The Effect of Chromium on the Roasting Process of Vanadium Extraction
Abstract
:1. Introduction
2. Experimental Materials and Experimental Procedure
2.1. Materials
2.2. Experimentation
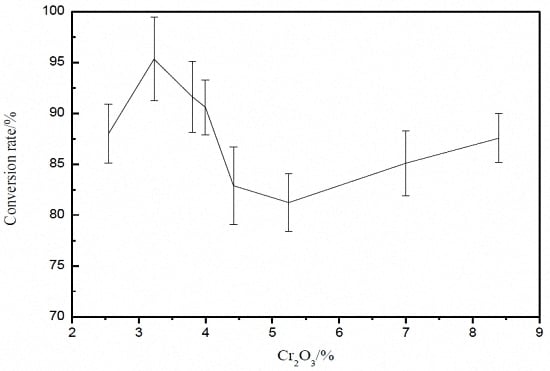
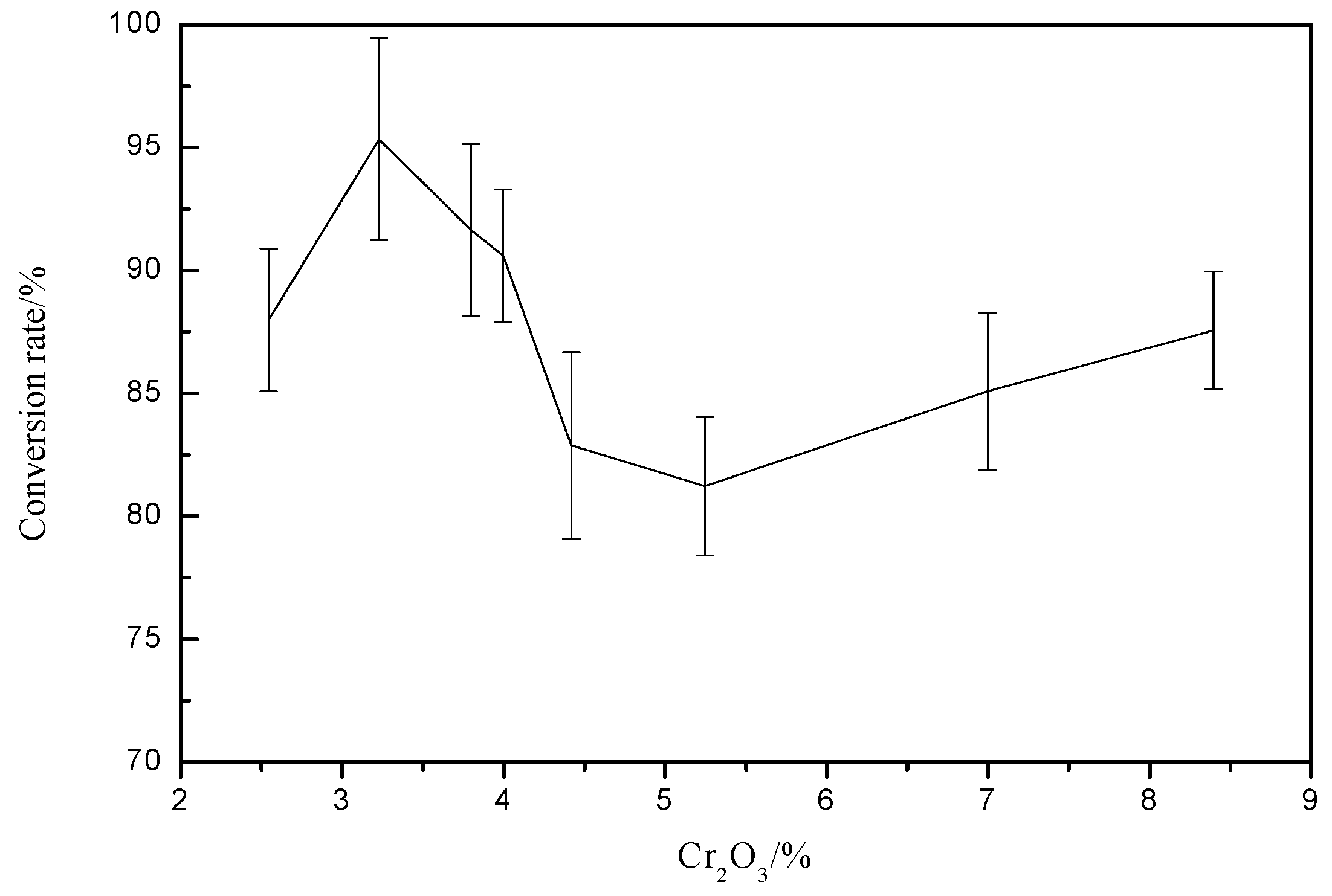
3. Effect of Cr2O3 on Vanadium Conversion Rate
4. The Mechanism of Chromium on in the Roasting of Vanadium Slag
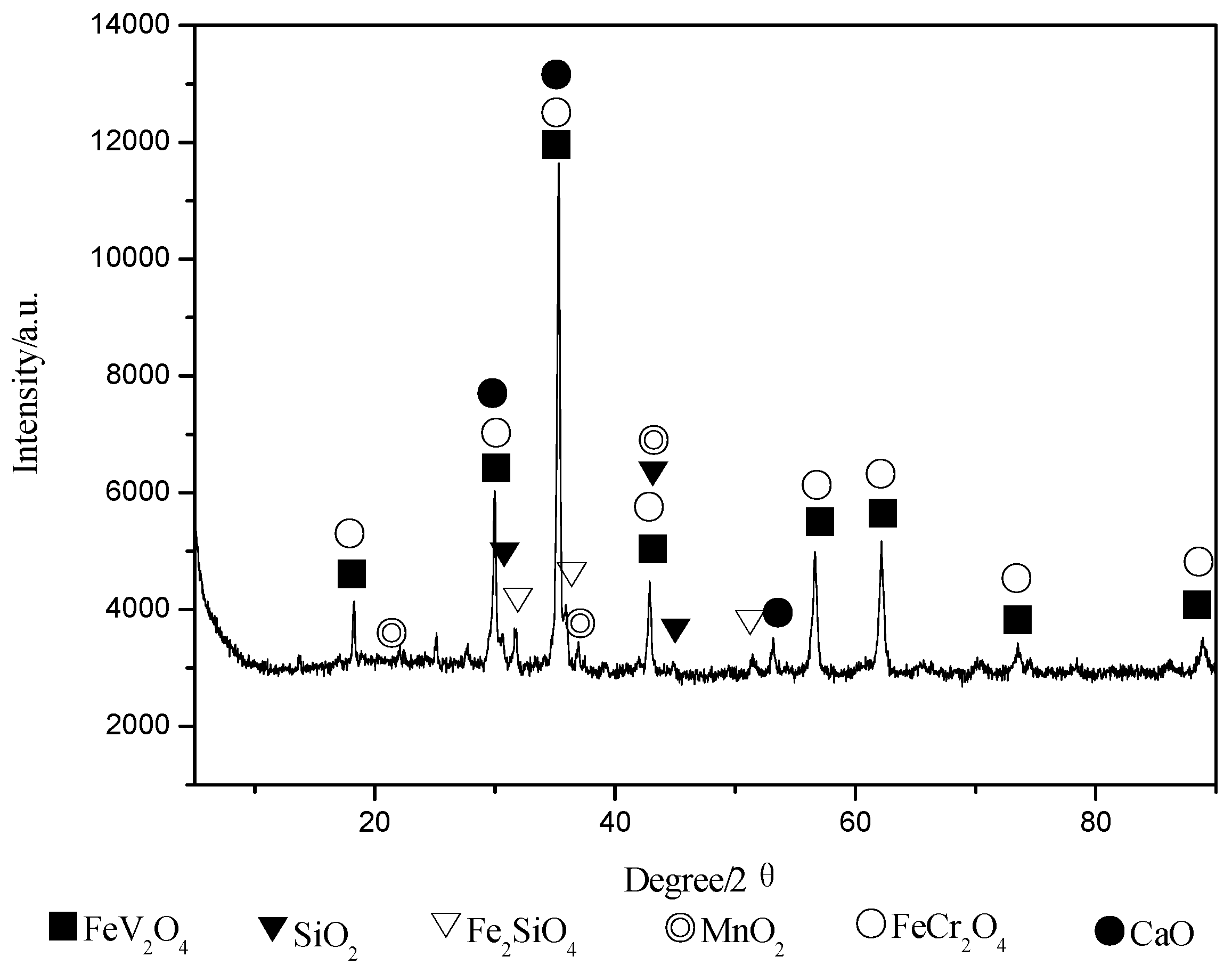
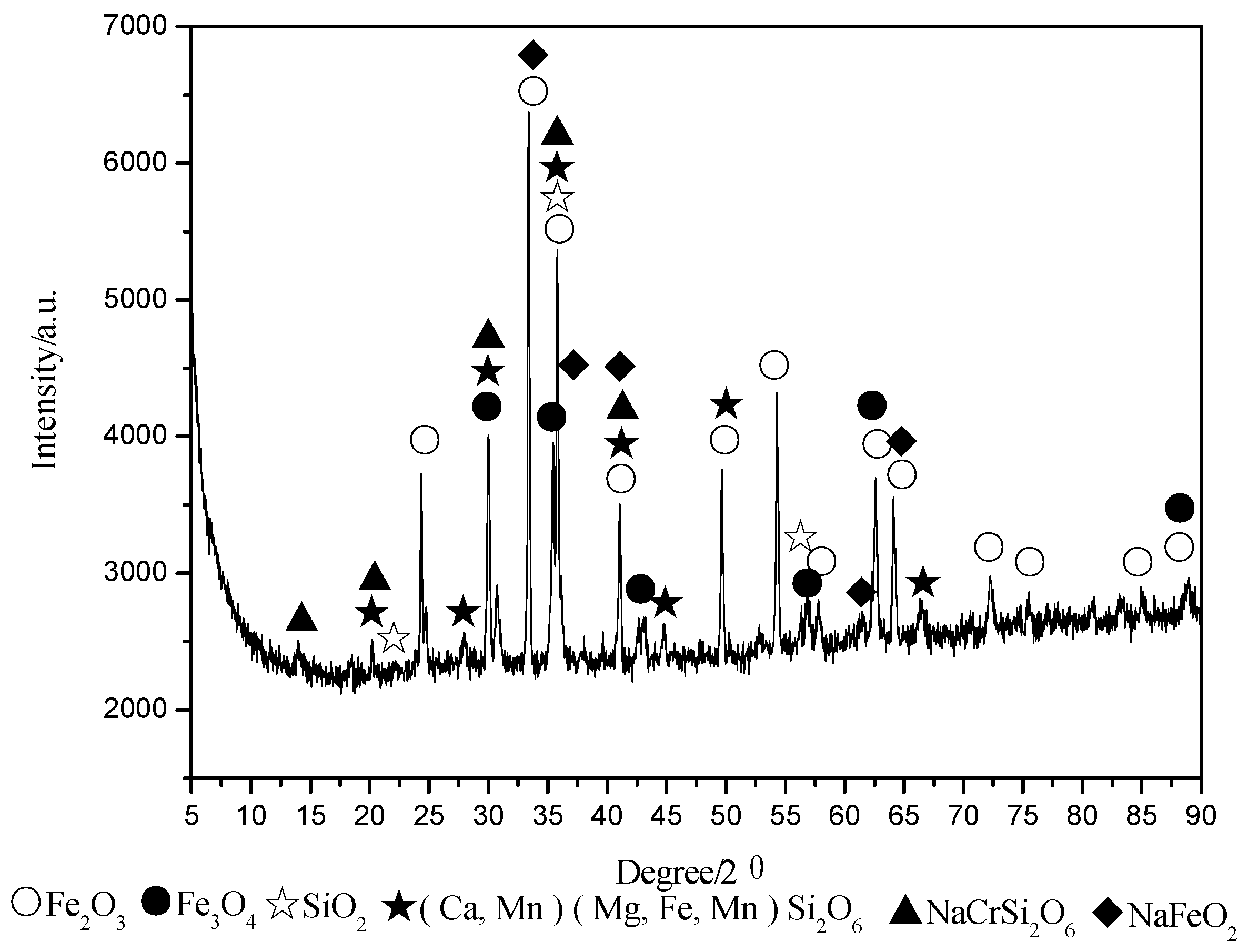
4.1. XRD Analysis
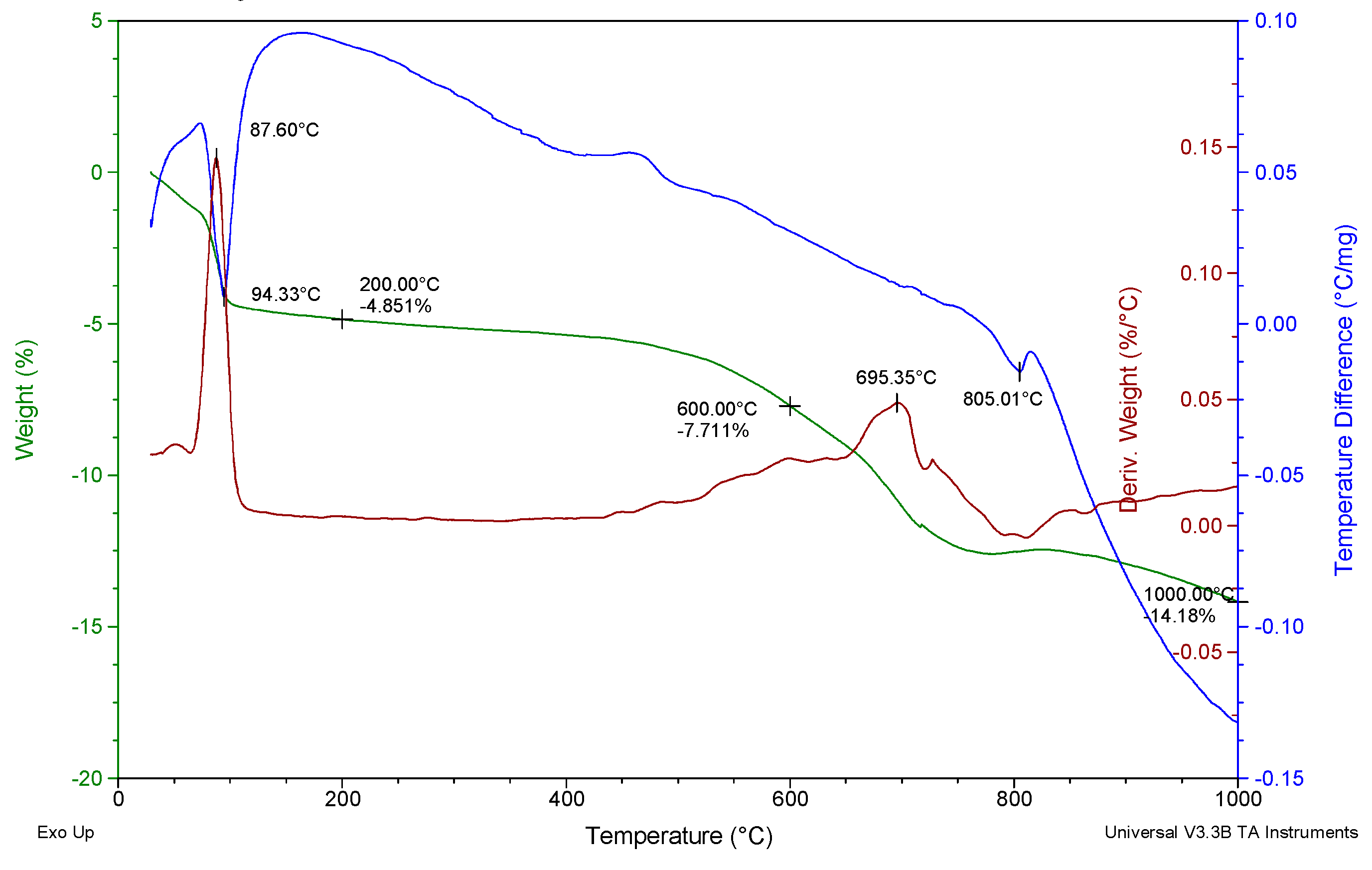
4.2. Comprehensive Thermal Analysis
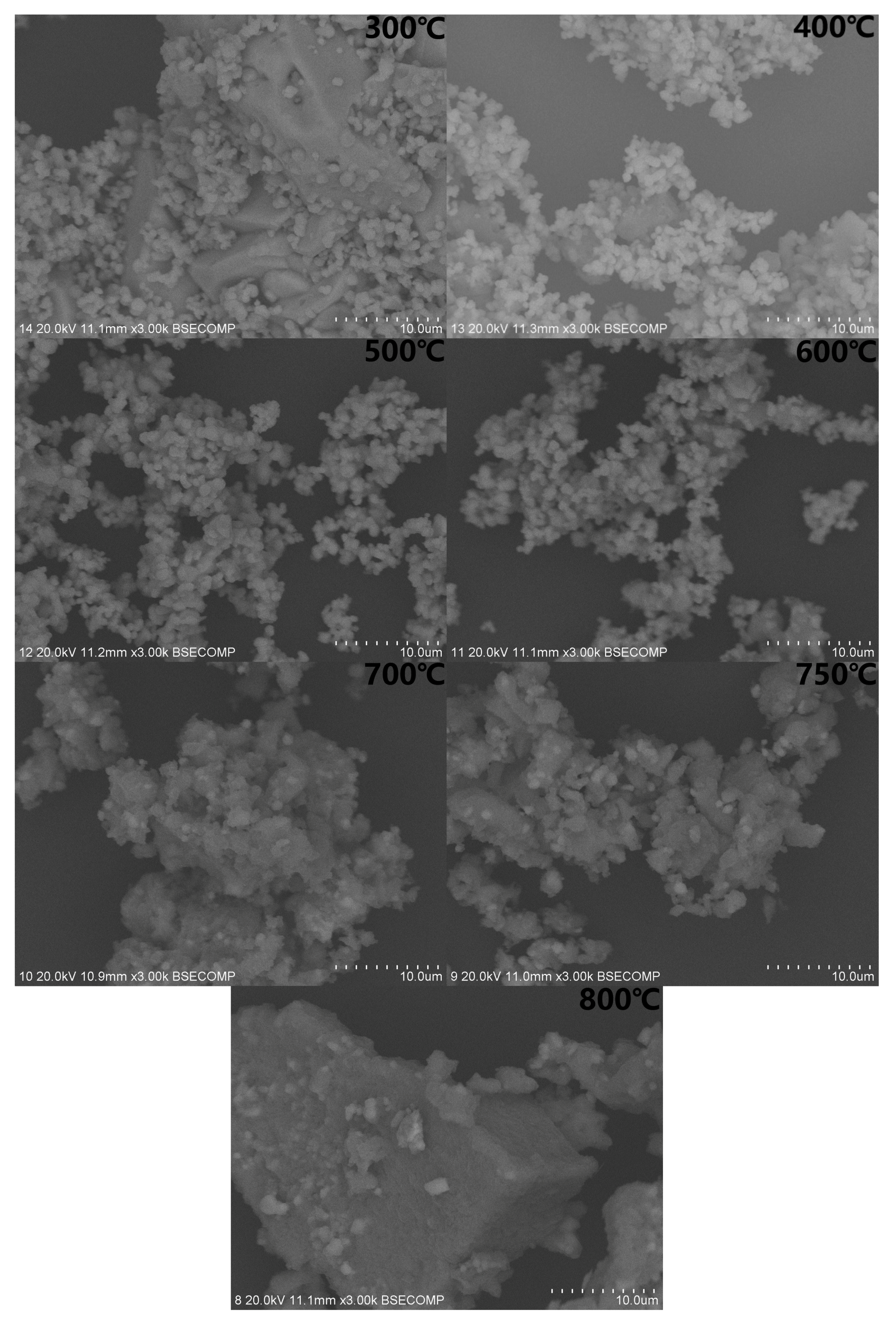
4.3. SEM Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, L.; Wang, J.; Xie, J.H. Technology on Vanadium Extraction from Bone Coal by Adding Sodium Chloride. Min. Metall. Eng. 2008, 28, 58–61. [Google Scholar]
- Cheng, L. Progress on Productive Technology of Vanadium Pentoxide. Gansu Metall. 2007, 29, 52–53. [Google Scholar]
- Yu, Q.; Long, M.L. Treatment of Pollution in Production of Vanadium Pentoxide with Stone Coal Containing Vanadium. Environ. Eng. 2007, 25, 96–97. [Google Scholar]
- Xi, X.; Yao, Q.; Hu, K.J. Present situation of production technology abroad about extraction of vanadium containing vanadium from oil slag. World Nonferrous Met. 2001, 5, 36–40. [Google Scholar]
- Raja, B.V.R. Vanadium market in the world. Steelworld 2007, 13, 19–22. [Google Scholar]
- Lin, H.L. Study on vanadium extraction from Fangshankou bone coal in GansuDunhuang; Chengdu University of Technology: Chengdu, China, 2000. [Google Scholar]
- Zhang, Y.; Fan, B.W.; Peng, D.P. Research of Precipitation Poly-ammonium Vandate from Extraction Solution of Acid-leaching Bone Coal. Chin. J. Rare Met. 2001, 25, 157–160. [Google Scholar]
- Chmielewski, A.G.; Urbaski, T.S.; Migda, W. Separation technologies for metals recovery from industrial wastes. Hydrometallurgy 1997, 45, 333–344. [Google Scholar] [CrossRef]
- Gabra, G.; Malinsky, I. A comparative study of the extraction of vanadium from titaniferous magnetite and slag. In Proceedings of the 110th AIME Meeting, Metallurgical Society of AIME, Chicago, IL, USA, 22–26 February 1981; pp. 167–189.
- Goddard, J.B.; Fox, J.S. Salt roasting of vanadium ores. In Proceedings of the 110th AIME Meeting, Chicago, IL, USA, 22–26 February 1981; pp. 127–145.
- Lu, M.; Zhang, Y.M.; Liu, T.; Yang, D. Sintering Action of NaCl Calcination during Extracting Vanadium from Stone Coal. Chin. J. Rare Met. 2009, 33, 894–897. [Google Scholar]
- Wang, M.Y.; Wang, X.W. Research Status and Prospect of Vanadium Leaching Processes from Stone Coal. Chin. J. Rare Met. 2010, 34, 90–97. [Google Scholar]
- Shlewit, H.; Alibrahim, M. Extraction of Sulfur and Vanadium from Petroleum Coke by Means of Salt-roasting Treatment. Fuel 2006, 85, 878–880. [Google Scholar] [CrossRef]
- Ji, Z. A summary of the harmfulness of chrome residue and its treatment. Inorg. Chem. Ind. 2003, 35, 1–4. [Google Scholar]
- Ji, Z. The two key factors for disposal of chrome residue. Inorg. Chem. Ind. 2004, 36, 1–4. [Google Scholar]
- Jagupilla, S.C.; Moon, D.H.; Waznea, M.; Christodoulatos, C.; Kim, M.G. Effects of particle size and acid addition on the remediation of chromite ore processing residue using ferrous sulfate. J. Hazard. Mater. 2009, 168, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Geelhoed, J.S.; Meeussen, J.C.; Hillier, S.; Lumsdon, D.G.; Thomas, R.P.; Farmer, J.G.; Paterson, E. Identification and geochemical modeling of processes controlling leaching of Cr(VI) and other major elements from chromite ore processing residue. Geochim. Cosmochim. Acta 2002, 66, 3927–3942. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, T.L.; Zhou, Z.Q.; Pan, P.; Sun, X.H. Preparation V2O5 from Low Grade Vanadium bearing Slag of High Calcium and High Phosphor. Ferro-Alloys 2007, 38, 18–23. [Google Scholar]
- Huang, D.X. Vanadium Extraction and Steelmaking; Metallurgical Industry Press: Beijing, China, 2000. [Google Scholar]
- Wang, J.C.; Chen, H.S.; Li, G.S.; Xie, Q.L.; Deng, X.B. Production process from vanadium slag smelted in Pyongyang converter. Iron. Steel Vanadium Titanium 1998, 19, 41–46. [Google Scholar]
- Wu, B. Extracting vanadium pentoxide from high silicon low-vanadium vanadium slag. Ferro-Alloys 2008, 200, 5–8. [Google Scholar]
- Qiu, S.X.; Liu, X.S.; Zhou, D.; Gao, H.M.; Wang, P.P.; Jia, D.N.; Hu, F. Study on extracting vanadium pentoxide in vanadium slag. Inorg. Chem. Ind. 2010, 42, 46–48. [Google Scholar]
- Li, H.; Zhou, R.Z.; Wang, W.J.; Li, J.J.; Wang, X. Method for Extracting V2O5 from Vanadium Slag. CN92108960.0, 23 February 1994. [Google Scholar]
- Kozlov, V.A.; Demidov, A.E. Chemical principles of a technology for making pure vanadium pentoxide. Metallurgist 2000, 44, 428–433. [Google Scholar] [CrossRef]
- Wen, S.D.; Ding, Y.X. Exploration of the factors of roasting conversion ratio for vanadium slag. J. Chengde Petrol. Coll. 1999, 1, 9–12. [Google Scholar]
- Fu, Z.B.; Deng, J.B.; Zhang, L.; Gao, G.J.; Chen, H.J.; Gu, J.Y. Method for Extracting Vanadium from Sodium Roasting Clinker. ZL 200910176895.3, 23 September 2009. [Google Scholar]
- Li, X.-S.; Xie, B.; Wang, G.-E.; Li, X.-J. Oxidation process of low-grade vanadium slag in presence of Na2CO3. Trans. Nonferrous Met. Soc. China 2011, 21, 1860–1867. [Google Scholar] [CrossRef]
- Li, H.-Y.; Fang, H.-X.; Wang, K.; Zhou, W.; Yang, Z.; Yan, X.-M.; Ge, W.-S.; Li, Q.-W.; Xie, B. Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting-water leaching. Hydrometallurgy 2015, 156, 124–135. [Google Scholar] [CrossRef]
- Chen, D.H. Study on vanadium extraction from vanadium extraction residue. Inorg. Chem. Ind. 1993, 4, 28–32. [Google Scholar]

| Component | TFe | V | SiO2 | CaO | Mn | MgO | TiO2 | Cr2O3 | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | 31.12 | 7.68 | 16.65 | 2.04 | 5.4 | 3.07 | 8.49 | 8.8 | 0.089 | |
| Component | TFe | V | SiO2 | CaO | Mn | MgO | TiO2 | Cr2O3 | P |
|---|---|---|---|---|---|---|---|---|---|
| wt. % | 33.36 | 0.935 | 15.83 | 2.16 | 5.77 | 2.84 | 8.82 | 6.58 | 0.087 |
| No. | Cr2O3% | Temperature/°C | Time/min | Alkali Vanadium Ratio | Alkali Salt Ratio |
|---|---|---|---|---|---|
| 1 | 8.39 | 780 | 100 | 1.2 | 3.5/1 |
| 2 | 7.00 | 780 | 100 | 1.2 | 3.5/1 |
| 3 | 5.25 | 780 | 100 | 1.2 | 3.5/1 |
| 4 | 4.42 | 780 | 100 | 1.2 | 3.5/1 |
| 5 | 4.00 | 780 | 100 | 1.2 | 3.5/1 |
| 6 | 3.80 | 780 | 100 | 1.2 | 3.5/1 |
| 7 | 3.23 | 780 | 100 | 1.2 | 3.5/1 |
| 8 | 2.54 | 780 | 100 | 1.2 | 3.5/1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Xue, X.; Yang, H. The Effect of Chromium on the Roasting Process of Vanadium Extraction. Metals 2016, 6, 157. https://doi.org/10.3390/met6070157
Liu D, Xue X, Yang H. The Effect of Chromium on the Roasting Process of Vanadium Extraction. Metals. 2016; 6(7):157. https://doi.org/10.3390/met6070157
Chicago/Turabian StyleLiu, Dong, Xiangxin Xue, and He Yang. 2016. "The Effect of Chromium on the Roasting Process of Vanadium Extraction" Metals 6, no. 7: 157. https://doi.org/10.3390/met6070157