DFT Investigation of the Effects of Coexisting Cations and Complexing Reagents on Ni(II) Adsorption by a Polyvinylidene Fluoride-Type Chelating Membrane Bearing Poly(Amino Phosphonic Acid) Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Chelating Membrane
2.3. Characterization of the Membrane
2.4. Adsorption Experiments
2.5. Computational Details
3. Results and Discussion
3.1. Characterization of the Chelating Membrane
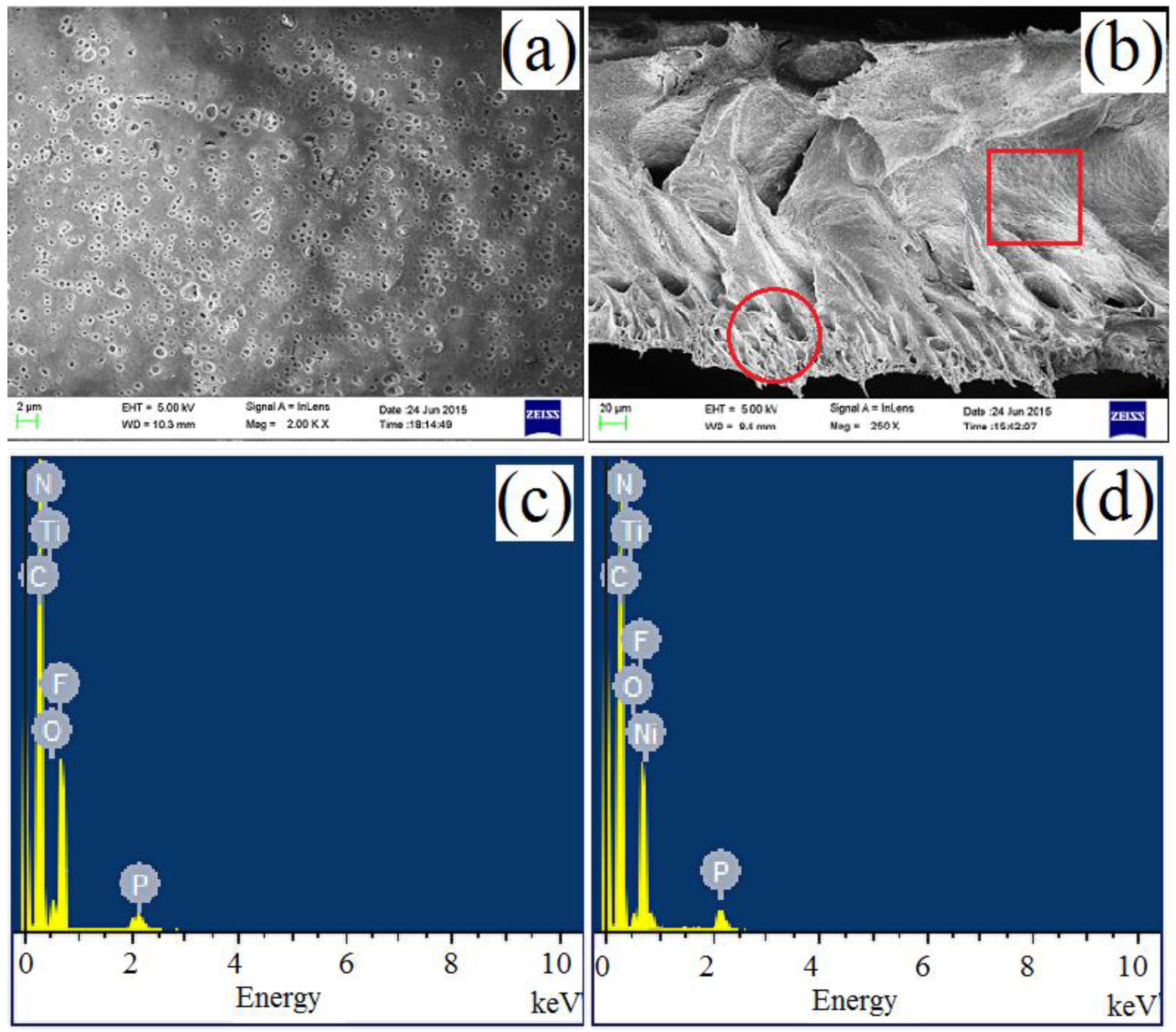
3.1.1. Analyses of FE-SEM and EDS
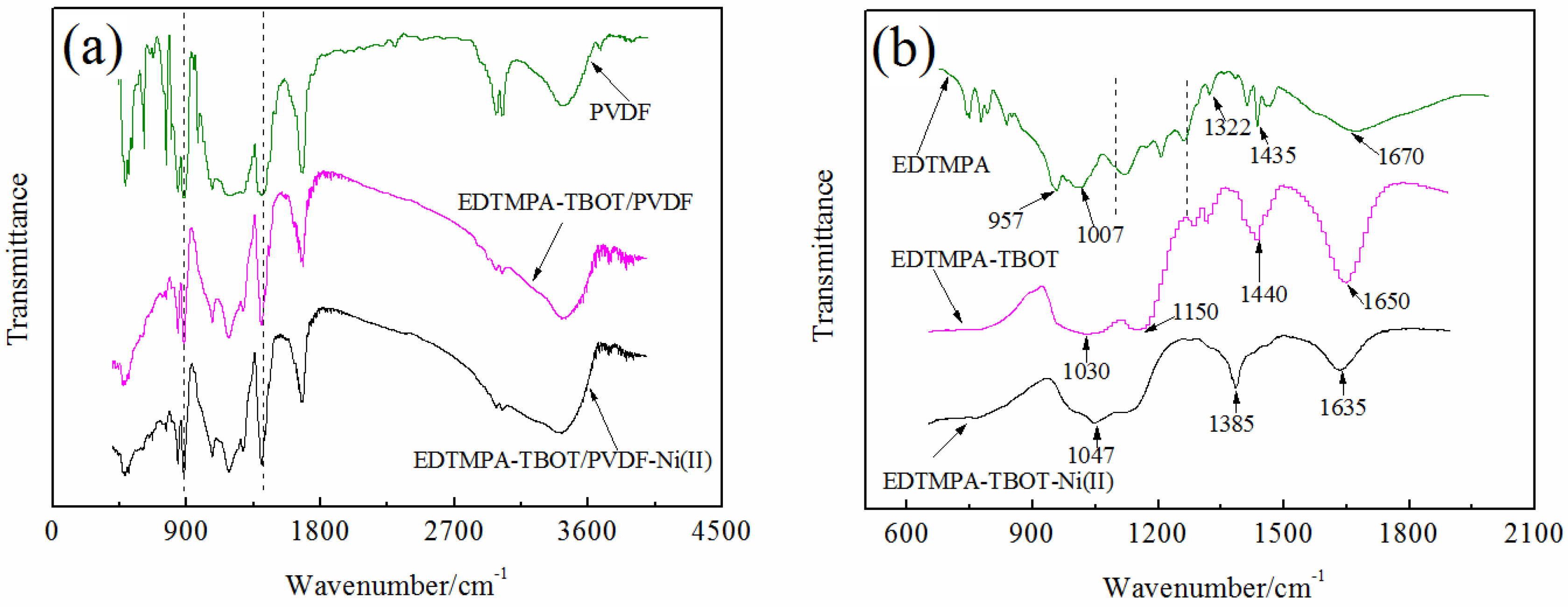
3.1.2. FTIR Spectra
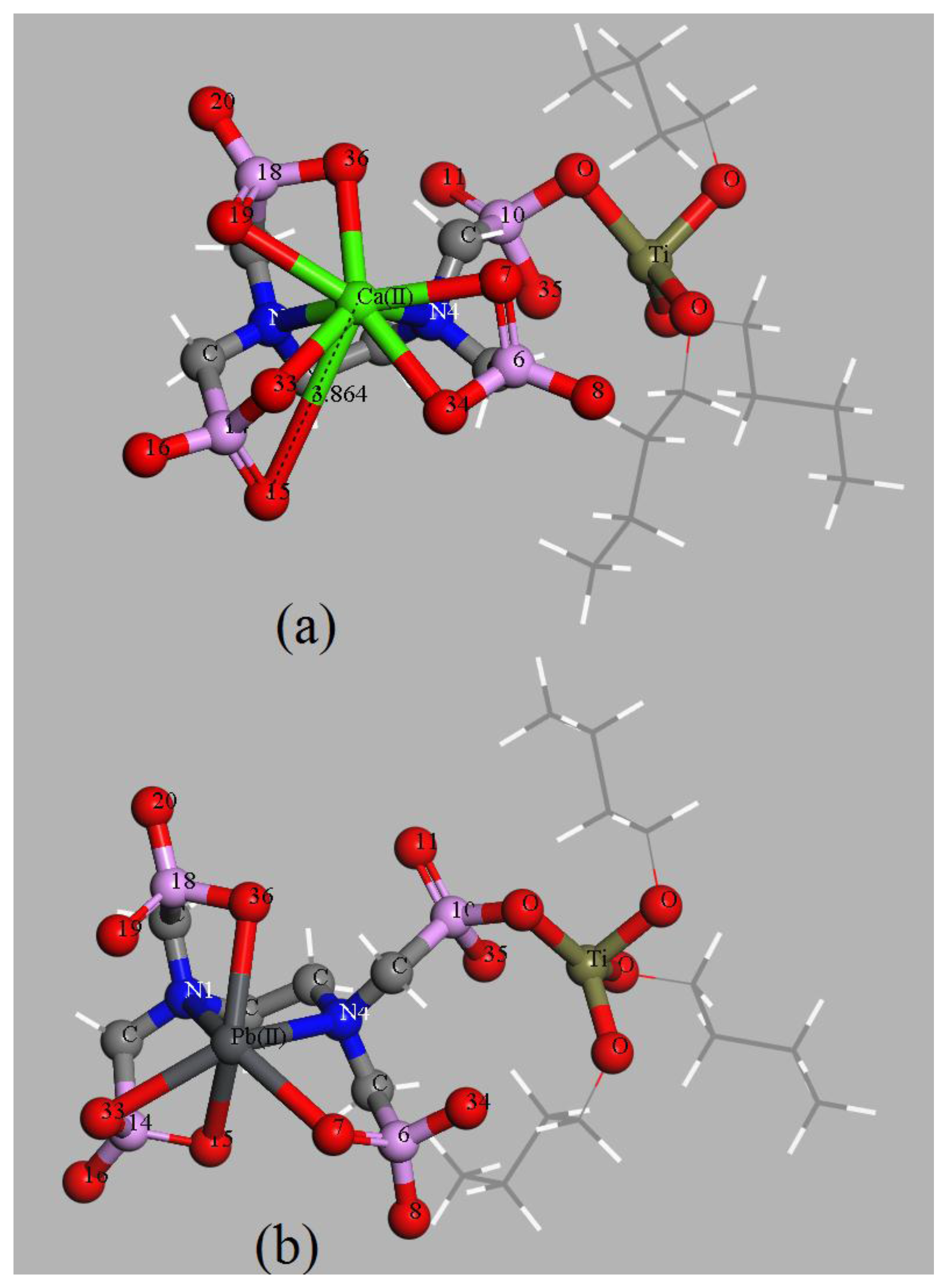
3.2. Effects of Coexisting Cations
3.3. Effects of Coexisting Complexing Reagents
3.4. DFT Simulations
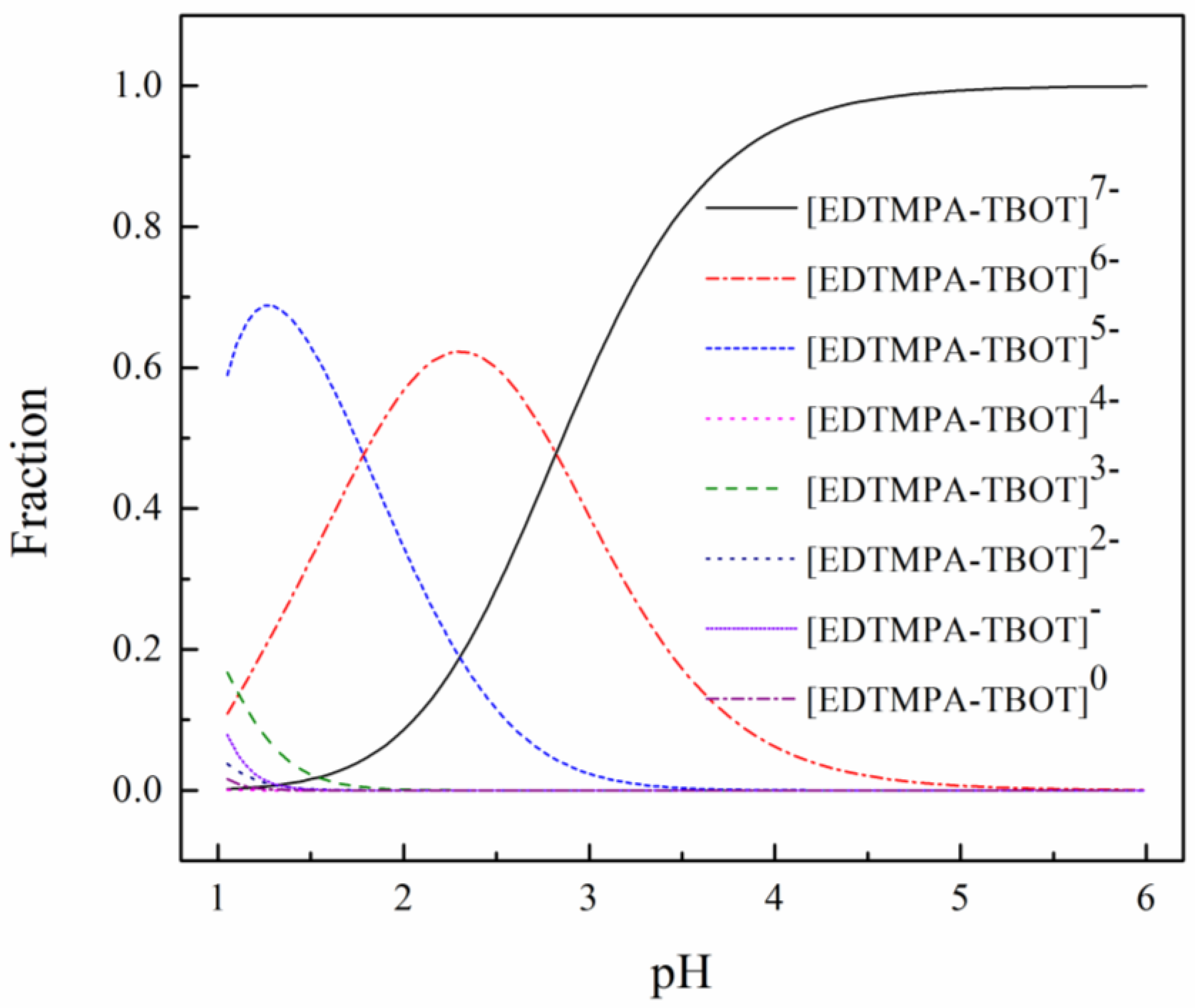
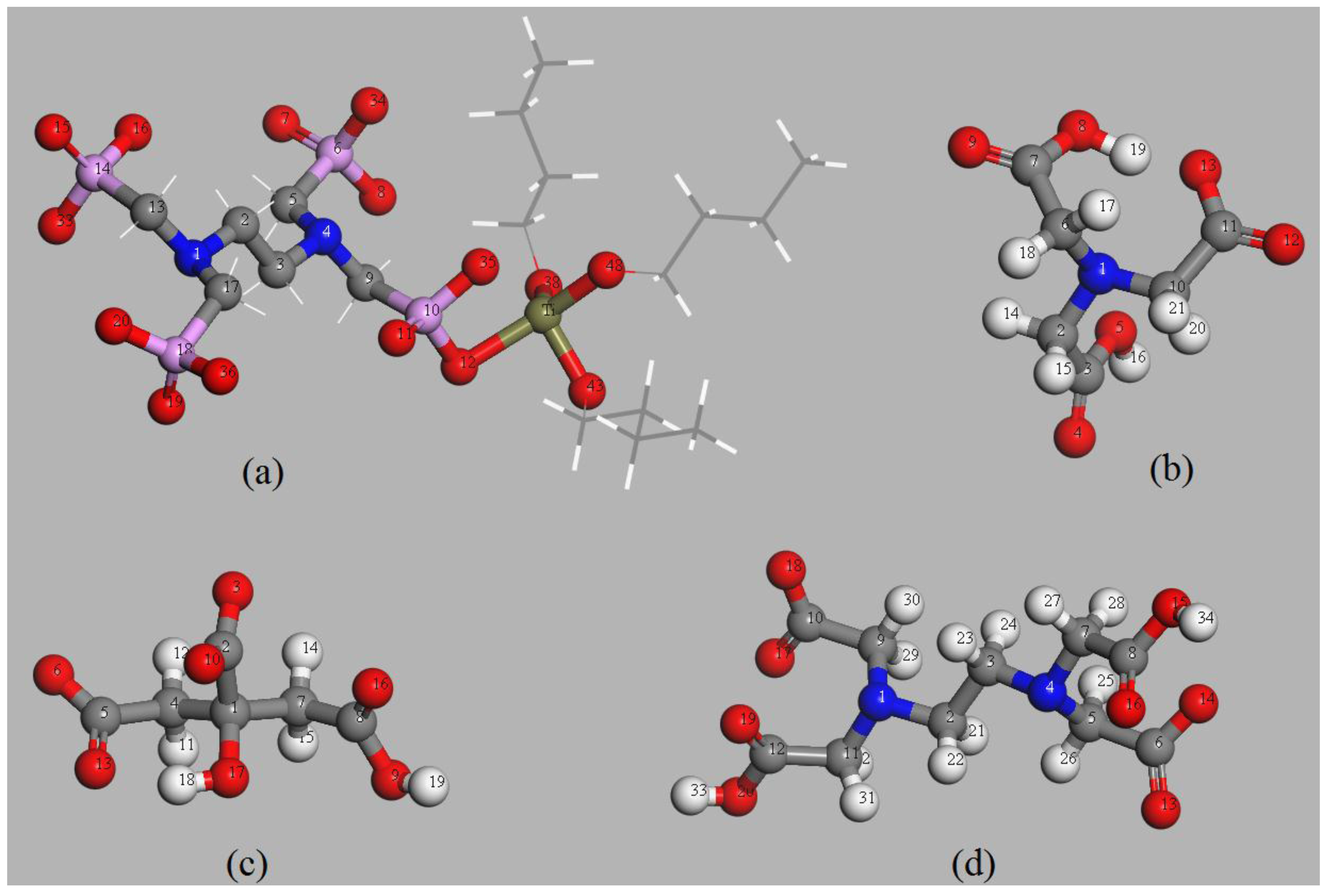
3.4.1. Structure Analysis of the EDTMPA-TBOT Ligand
3.4.2. The Chemical Reactivity Descriptors
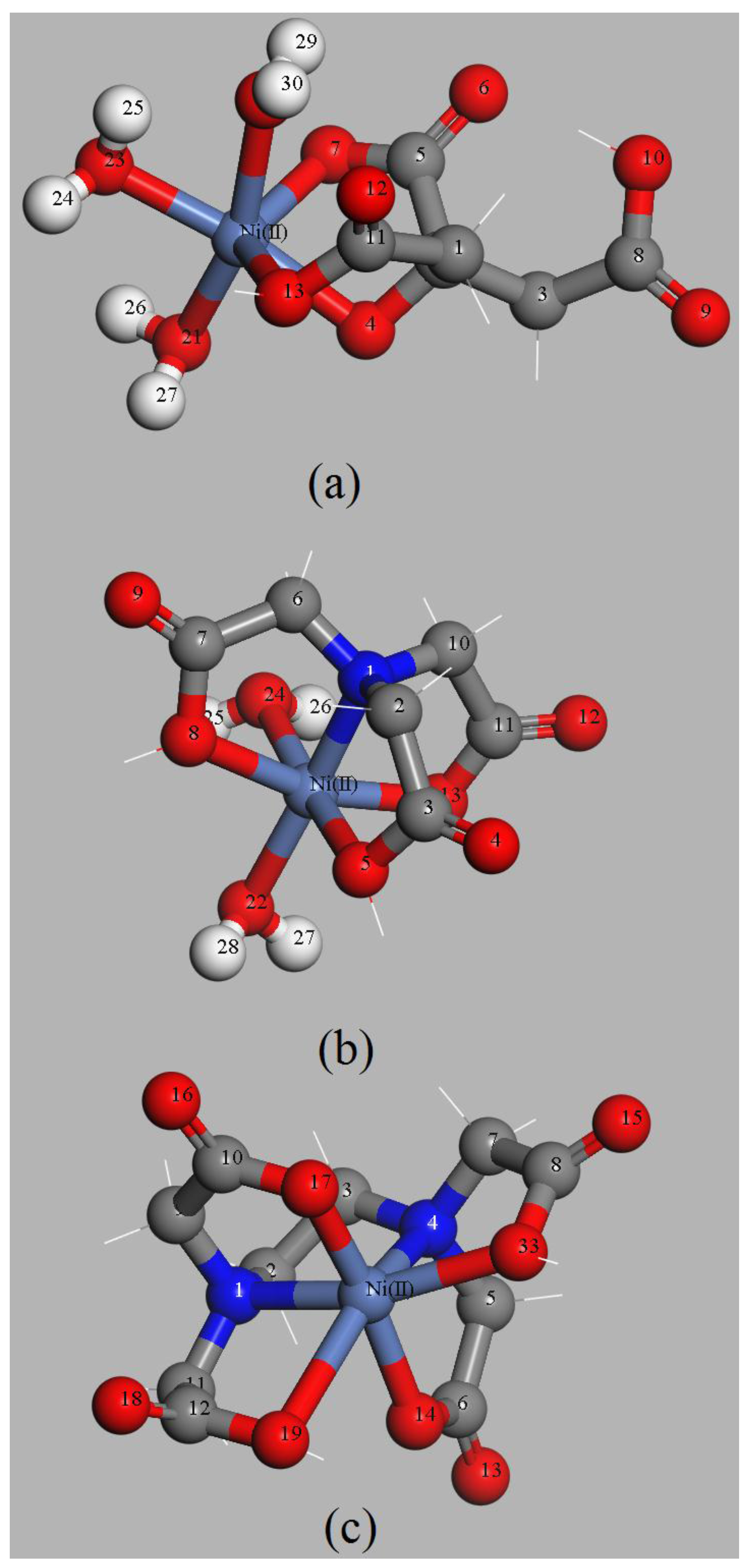
3.4.3. Complexing Sites of the EDTMPA-TBOT Ligand
3.4.4. Analyses of the Adsorption Energy and the Gibbs Free Energy of Adsorption
4. Conclusions
- (1)
- The combination of DFT simulations and the adsorption experiments is suitable for evaluating the adsorption process between Ni(II) and the EDTMPA-TBOT/PVDF chelating membrane.
- (2)
- The coexistent Pb(II) shows a stronger interferential effect on Ni(II) uptake than Ca(II). Furthermore, the disturbance of EDTA on the Ni(II) uptake of the EDTMPA-TBOT/PVDF membrane is more remarkable than those of NTA and citrate.
- (3)
- The large absolute value of charge transfer, the negative values of adsorption energy and Gibbs free energy of adsorption for the [Ni(II)-(EDTMPA-TBOT)]5− and [Pb(II)-(EDTMPA-TBOT)]5− complexes suggests that the fabricated EDTMPA-TBOT/PVDF chelating membrane will be qualified for the removal heavy metals with a high biotoxicity from the aqueous solution.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cempel, M.; Nikel, G. Nickel: A review of its sources and environmental toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Waalkes, M.P.; Liu, J.; Kasprzak, K.S.; Diwan, B.A. Minimal influence of metallothionein over-expression on nickel carcinogenesis in mice. Toxicol. Lett. 2004, 153, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, R.T.; Teng, Y. Study on the toxic interactions of Ni2+ with DNA using neutral red dye as a fluorescence probe. J. Lumin. 2011, 131, 705–709. [Google Scholar] [CrossRef]
- Song, L.Z.; He, J.; Wang, X.L.; Wang, T. Thermodynamic and kinetic investigations of a chelating membrane bearing polyaminecarboxylate groups towards Ni(II). Toxicol. Environ. Chem. 2012, 94, 1902–1915. [Google Scholar] [CrossRef]
- Shih, Y.J.; Lin, C.P.; Huang, Y.H. Application of Fered-Fenton and chemical precipitation process for the treatment of electroless nickel plating wastewater. Sep. Purif. Technol. 2013, 104, 100–105. [Google Scholar] [CrossRef]
- Franco, P.E.; Veit, M.T.; Borba, C.E.; Gonçalves, G.D.C.; Fagundes-Klen, M.R.; Bergamasco, R.; Silva, E.A.D.; Suzaki, P.Y.R. Nickel(II) and zinc(II) removal using Amberlite IR-120 resin: Ion exchange equilibrium and kinetics. Chem. Eng. J. 2013, 221, 426–435. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nadeem, R.; Bhatti, H.N.; Ahmad, N.R.; Ansari, T.M. Ni(II) biosorption by Cassia fistula (Golden Shower) biomass. J. Hazard. Mater. 2007, 139, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.E.; Paiva, A.P.; Soares, D.; Labrincha, A.; Castro, F. Solvent extraction applied to the recovery of heavy metals from galvanic sludge. J. Hazard. Mater. 2005, 120, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hoseinian, F.S.; Irannajad, M.; Nooshabadi, A.J. Ion flotation for removal of Ni(II) and Zn(II) ions from wastewaters. Int. J. Miner. Process. 2015, 143, 131–137. [Google Scholar] [CrossRef]
- Dermentzis, K.; Christoforidis, A.; Valsamidou, E. Removal of nickel, copper, zinc and chromium from synthetic and industrial wastewater by electrocoagulation. Int. J. Environ. Sci. 2011, 1, 697–710. [Google Scholar]
- Gupta, B.S.; Curran, M.; Hasan, S.; Ghosh, T.K. Adsorption characteristics of Cu and Ni on Irish peat moss. J. Environ. Manag. 2009, 90, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.P.; Tang, W.Z.; Zhao, D.B.; Meng, Y.; Yin, D.L.; Sillanpää, M. Adsorption kinetics, isotherms and mechanisms of Cd(II), Pb(II), Co(II) and Ni(II) by a modified magnetic polyacrylamide microcomposite adsorbent. J. Water Process Eng. 2014, 4, 47–57. [Google Scholar] [CrossRef]
- Nabarlatz, D.; Celis, J.D.; Bonelli, P.; Cukierman, A.L. Batch and dynamic sorption of Ni(II) ions by activated carbon based on a native lignocellulosic precursor. J. Environ. Manag. 2012, 97, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.W.; Othaman, R.; Hilal, N. Potential use of nanofiltration membranes in treatment of industrial wastewater from Ni-P electroless plating. Desalination 2004, 168, 241–252. [Google Scholar] [CrossRef]
- Song, L.Z.; Yang, M.; Fu, J.; Lu, P.P.; Wang, X.L.; He, J. Adsorption performance of APTMS-DTPA/PVDF chelating membrane toward Ni(II) with the presence of Ca(II), NH4+, lactic acid, and citric acid. Desalin. Water Treat. 2015, 53, 158–170. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P.; Raguime, J.A.; Raajenthiren, M.; Mohan, D. Metal ion separation and protein removal from aqueous solutions using modified cellulose acetate membranes: Role of polymeric additives. Sep. Purif. Technol. 2007, 55, 8–15. [Google Scholar] [CrossRef]
- Dermentzis, K. Removal of nickel from electroplating rinse waters using electrostatic shielding electrodialysis/electrodeionization. J. Hazard. Mater. 2010, 173, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Kumbasar, R.A.; Kasap, S. Selective separation of nickel from cobalt in ammoniacal solutions by emulsion type liquid membranes using 8-hydroxyquinoline (8-HQ) as mobile carrier. Hydrometallurgy 2009, 95, 121–126. [Google Scholar] [CrossRef]
- Barakat, M.A.; Schmidt, E. Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. Desalination 2010, 256, 90–93. [Google Scholar] [CrossRef]
- Richards, L.A.; Richards, B.S.; Schafer, A.I. Renewable energy powered membrane technology: Salt and inorganic contaminant removal by nanofiltration/reverse osmosis. J. Membr. Sci. 2011, 369, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-C.; Wang, C.-C. Adsorption characteristics of metal complexes by chelated copolymers with amino group. React. Funct. Polym. 2006, 66, 343–356. [Google Scholar] [CrossRef]
- Vetriselvi, V.; Santhi, R.J. Redox polymer as an adsorbent for the removal of chromium(VI) and lead(II) from the tannery effluents. Water Res. Ind. 2015, 10, 39–52. [Google Scholar] [CrossRef]
- Long, H.; Wu, P.X.; Zhu, N.W. Evaluation of Cs+ removal from aqueous solution by adsorption on ethylamine-modified montmorillonite. Chem. Eng. J. 2013, 225, 237–244. [Google Scholar] [CrossRef]
- Deng, S.B.; Ting, Y.P. Characterization of PEI-modified biomass and biosorption of Cu(II), Pb(II) and Ni(II). Water Res. 2005, 39, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kwak, S.Y. Surface functionalization of PTFE membranes with hyperbranched poly (amidoamine) for the removal of Cu2+ ions from aqueous solution. J. Membr. Sci. 2013, 448, 125–134. [Google Scholar] [CrossRef]
- Adewuyi, A.; Pereira, F.V. Nitrilotriacetic acid functionalized Adansonia digitata biosorbent: Preparation, characterization and sorption of Pb(II) and Cu(II) pollutants from aqueous solution. J. Adv. Res. 2016, 7, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Baraka, A.; Hall, P.J.; Heslop, M.J. Preparation and characterization of melamine–formaldehyde–DTPA chelating resin and its use as an adsorbent for heavy metals removal from wastewater. React. Funct. Polym. 2007, 67, 585–600. [Google Scholar] [CrossRef]
- Zhao, X.D.; Song, L.Z.; Fu, J.; Tang, P.; Liu, F. Adsorption characteristics of Ni(II) onto MA–DTPA/PVDF chelating membrane. J. Hazard. Mater. 2011, 189, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Zhang, X.J.; Yuan, Z.Y. Hierarchically meso-/macroporous titanium tetraphosphonate materials: Synthesis, photocatalytic activity and heavy metal ion adsorption. Microporous Mesoporous Mater. 2009, 123, 234–242. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Sillanpää, M. Heavy metals adsorption by novel EDTA-modified chitosan-silica hybrid materials. J. Colloid Interface Sci. 2011, 358, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Flores-Holguín, N.; Aguilar-Elguézabal, A.; Rodríguez-Valdez, L.M.; Glossman-Mitnik, D. Theoretical study of chemical reactivity of the main species in the α-pinene isomerization reaction. J. Mol. Struct. Theochem. 2008, 854, 81–88. [Google Scholar] [CrossRef]
- Srivastava, A.; Rawat, P.; Tandon, P.; Singh, R.N. A computational study on conformational geometries, chemical reactivity and inhibitor property of an alkaloid bicuculline with γ-aminobutyric acid (GABA) by DFT. Comput. Theor. Chem. 2012, 993, 80–89. [Google Scholar] [CrossRef]
- Yeh, C.-W.; Chen, J.-D. Role of ligand conformation in the structural diversity of divalent complexes containing phosphinic amide ligand. Inorg. Chem. Commun. 2011, 14, 1212–1216. [Google Scholar] [CrossRef]
- Romanowski, Z.; Kempisty, P.; Sakowski, K.; Krukowski, S. Polarization analysis and Density Functional Theory (DFT) simulations of the electric field in InN/GaN multiple quantum wells (MQWs). J. Phys. Chem. C 2009, 114, 14410–14416. [Google Scholar] [CrossRef]
- Savizi, I.S.P.; Janik, M.J. Acetate and phosphate anion adsorption linear sweep voltammograms simulated using density functional theory. Electrochim. Acta 2011, 56, 3996–4006. [Google Scholar] [CrossRef]
- Rahnemaie, R.; Hiemstra, T.; Riemsdijk, W.H. Carbonate adsorption on goethite in competition with phosphate. J. Colloid Interface Sci. 2007, 315, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Acelas, N.Y.; Mejia, S.M.; Mondragón, F.; Flórez, E. Density functional theory characterization of phosphate and sulfate adsorption on Fe-(hydr)oxide: Reactivity, pH effect, estimation of Gibbs free energies, and topological analysis of hydrogen bonds. Comput. Theor. Chem. 2013, 1005, 16–24. [Google Scholar] [CrossRef]
- Ren, X.M.; Yang, S.T.; Tan, X.L.; Chen, C.L.; Sheng, G.D.; Wang, X.K. Mutual effects of copper and phosphate on their interaction with γ-Al2O3: Combined batch macroscopic experiments with DFT calculations. J. Hazard. Mater. 2012, 237–238, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Song, L.Z.; Zhao, X.D.; Fu, J.; Wang, X.L.; Sheng, Y.P.; Liu, X.W. DFT investigation of Ni(II) adsorption onto MA-DTPA/PVDF chelating membrane in the presence of coexistent cations and organic acids. J. Hazard. Mater. 2012, 199–200, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.S.; Zhou, X.S.; Yue, Y.L. Determination of pore size and pore size distribution on the surface of hollow-fiber filtration membranes: A review of methods. Desalination 2000, 129, 107–123. [Google Scholar] [CrossRef]
- Wang, X.L.; Song, L.Z.; Yang, F.F.; He, J. Investigation of phosphate adsorption by a polyethersulfone-type affinity membrane using experimental and DFT methods. Desalin. Water Treat. 2016, 57, 25036–25056. [Google Scholar] [CrossRef]
- Chen, C.; Shen, C.H.; Kong, G.J.; Gao, S.J. High temperature proton exchange membranes prepared from epoxycyclohexylethyltrimethoxysilane and amino trimethylene phosphonic acid as anhydrous proton conductors. Mater. Chem. Phys. 2013, 140, 24–30. [Google Scholar] [CrossRef]
- Xu, J.F.; Wu, X.Y.; Fu, G.T.; Liu, X.Y.; Chen, Y.; Zhou, Y.M.; Tang, Y.W.; Lu, T.H. Fabrication of phosphonate functionalized platinum nanoclusters and their application in hydrogen peroxide sensing in the presence of oxygen. Electrochim. Acta 2012, 80, 233–239. [Google Scholar] [CrossRef]
- Ma, T.Y.; Li, H.; Tang, A.N.; Yuan, Z.Y. Ordered, mesoporous metal phosphonate materials with microporous crystalline walls for selective separation techniques. Small 2011, 7, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Ferrah, N.; Abderrahim, O.; Didi, M.A.; Villemin, D. Removal of copper ions from aqueous solutions by a new sorbent: Polyethyleneiminemethylene phosphonic acid. Desalination 2011, 269, 17–24. [Google Scholar] [CrossRef]
- Song, L.Z.; Zhao, R.F.; Yun, D.D.; Lu, P.P.; He, J.; Wang, X.L. Influence of Co(II), Ni(II), tartrate, and ethylenediaminetetraacetic acid on Cu(II) adsorption onto a polyvinylidene fluoride-based chelating membrane. Toxicol. Environ. Chem. 2014, 96, 362–378. [Google Scholar] [CrossRef]
- Kumar, V.; Jain, G.; Kishor, S.; Ramaniah, L.M. Chemical reactivity analysis of some alkylating drug molecules—A density functional theory approach. Comput. Theor. Chem. 2011, 968, 18–25. [Google Scholar] [CrossRef]




| Chemical Species | EHOMO/Ha | ELUMO/Ha | ΔELUMO-HOMO/Ha | μ/Ha | η/Ha | ω/Ha |
|---|---|---|---|---|---|---|
| [EDTMPA-TBOT]7− | −0.1298 | −0.0650 | 0.0648 | −0.0974 | 0.0324 | 0.1464 |
| [Ni(II)(H2O)6]2+ | −0.2593 | −0.1401 | 0.1192 | −0.1997 | 0.0596 | 0.3346 |
| [Pb(II)(H2O)2]2+ | −0.3387 | −0.1459 | 0.1928 | −0.2423 | 0.0964 | 0.3045 |
| [Ca(II)(H2O)8]2+ | −0.2957 | −0.0477 | 0.2480 | −0.1717 | 0.1240 | 0.1189 |
| [H2Cit]− | −0.1797 | −0.0429 | 0.1368 | −0.1113 | 0.0684 | 0.0906 |
| [H2NTA]− | −0.1782 | −0.0434 | 0.1348 | −0.1108 | 0.0674 | 0.0911 |
| [H2EDTA]2− | −0.1631 | −0.0392 | 0.1239 | −0.1012 | 0.0620 | 0.0826 |
| Reactant | [Ni(II)(H2O)6]2+ | [Pb(II)(H2O)2]2+ | [Ca(II)(H2O)8]2+ |
|---|---|---|---|
| [EDTMPA-TBOT]7− | −0.5560 | −0.5621 | −0.2375 |
| [H2Cit]− | −0.3453 | -- | -- |
| [H2NTA]− | −0.3500 | -- | -- |
| [H2EDTA]2− | −0.4054 | -- | -- |
| Reactant | ΔEads/(kJ/mol) | ΔGads/(kJ/mol) |
|---|---|---|
| [Ni(II)-(EDTMPA-TBOT)]5− | −58.30 | −114.20 |
| [Pb(II)-(EDTMPA-TBOT)]5− | −112.47 | −117.85 |
| [Ca(II)-(EDTMPA-TBOT)]5− | −12.88 | −91.97 |
| [Ni(II)-citrate]+ | 16.88 | −24.71 |
| [Ni(II)-NTA]+ | 8.07 | −3.55 |
| [Ni(II)-EDTA]0 | −6.41 | −59.51 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Song, L.; Tian, C.; He, J.; Wang, S.; Wang, J.; Li, C. DFT Investigation of the Effects of Coexisting Cations and Complexing Reagents on Ni(II) Adsorption by a Polyvinylidene Fluoride-Type Chelating Membrane Bearing Poly(Amino Phosphonic Acid) Groups. Metals 2017, 7, 61. https://doi.org/10.3390/met7020061
Wang X, Song L, Tian C, He J, Wang S, Wang J, Li C. DFT Investigation of the Effects of Coexisting Cations and Complexing Reagents on Ni(II) Adsorption by a Polyvinylidene Fluoride-Type Chelating Membrane Bearing Poly(Amino Phosphonic Acid) Groups. Metals. 2017; 7(2):61. https://doi.org/10.3390/met7020061
Chicago/Turabian StyleWang, Xiuli, Laizhou Song, Caili Tian, Jun He, Shuaijie Wang, Jinbo Wang, and Chunyu Li. 2017. "DFT Investigation of the Effects of Coexisting Cations and Complexing Reagents on Ni(II) Adsorption by a Polyvinylidene Fluoride-Type Chelating Membrane Bearing Poly(Amino Phosphonic Acid) Groups" Metals 7, no. 2: 61. https://doi.org/10.3390/met7020061





