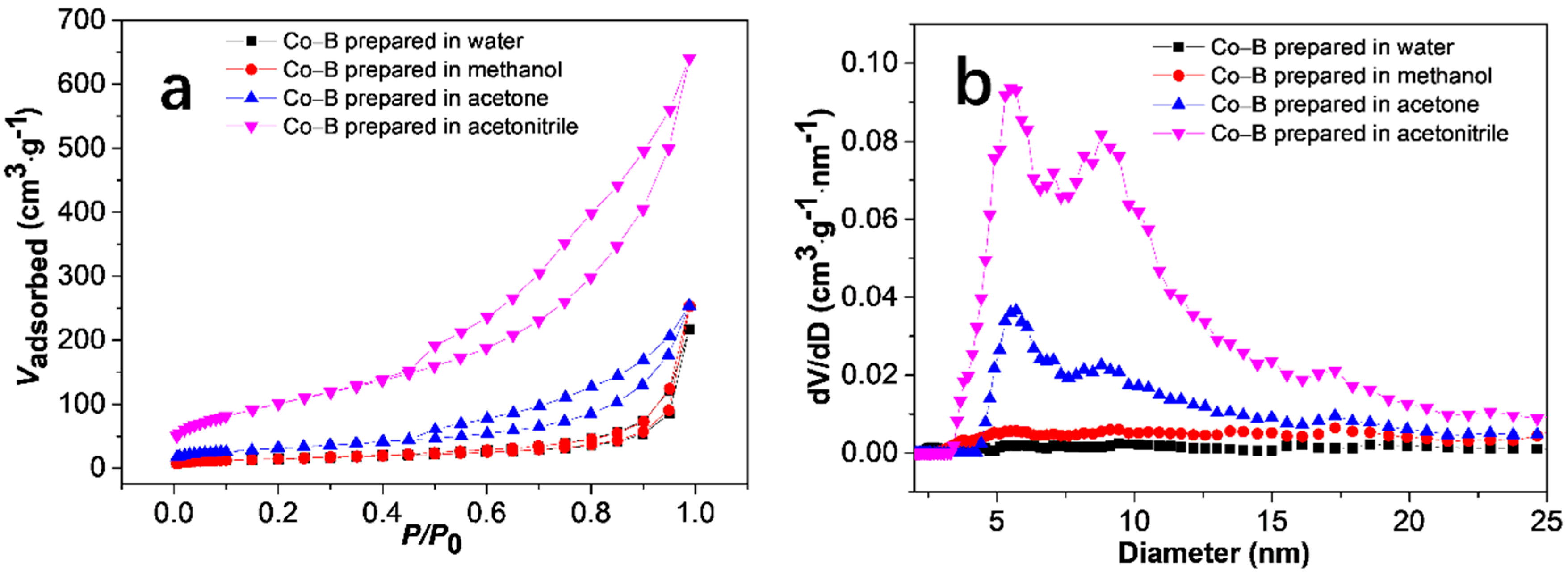
3.1. Catalyst Characterization
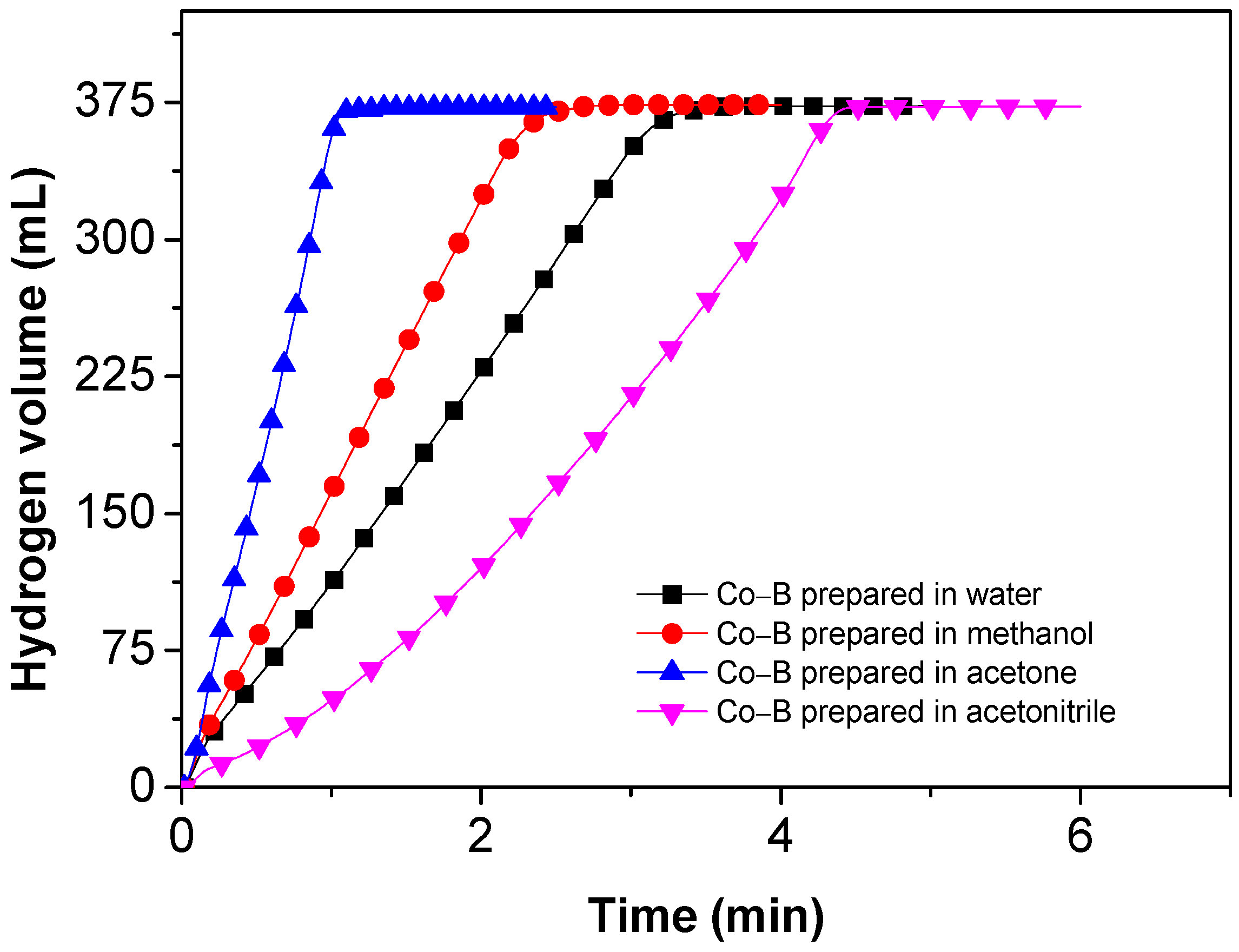
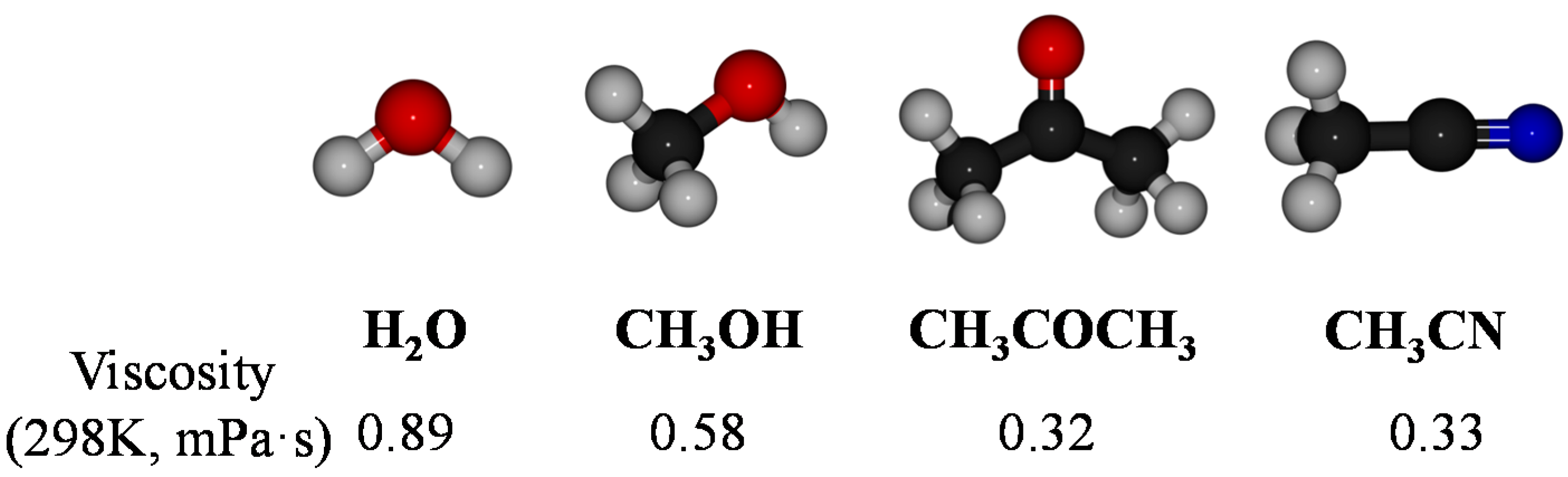
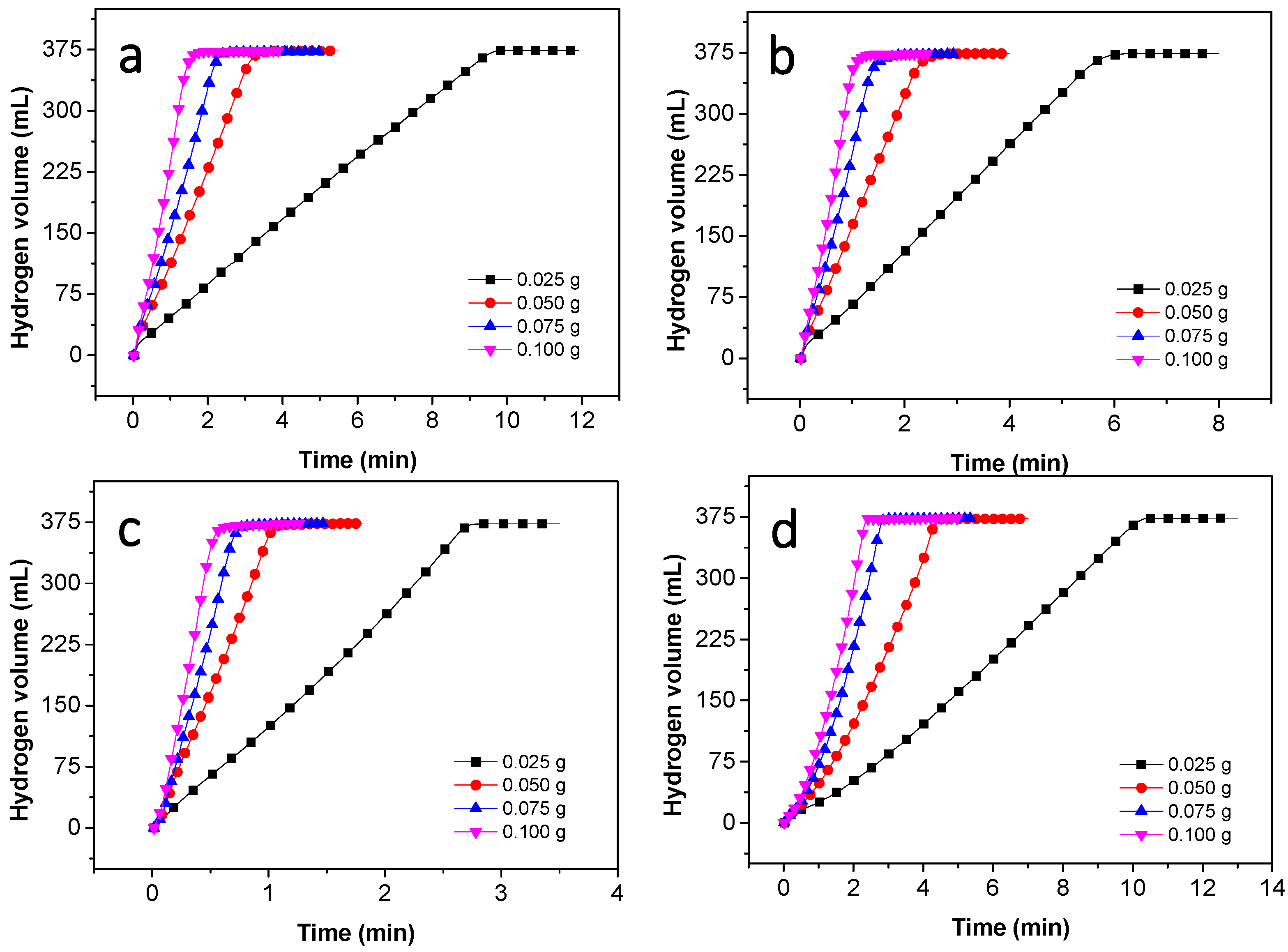
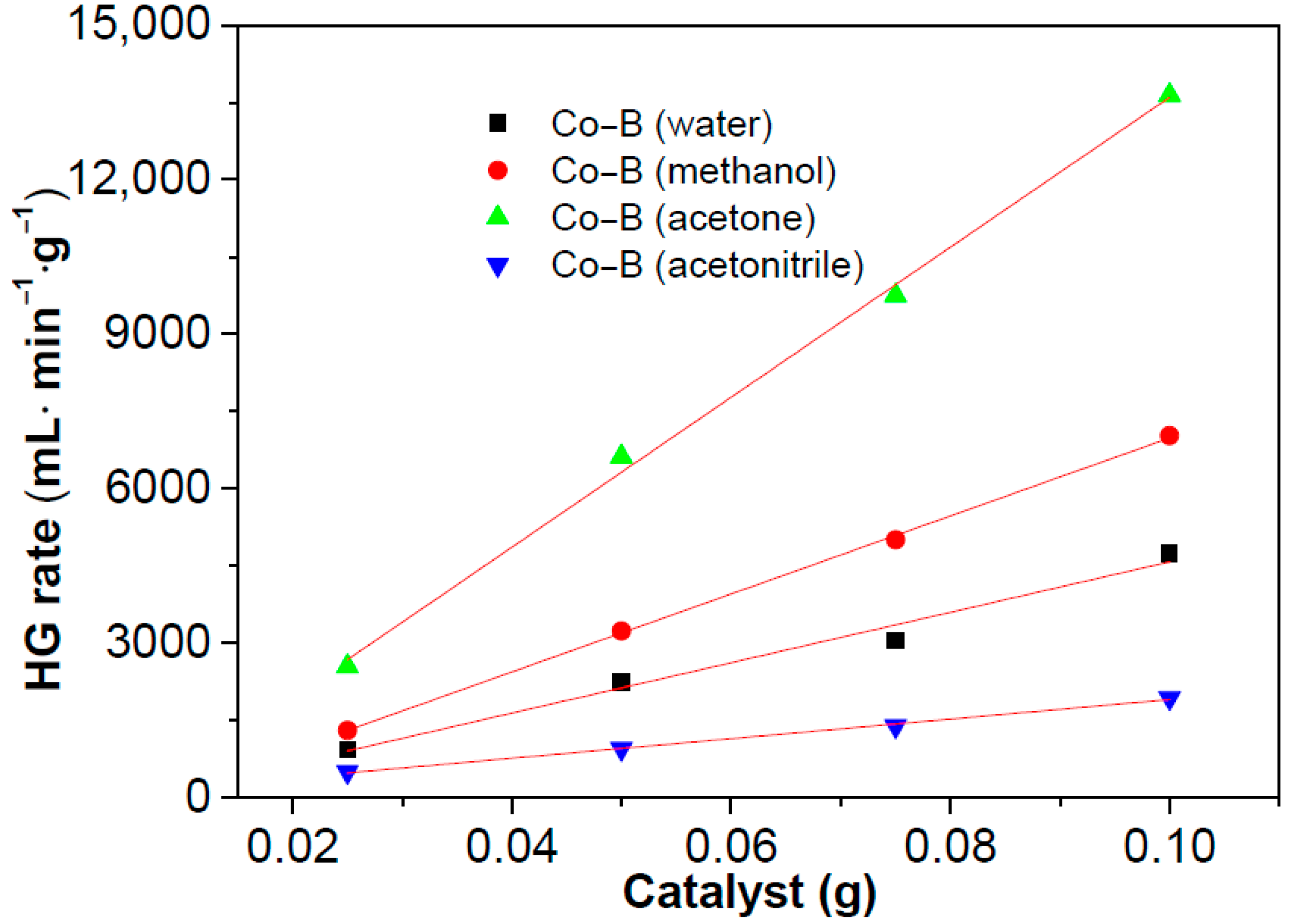
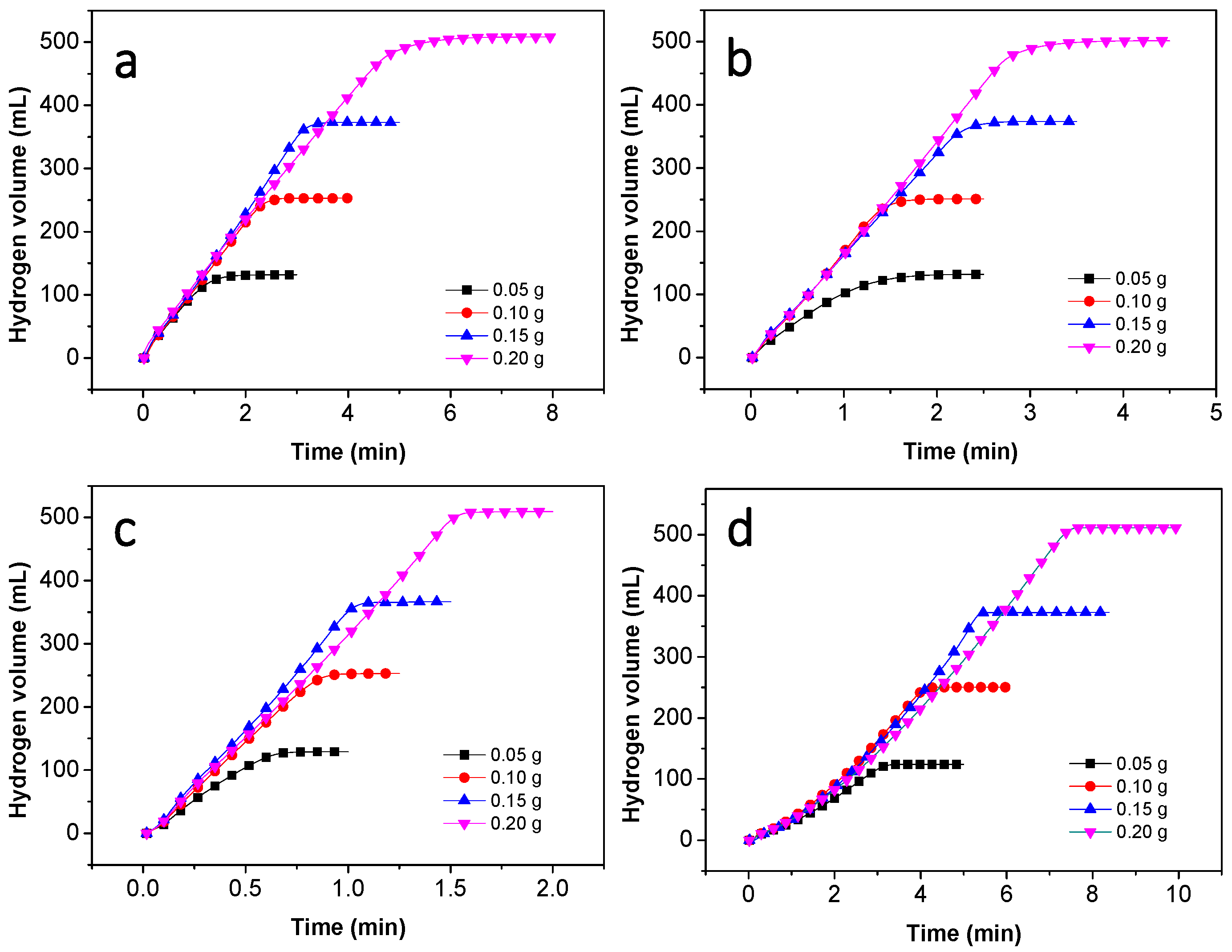
3.2. Hydrolysis of NaBH4 Catalyzed by Co–B Alloy
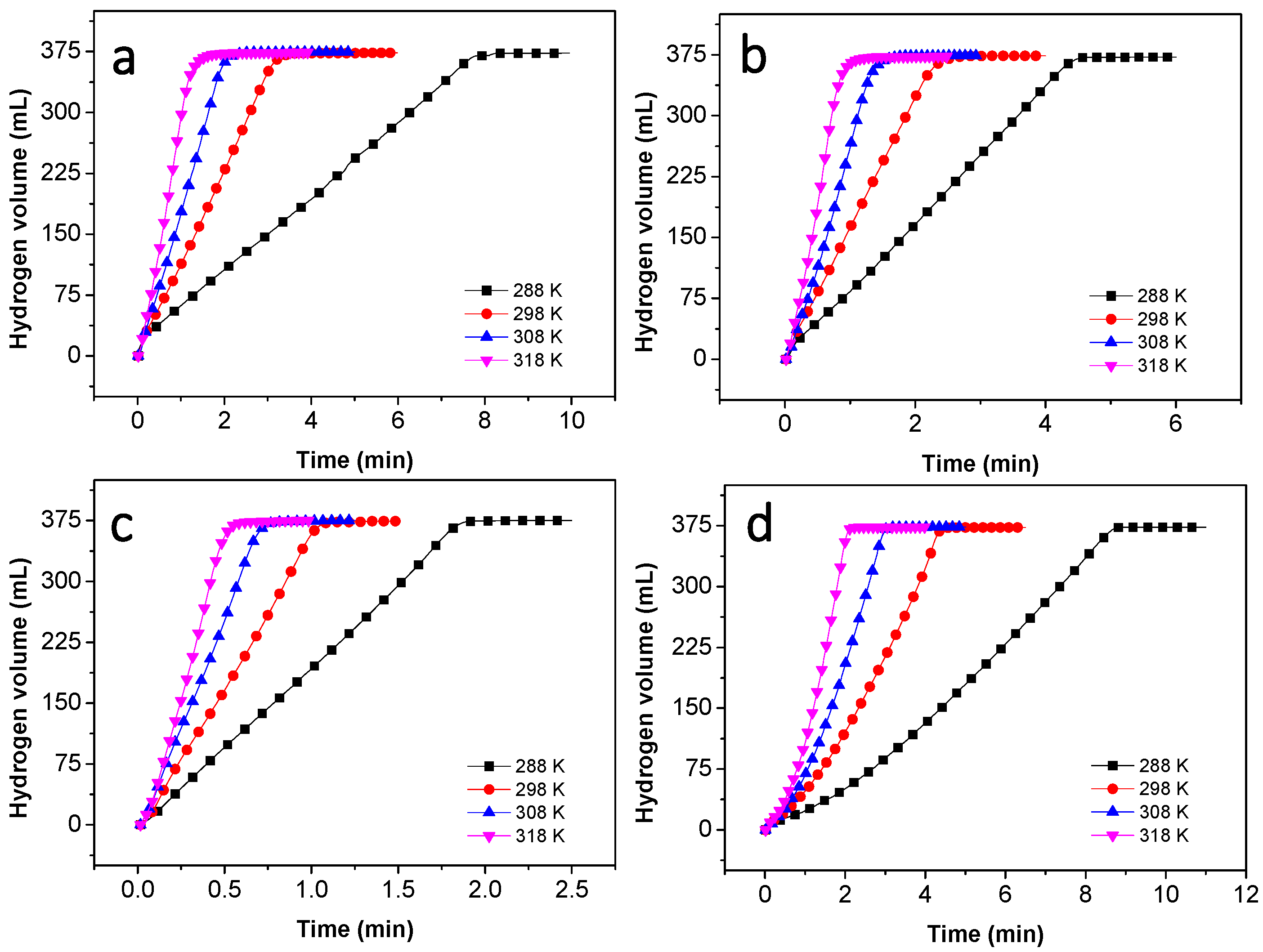
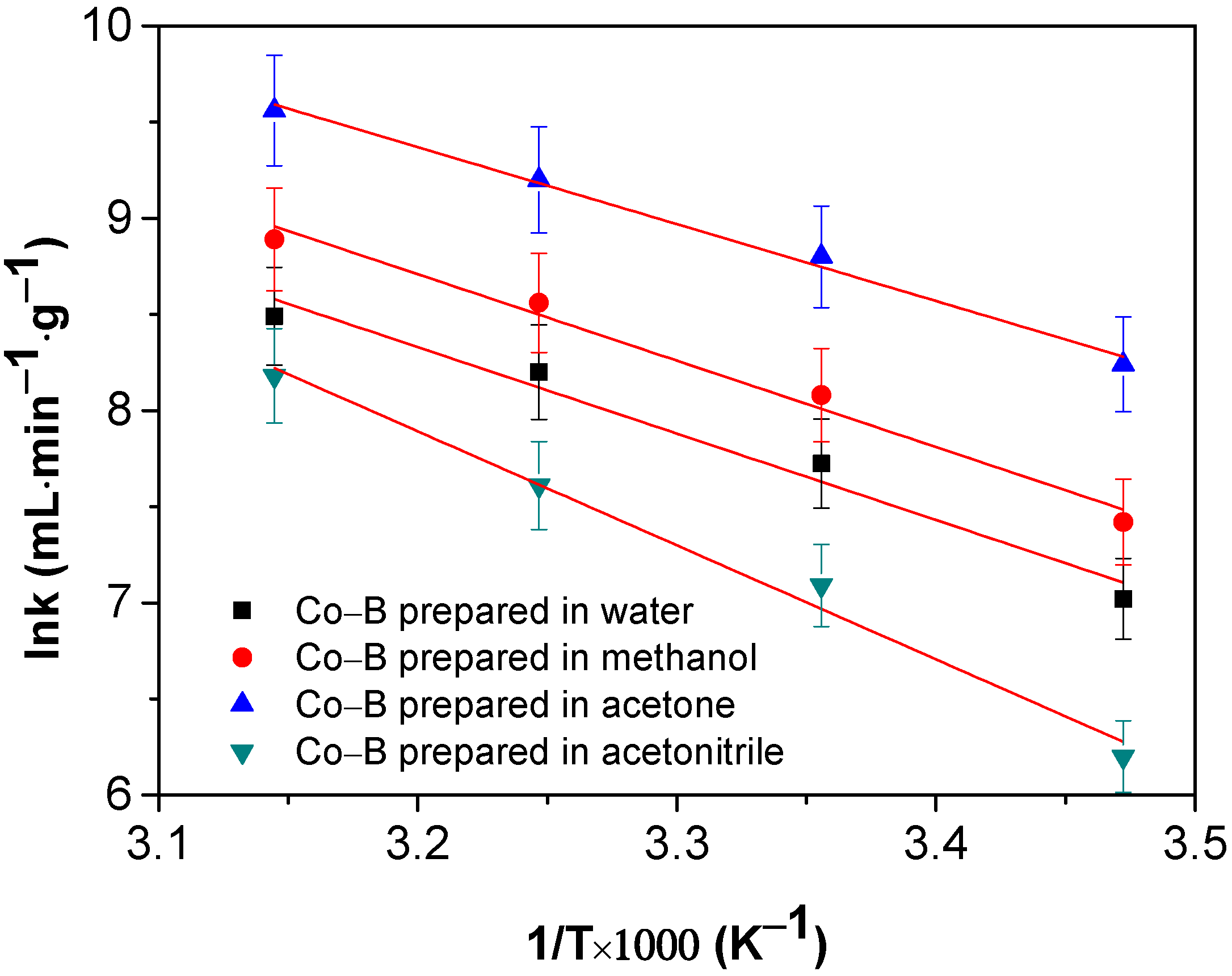
3.3. Activation Energy of NaBH4 Hydrolysis Catalyzed by Co–B
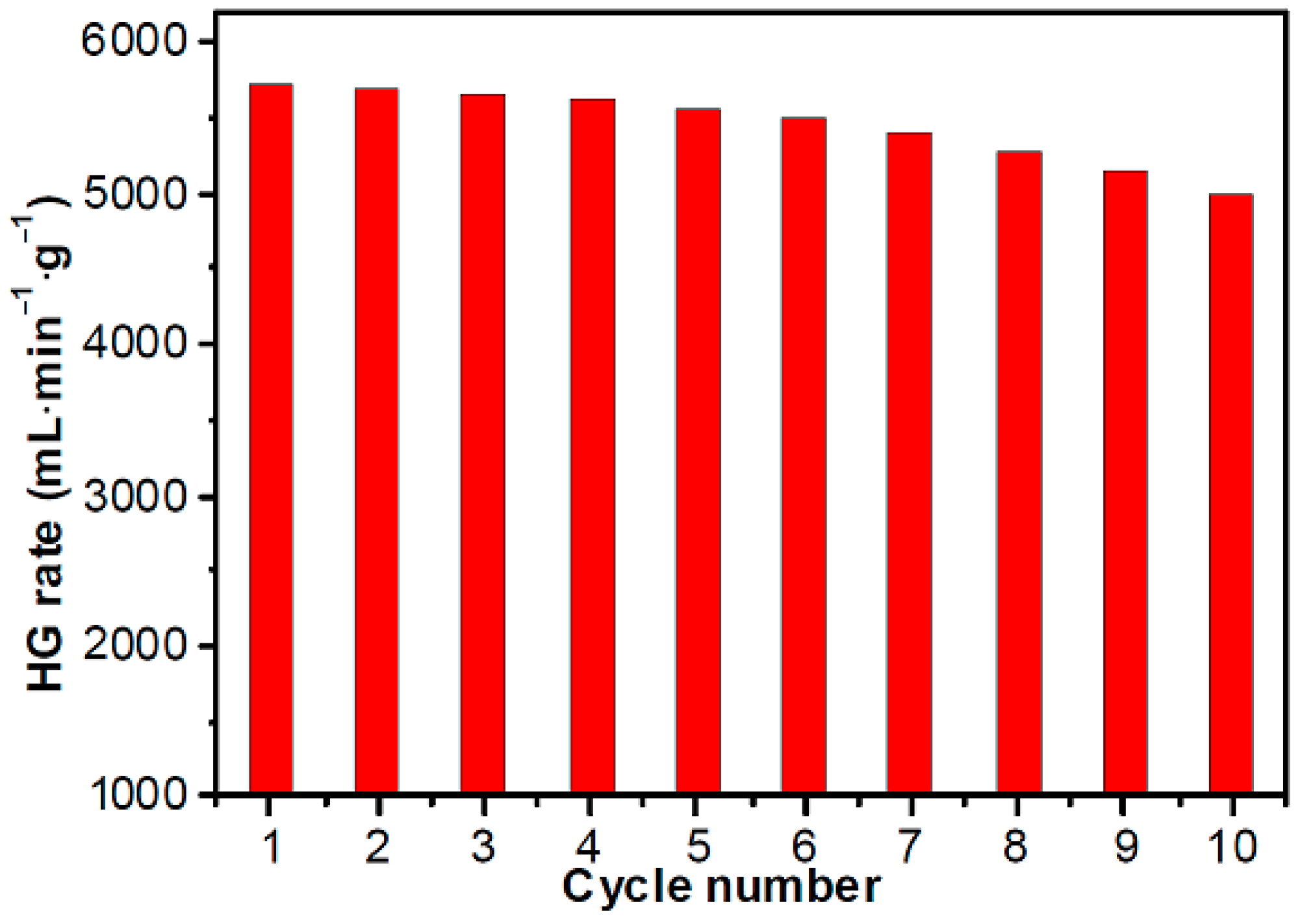
3.4. Stability of Co–B for Hydrolysis of NaBH4
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liang, Z.; Li, Q.; Li, F.; Zhao, S.; Xia, X. Hydrogen generation from hydrolysis of NaBH4 based on high stable NiB/NiFe2O4 catalyst. Int. J. Hydrog. Energy 2017, 42, 3971–3980. [Google Scholar] [CrossRef]
- Sahiner, N.; Yasar, A.O.; Aktas, N. An alternative to metal catalysts: Poly(4-vinyl pyridine)-based polymeric ionic liquid catalyst for H2 generation from hydrolysis and methanolysis of NaBH4. Int. J. Hydrog. Energy 2016, 41, 20562–20572. [Google Scholar] [CrossRef]
- Cao, Z.; Ouyang, L.; Wang, H.; Liu, J.; Felderhoff, M.; Zhu, M. Reversible hydrogen storage in yttrium aluminum hydride. J. Mater. Chem. A 2017, 5, 6042–6046. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Guo, Q.; Ji, S. Catalytic behavior of carbon supported Ni–B, Co–B and Co–Ni–B in hydrogen generation by hydrolysis of KBH4. Fuel Process. Technol. 2011, 92, 1606–1610. [Google Scholar] [CrossRef]
- Ma, M.; Duan, R.; Ouyang, L.; Zhu, X.; Chen, Z.; Peng, C.; Zhu, M. Hydrogen storage and hydrogen generation properties of CaMg2-based alloys. J. Alloys Compd. 2017, 691, 929–935. [Google Scholar] [CrossRef]
- Peng, S.; Fan, X.; Zhang, J.; Wang, F. A highly efficient heterogeneous catalyst of Ru/MMT: Preparation, characterization, and evaluation of catalytic effect. Appl. Catal. B 2013, 140–141, 115–124. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Qi, K.; Cao, Z.; Zhang, K.; Wu, S. Nanostructured cobalt-phosphorous catalysts for hydrogen generation from hydrolysis of sodium borohydride solution. Renew. Energy 2016, 89, 285–294. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Wang, D.; Wu, S.; Cao, Z.; Zhang, K.; Liu, H.; Xin, S. Hydrogen generation from hydrolysis of sodium borohydride using nanostructured Ni–B catalysts. Int. J. Hydrog. Energy 2016, 41, 16077–16086. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, F.; Liang, J.; Chen, J. Hydrogen generation by hydrolysis of ammonia borane with a nanoporous cobalt-tungsten-boron-phosphorus catalyst supported on Ni foam. Int. J. Hydrog. Energy 2011, 36, 1411–1417. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Qi, K.; Cao, Z.; Zhang, K.; Wu, S. Preparation and characterization of fishbone-like Co–B nanoparticles with high catalytic activity for hydrogen generation from NaBH4 solution. Mater. Lett. 2016, 165, 147–152. [Google Scholar] [CrossRef]
- Patel, N.; Fernandes, R.; Bazzanella, N.; Miotello, A. Enhanced hydrogen production by hydrolysis of NaBH4 using “Co-B nanoparticles supported on Carbon film” catalyst synthesized by pulsed laser deposition. Catal. Today 2011, 170, 20–26. [Google Scholar] [CrossRef]
- Zou, Y.; Cheng, J.; Wang, Q.; Xiang, C.; Chu, H.; Qiu, S.; Zhang, H.; Xu, F.; Liu, S.; Tang, C.; Sun, L. Cobalt-boron/nickel-boron nanocomposite with improved catalytic performance for the hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2015, 40, 13423–13430. [Google Scholar] [CrossRef]
- Lale, A.; Wasan, A.; Kumar, R.; Miele, P.; Demirci, U.B.; Bernard, S. Organosilicon polymer-derived mesoporous 3D silicon carbide, carbonitride and nitride structures as platinum supports for hydrogen generation by hydrolysis of sodium borohydride. Int. J. Hydrog. Energy 2016, 41, 15477–15488. [Google Scholar] [CrossRef]
- Hsueh, C.L.; Chen, C.Y.; Ku, J.R.; Tsai, S.F.; Hsu, Y.Y.; Tsau, F.; Jeng, M.S. Simple and fast fabrication of polymer template-Ru composite as a catalyst for hydrogen generation from alkaline NaBH4 solution. J. Power Sources 2008, 177, 485–492. [Google Scholar] [CrossRef]
- Şahin, Ö.; Kılınç, D.; Saka, C. Hydrogen generation from hydrolysis of sodium borohydride with a novel palladium metal complex catalyst. J. Energy Inst. 2016, 89, 182–189. [Google Scholar] [CrossRef]
- Larichev, Y.V.; Netskina, O.V.; Komova, O.V.; Simagina, V.I. Comparative XPS study of Rh/Al2O3 and Rh/TiO2 as catalysts for NaBH4 hydrolysis. Int. J. Hydrog. Energy 2010, 35, 6501–6507. [Google Scholar] [CrossRef]
- Patel, N.; Miotello, A. Progress in Co–B related catalyst for hydrogen production by hydrolysis of boron-hydrides: A review and the perspectives to substitute noble metals. Int. J. Hydrog. Energy 2015, 40, 1429–1464. [Google Scholar] [CrossRef]
- Qiu, F.; Liu, G.; Li, L.; Wang, Y.; Xu, C.; An, C.; Chen, C.; Xu, Y.; Huang, Y.; Wang, Y.; et al. Synthesis of triple-layered Ag@Co@Ni core–shell nanoparticles for the catalytic dehydrogenation of ammonia borane. Chem. Eur. J. 2014, 20, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Simagina, V.I.; Ozerova, A.M.; Komova, O.V.; Odegova, G.V.; Kellerman, D.G.; Fursenko, R.V.; Odintsov, E.S.; Netskina, O.V. Cobalt boride catalysts for small-scale energy application. Catal. Today 2015, 242, 221–229. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, K.; Chen, T.; Tan, X.; Li, C.M. Hydrogen storage in Ni–B nanoalloy-doped 2D graphene. Int. J. Hydrog. Energy 2011, 36, 12950–12954. [Google Scholar] [CrossRef]
- Tang, M.; Huang, G.; Gao, C.; Li, X.; Qiu, H. Co nanoparticles supported 3D structure for catalytic H2 production. Mater. Chem. Phys. 2017, 191, 6–12. [Google Scholar] [CrossRef]
- Aydin, M.; Hasimoglu, A.; Ozdemir, O.K. Kinetic properties of Cobalt-Titanium-Boride (Co–Ti–B) catalysts for sodium borohydride hydrolysis reaction. Int. J. Hydrog. Energy 2016, 41, 239–248. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Wu, C.; Chen, Y.; Li, J.; Li, Z. Hydrogen generation from hydrolysis of NaBH4-NH3BH3 composite promoted by AlCl3. Int. J. Hydrog. Energy 2016, 41, 16344–16351. [Google Scholar] [CrossRef]
- Wang, X.; Wu, C.; Zhang, Y.; Chen, Y. Hydrogen generation behaviors of LiAlH4 and NH4Cl in Et2O, THF or DME. Int. J. Hydrog. Energy 2016, 41, 6825–6832. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Xie, S.; Qiao, M.; Dai, W.; Li, H. Highly active Co–B amorphous alloy catalyst with uniform nanoparticles prepared in oil-in-water mircoemulsion. J. Catal. 2008, 259, 104–110. [Google Scholar] [CrossRef]
- Patel, N.; Fernandes, R.; Guella, G.; Miotello, A. Nanoparticle-assembled Co-B thin film for the hydrolysis of ammonia borane: A highly active catalyst for hydrogen production. Appl. Catal. B Environ. 2010, 95, 137–143. [Google Scholar] [CrossRef]
- Şahin, Ö.; İzgi, M.S.; Onat, E.; Saka, C. Influence of the using of methanol instead of water in the preparation of Co–B–TiO2 catalyst for hydrogen production by NaBH4 hydrolysis and plasma treatment effect on the Co–B–TiO2 catalyst. Int. J. Hydrog. Energy 2016, 41, 2539–2546. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Gao, M.; Zhao, B.; Ding, W. Solvent effects in the synthesis of CoB catalysts on hydrogen generation from hydrolysis of sodium borohydride. Chin. J. Catal. 2013, 34, 979–985. [Google Scholar] [CrossRef]
- Niu, W.; Ren, D.; Han, Y.; Wu, Y.; Gou, X. Optimizing preparation of carbon supported cobalt catalyst for hydrogen generation from NaBH4 hydrolysis. J. Alloys Compd. 2012, 543, 159–166. [Google Scholar] [CrossRef]
- Patel, N.; Fernandes, R.; Gupta, S.; Edla, R.; Kothari, D.C.; Miotello, A. Co–B catalyst supported over mesoporous silica for hydrogen production by catalytic hydrolysis of ammonia borane: A study on influence of pore structure. Appl. Catal. B Environ. 2013, 140–141, 125–132. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, H.; Chen, J. Improved hydrogen generation from alkaline NaBH4 solution using carbon-supported Co–B as catalysts. Int. J. Hydrog. Energy 2007, 32, 4711–4716. [Google Scholar] [CrossRef]
- Ma, H.; Ji, W.; Zhao, J.; Liang, J.; Chen, J. Preparation, characterization and catalytic NaBH4 hydrolysis of Co-B hollow spheres. J. Alloys Compd. 2009, 474, 584–589. [Google Scholar] [CrossRef]
- Wu, Z.; Ge, S. Facile synthesis of a Co–B nanoparticle catalyst for efficient hydrogen generation via borohydride hydrolysis. Catal. Commun. 2011, 13, 40–43. [Google Scholar] [CrossRef]
| Solvent | Co (wt %) | B (wt %) | O (wt %) | Standard Deviation (%) |
|---|---|---|---|---|
| Water | 79.616 | 4.117 | 16.267 | 0.82 |
| Methanol | 57.739 | 4.458 | 37.803 | 0.47 |
| Acetone | 62.494 | 5.561 | 31.945 | 0.66 |
| Acetonitrile | 43.267 | 13.561 | 43.217 | 0.58 |
| Solvent | Surface Area (m2·g−1) | Pore Volumea (cm3·g−1) | Pore Diameter (nm) |
|---|---|---|---|
| Water | 39.7 | 0.214 | 10.23 |
| Methanol | 51.3 | 0.249 | 18.27 |
| Acetone | 117.7 | 0.329 | 6.83 |
| Acetonitrile | 364.7 | 0.879 | 5.74 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Gao, Y.; Huang, P.; Xiang, C.; Chu, H.; Qiu, S.; Yan, E.; Xu, F.; Sun, L. Effects of the Preparation Solvent on the Catalytic Properties of Cobalt–Boron Alloy for the Hydrolysis of Alkaline Sodium Borohydride. Metals 2017, 7, 365. https://doi.org/10.3390/met7090365
Zou Y, Gao Y, Huang P, Xiang C, Chu H, Qiu S, Yan E, Xu F, Sun L. Effects of the Preparation Solvent on the Catalytic Properties of Cobalt–Boron Alloy for the Hydrolysis of Alkaline Sodium Borohydride. Metals. 2017; 7(9):365. https://doi.org/10.3390/met7090365
Chicago/Turabian StyleZou, Yongjin, Yubo Gao, Pengru Huang, Cuili Xiang, Hailiang Chu, Shujun Qiu, Erhu Yan, Fen Xu, and Lixian Sun. 2017. "Effects of the Preparation Solvent on the Catalytic Properties of Cobalt–Boron Alloy for the Hydrolysis of Alkaline Sodium Borohydride" Metals 7, no. 9: 365. https://doi.org/10.3390/met7090365






