The Morphologies of Different Types of Fe2SiO4–FeO in Si-Containing Steel
Abstract
:1. Introduction
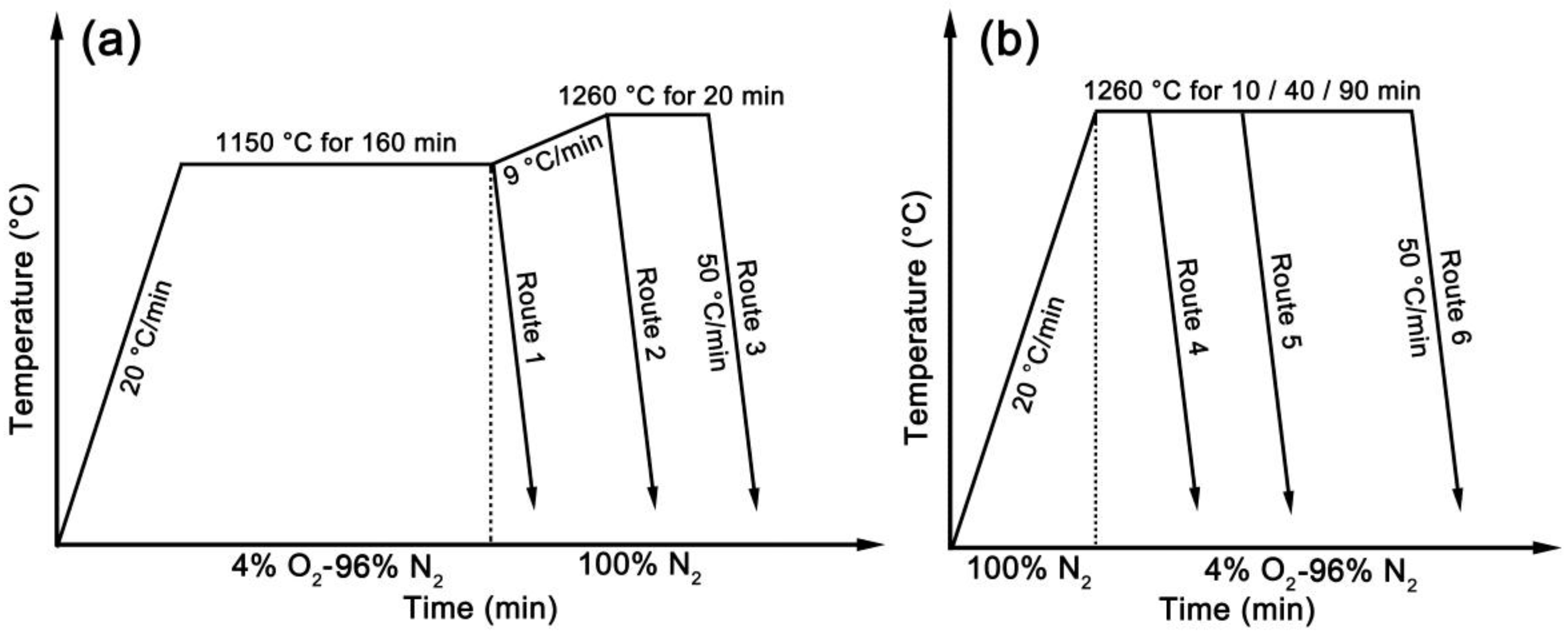
2. Materials and Methods
3. Results and Discussions
3.1. In Situ Observation
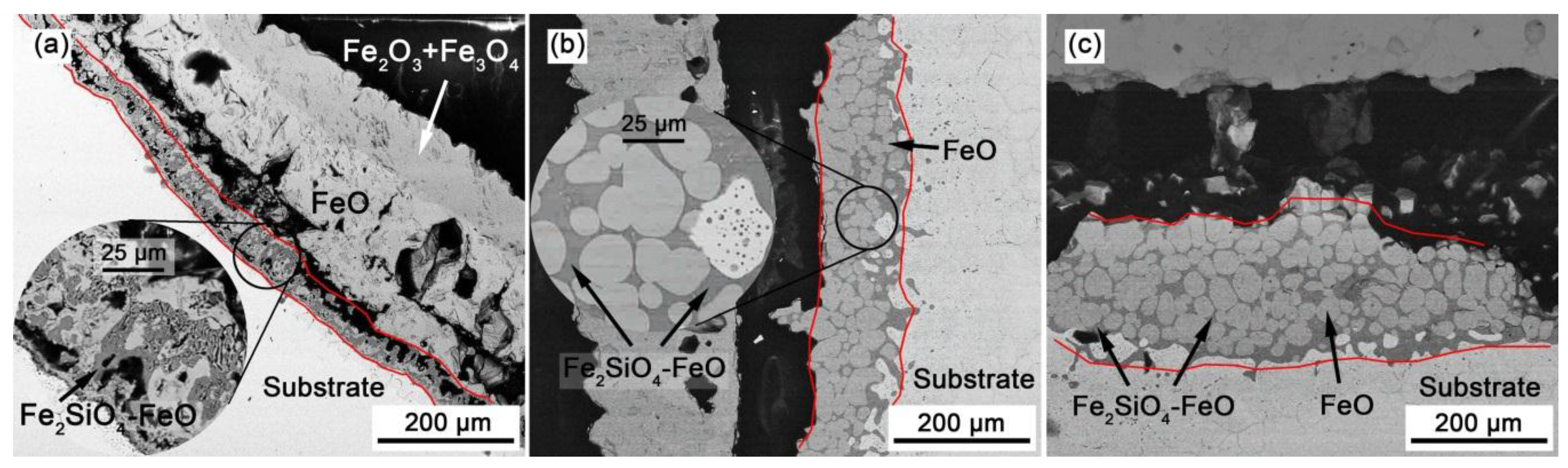
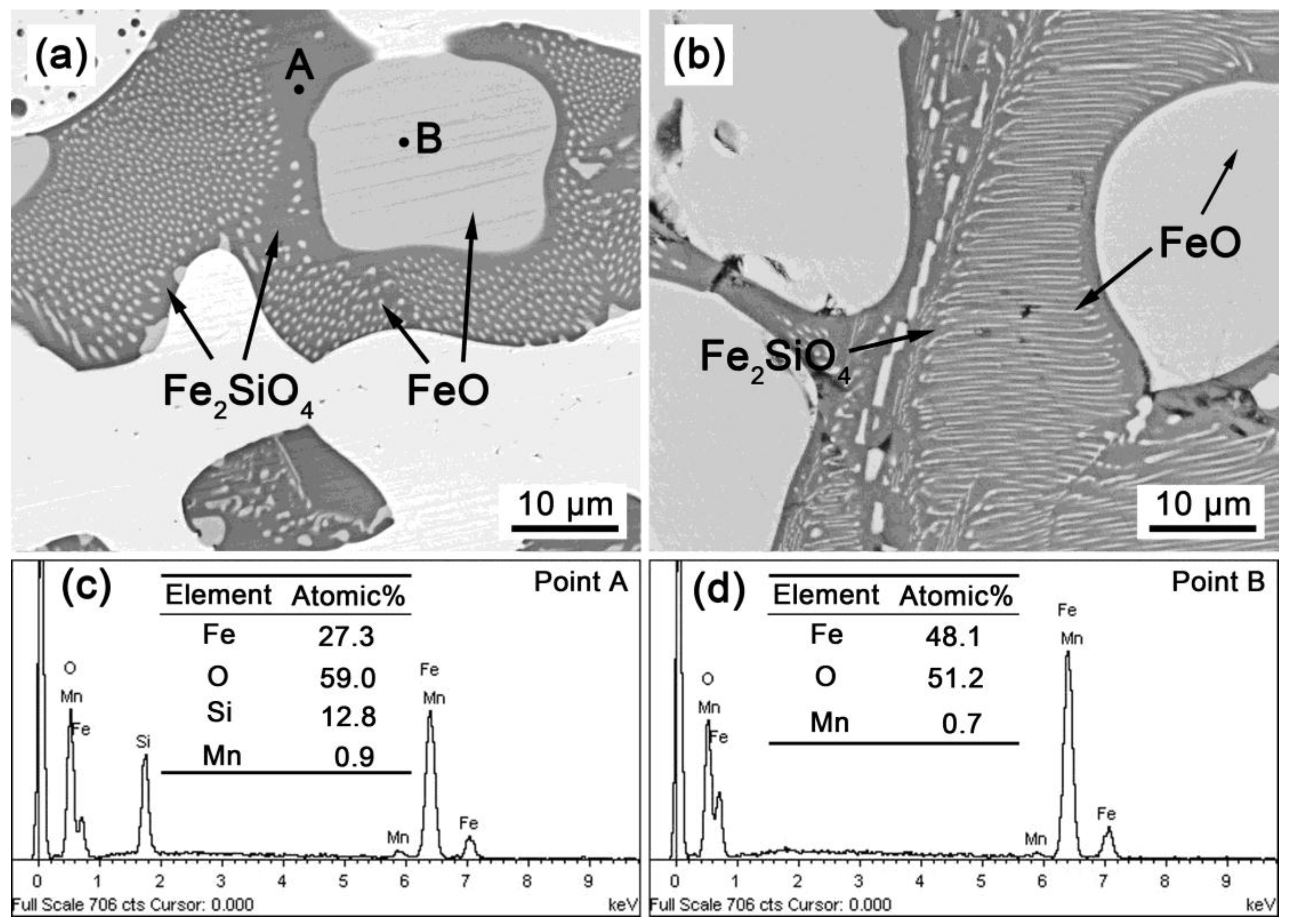
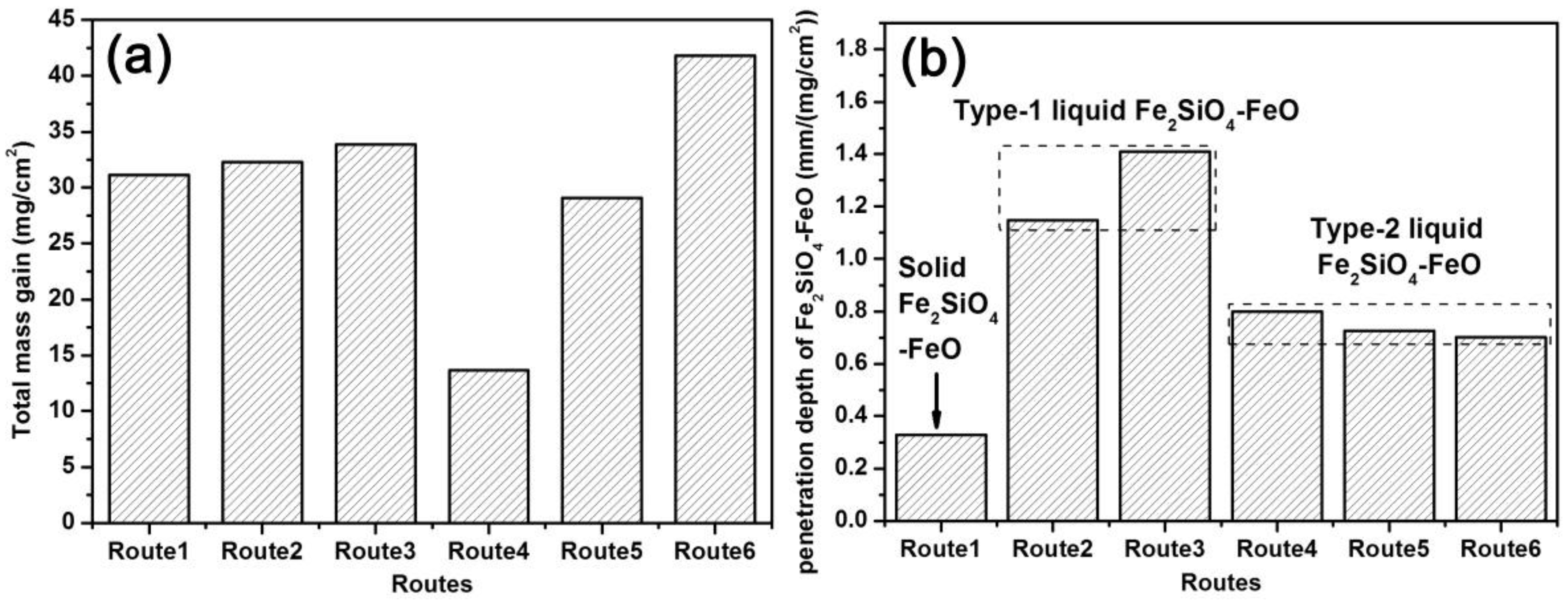
3.2. SEM Observations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| STA | simultaneous thermal analyzer |
| LSCM | high temperature laser scanning confocal microscopy |
| SEM | scanning electron microscope |
References
- Hu, H.J.; Xu, G.; Wang, L.; Xue, Z.L.; Zhang, Y.; Liu, G. The effects of Nb and Mo addition on transformation and properties in low carbon bainitic steels. Mater. Des. 2015, 84, 95–99. [Google Scholar] [CrossRef]
- Hu, H.J.; Xu, G.; Zhou, M.X.; Yuan, Q. Effect of Mo Content on Microstructure and Property of Low-Carbon Bainitic Steels. Metals 2016, 6, 173–182. [Google Scholar] [CrossRef]
- Zhou, M.X.; Xu, G.; Wang, L.; Yuan, Q. The Varying Effects of Uniaxial Compressive Stress on the Bainitic Transformation under Different Austenitization Temperatures. Metals 2016, 6, 119–130. [Google Scholar] [CrossRef]
- Takeda, M.; Onishi, T. Oxidation behavior and scale properties on the Si containing steels. Mater. Sci. Forum. 2006, 522, 477–488. [Google Scholar] [CrossRef]
- Okada, H.; Fukagawa, T.; Ishihara, H. Prevention of red scale formation during hot rolling of steels. ISIJ Int. 1995, 35, 886–891. [Google Scholar] [CrossRef]
- Yang, Y.L.; Yang, C.H.; Lin, S.N.; Chen, C.H.; Tsai, W.T. Effect of Si and its content on the scale formation on hot-rolled steel strips. Mater. Chem. Phys. 2008, 112, 566–571. [Google Scholar] [CrossRef]
- Liu, X.J.; Cao, G.M.; He, Y.Q.; Jia, T.; Liu, Z.Y. Effect of temperature on scale morphology of Fe-1.5Si Alloy. J. Iron Steel Res. Int. 2013, 20, 73–78. [Google Scholar] [CrossRef]
- Garnaud, G.; Rapp, R.A. Thickness of the oxide layers formed during the oxidation of iron. Oxid. Met. 1977, 11, 193–198. [Google Scholar] [CrossRef]
- Liu, X.J.; Cao, G.M.; Nie, D.M.; Liu, Z.Y. Mechanism of black strips generated on surface of CSP hot-rolled silicon steel. J. Iron. Steel Res. Int. 2013, 20, 54–59. [Google Scholar] [CrossRef]
- Fukagawa, T.; Okada, H.; Maeharara, Y. Mechanical of red scale defect formation in Si-added hot-rolled steels. ISIJ Int. 1994, 34, 906–911. [Google Scholar] [CrossRef]
- Yuan, Q.; Xu, G.; Zhou, M.X.; He, B. The effect of the Si content on the morphology and amount of Fe2SiO4 in low carbon steels. Metals 2016, 6, 94–103. [Google Scholar] [CrossRef]
- Mouayd, A.A.; Koltsov, A.; Sutter, E.; Tribollet, B. Effect of silicon content in steel and oxidation temperature on scale growth and morphology. Mater. Chem. Phys. 2014, 143, 996–1004. [Google Scholar] [CrossRef]
- Suarez, L.; Schneider, J.; Houbaert, Y. High-Temperature oxidation of Fe-Si alloys in the temperature range 900–1250 °C. Defect. Diffus. Forum. 2008, 273–276, 661–666. [Google Scholar] [CrossRef]
- He, B.; Xu, G.; Zhou, M.X.; Yuan, Q. Effect of Oxidation Temperature on the Oxidation Process of Silicon-Containing Steel. Metals 2016, 6, 137–145. [Google Scholar] [CrossRef]
- Yuan, Q.; Xu, G.; Zhou, M.X.; He, B. New insights into the effects of silicon content on the oxidation process in silicon-containing steels. Int. J. Min. Met. Mater. 2016, 23, 1–8. [Google Scholar] [CrossRef]
- Staettle, R.W.; Fontana, M.G. Advances in Corrosion Science and Technology; Springer: New York, NY, USA, 1974; pp. 239–356. [Google Scholar]
- Abuluwefa, H.; Guthrie, R.I.L.; Ajersch, F. The Effect of Oxygen Concentration on the Oxidation of Low-Carbon Steel in the Temperature Range 1000 to 1250 °C. Oxid. Met. 1996, 46, 423–440. [Google Scholar] [CrossRef]
- Chen, R.Y.; Yuen, W.Y.D. Review of the High-Temperature Oxidation of Iron and Carbon Steels in Air or Oxygen. Oxid. Met. 2003, 59, 433–468. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Xu, G.; Hu, H.; Yuan, Q.; Tian, J. The Morphologies of Different Types of Fe2SiO4–FeO in Si-Containing Steel. Metals 2017, 7, 8. https://doi.org/10.3390/met7010008
Zhou M, Xu G, Hu H, Yuan Q, Tian J. The Morphologies of Different Types of Fe2SiO4–FeO in Si-Containing Steel. Metals. 2017; 7(1):8. https://doi.org/10.3390/met7010008
Chicago/Turabian StyleZhou, Mingxing, Guang Xu, Haijiang Hu, Qing Yuan, and Junyu Tian. 2017. "The Morphologies of Different Types of Fe2SiO4–FeO in Si-Containing Steel" Metals 7, no. 1: 8. https://doi.org/10.3390/met7010008
APA StyleZhou, M., Xu, G., Hu, H., Yuan, Q., & Tian, J. (2017). The Morphologies of Different Types of Fe2SiO4–FeO in Si-Containing Steel. Metals, 7(1), 8. https://doi.org/10.3390/met7010008






